Abstract
We developed a novel titanium coating that has applications for preventing infection-related implant failures in dentistry and orthopedics. The coating incorporates an antimicrobial peptide, GL13K, derived from parotid secretory protein, which has been previously shown to be bactericidal and bacteriostatic in solution. We characterized the resulting physicochemical properties, resistance to degradation, activity against Porphyromonas gingivalis, and in vitro cytocompatibility. P. gingivalis is a pathogen associated with dental peri-implantitis, an inflammatory response to bacteria resulting in bone loss and implant failure. Our surface modifications obtained a homogeneous, highly hydrophobic, and strongly-anchored GL13K-coating that was resistant to mechanical, thermochemical, and enzymatic degradation. The GL13K-coatings had bactericidal effect and thus, significantly reduced the number of viable bacteria compared to control surfaces. Finally, adequate proliferation of osteoblasts and human-gingival-fibroblasts demonstrated the GL13K-coating’s cytocompatibility. The robustness, antimicrobial activity, and cytocompatibility of GL13K-biofunctionalized titanium make it a promising candidate for sustained inhibition of bacterial biofilm growth. This surface chemistry provides a basis for development of multifunctional bioactive surfaces to reduce patient morbidities and improve long-term clinical efficacy of metallic dental and orthopedic implants.
1. INTRODUCTION
Dental implants machined from commercially-pure titanium are increasingly becoming the treatment of choice for replacing missing teeth with 10–15 year survival rates of 89%+ [1]. Despite recent improvement in implant survival rates, there remains a significant demand for improving osseointegration and maintaining a perimucosal seal. In particular, the long-term clinical efficacy of titanium (Ti) dental implants is influenced by peri-implantitis, an inflammatory response resulting in bone loss and implant failure [2]. Peri-implantitis or infection can affect up to 14% of implants after 5 years, although the incidence may be higher due to poor clinical diagnosis and the limited data and short duration of reporting clinical studies [1, 3, 4]. The trans-mucosal placement of dental implants presents unique challenges for designing surface modifications capable of decreasing the formation of bacterial biofilm. The coronal implant surface is exposed to the mucosal sulcus, capable of harboring biofilms. Similar to periodontitis, in peri-impantitis local and host factors cause an ecologic shift toward anaerobic, gram-negative and gram-positive bacteria associated with inflammation and bone loss, including Porphyromonas gingivalis, Eikenella corrodens, Fusobacterium nucleatum, and Peptostreptococcus micros [5], as well as microorganisms not commonly associated with periodontitis, such as Staphylococcus spp., enterics and Candida spp. [6]. Noninvasive strains of P. gingivalis, e.g., ATCC-33277, feature hydrophobic fimbriae which make the cell surface highly hydrophobic [7] and mediate binding to gingival epithelium and implant surfaces [8].
Cationic and histatin-derived antimicrobial peptides (AMPs) adsorbed to Ti have been shown to prevent biofilm formation [9, 10]. The physical driving forces behind the antibacterial activity include positive charge, hydrophobicity, and flexibility [11]. AMPs are hypothesized to bind to the bacterial cell membrane and disrupt its integrity by displacing positively-charged counterions and inducing a change in the membrane electrochemical potential, resulting in activation of autolytic enzymes [12]. AMPs have also been shown to modulate inflammatory responses of host cells [11].
The antimicrobial-peptide GL13K features a modified 13-amino-acid sequence based on the sequence of Parotid Secretory Protein (PSP; BPIFA2) [13], a potential dual-function host defense salivary protein with agglutination and anti-inflammatory activity [14]. The GL13K peptide exhibits bactericidal activity in vitro and anti-endotoxin activity in a mouse model. GL13K is bactericidal in solution against Pseudomonas aeruginosa, Escherichia coli, and Streptococcus gordonii, with a minimum inhibitory concentration of 5–10 μg/ml against P. aeruginosa and E. coli and 64 μg/ml against S. gordonii. GL13K also kills P. aeruginosa in biofilm and inhibits the lipopolysaccharide-stimulated secretion of TNFα from macrophages by 80% [14]. In contrast, GL13K is not effective in killing three strains of P. gingivalis (53977, W50, and DPG3), presumably due to gingipain proteases secreted by these bacteria [15]. These data support GL13K as a promising novel and efficacious antimicrobial agent for dental implants and restorative dentistry. While the potential for antimicrobial-peptide surfaces has been demonstrated [16, 17], an optimal surface for dental implants must retain its antimicrobial activity in a chronically microbial-challenged environment. Most antimicrobial-Ti surfaces have only been studied in vitro for 24–48 hours and existing approaches for surface modification of dental implants to reduce bacterial biofilm are not yet used clinically [17–19]. The only available treatments for peri-implantitis include mechanical debridement, surgical therapy, and non-surgical local or systemic antibiotic therapy [20, 21], but there is no reliable evidence suggesting which is the most effective intervention [22].
We present surface chemistry for stable immobilization of GL13K to Ti as a model antimicrobial bioactive Ti-surface for applications in dentistry and orthopaedics to reduce implant-associated infections and failure. We aim to fabricate a novel antimicrobial coating by covalently-anchoring the cationic antimicrobial peptide, GL13K, to a Ti-surface using a silane chemical-linker. We investigated mechanical and thermochemical stability, antimicrobial activity, and cytocompatibility of the antimicrobial coatings.
2. MATERIALS and METHODS
2.1 Materials
The antimicrobial GL13K (GKIIKLKASLKLL-CONH2, MW=1424 g/mol) and non-antimicrobial GK7-NH2 (GQIINLK-CONH2, MW=784 g/mol) peptides were obtained at >95% purity from the BioMedical Genomics Center-University of Minnesota. GL13K consists of a 13-amino-acid sequence of Parotid Secretory Protein (PSP 141–153) substituted with three lysine-residues in positions 2, 5 and 11 [15]. The negative-control peptide GK7-NH2 consists of the N-terminal seven amino-acids of GL13K without the lysine substitutions [13].
Titanium discs (10 mm diameter) were punched from sheets of commercially-pure titanium-Grade II (McMaster-Carr; Robbinsville, NJ). 3-(chloropropyl)-triethoxysilane (CPTES, 95%), other chemical reagents, and solvents were purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.2 Sample preparation
Ti-discs were ground with 600-grit SiC-discs and polished using 0.5 μm alumina suspensions (Buehler, Lake Bluff, IL). Samples were soaked in acetone overnight and then ultrasonicated in a series of solvents, and dried with N2-gas. Ti samples were etched (eTi) in 10 mL of 5 M NaOH overnight at 60°C, further cleaned with DI-water for 30-minutes (2x), and dried with N2-gas. A set of non-etched samples was instead O2-plasma treated (pTi) (PDC-32G, Harrick Plasma, Ithaca, NY, USA) for 5 minutes for conducting depth-profiling XPS analysis.
2.3 Silanization
Activated samples (pTi or eTi) were silanized by immersion in a solution of 7 mL anhydrous pentane, 1.2 mL 3-(chloropropyl)-triethoxysilane (CPTES), and 0.6 mL diisopropylethylamine (DIPEA) under saturated N2 atmosphere for 1-hour. After silanization, the samples were washed and dried with N2-gas.
2.4 Peptide immobilization
Solutions of GL13K (0.1 mM) or GK7-NH2 (0.25 mM) were prepared in 8 mL of Na2CO3 (0.5 mg/ml). The number of immobilization sites (free amines) was kept constant between the peptide solutions. Silanized eTi (eTi-Sil) and non-silanized eTi samples (eTi) were immersed in the appropriate peptide solutions overnight under argon in a desiccator and then rinsed with DI-water to produce eTi-Sil-GL13K (cov-GL13K), eTi-Sil-GK7-NH2 (cov-GK7-NH2), and eTiGL13K (phys-GL13K) samples.
2.5 Diffuse Reflectance Infrared Fourier Transform Spectroscopy (DRIFTS)
Surfaces were characterized with DRIFTS (Nicolet Series II Magna-IR System 750 FTIR, OMNIC software) following ultrasonication for 2-hours in DI-water to detect amide bonds. 32-scans per sample (2–4 cm−1 resolution) were averaged across replicates.
2.6 X-ray Photoelectron Spectroscopy (XPS)
XPS was performed (SSX-100, Al-Kα x-ray, 1 mm spot-size, 35° take-off angle) to characterize the atomic composition of the surface. Samples were ultrasonicated for 5-minutes, washed, and dried with N2-gas prior to measurements. Survey scans were done at 1 eV step-size for 3–4 scans/sample. High resolution scans of C1s, N1s, and O1s were taken at 0.1 eV step-size. The chemical shifts of high resolution scans were based on C1s at 285.0 eV. Depth profiling of pTi-samples used Ar+-sputtering 4 kV, 25 mA, and 5 mPa (2 nm/min SiO2) for 0–12 seconds (2-second intervals) with high-resolution O1s scans. The peak fittings and quantification of surface chemical composition were conducted using ESCA 2005 software provided with the XPS system.
2.7 Water contact angles
Advancing DI-water contact angles (θc) were measured with the sessile-drop (3 μL) method (DM-CE1 with FAMAS software, Kyowa Interface Science Co., Niiza, Japan). θc were determined at 1-second intervals for 35 seconds. θc were calculated by averaging the values of the last 5-seconds for each experiment. Samples were tested before and after ultrasonication in DI-water for 2 hours, as well as after 5 and 8-days of immersion in Phosphate Buffered Saline (PBS, pH 7.4, 37°C) following ultrasonication to determine the mechanical and thermochemical stability of the coatings, respectively.
2.8 Biofilm formation
A 48-hour culture of P. gingivalis ATCC 33277 was diluted to 1×106 cells/mL in Todd Hewitt Base (THB) broth with 4% Heat-Inactivated Fetal Bovin Serum (FBS), 5.0 μg/mL Hemin, and 0.5 μg/mL Menodine. The Ti-discs were cleaned with ethanol and placed in culture plates, and 1 mL of diluted P. gingivalis was added to each well. The culture was incubated under anaerobic conditions at 37°C for 8-days. After incubation medium was removed from the well, Ti-discs were removed to a new culture plate and washed with PBS. Discs were transferred to micro-centrifuge tubes with 300 μL pre-reduced oxygen PBS and sonicated for 60-seconds. 100 μL of obtained solutions were transferred to an opaque wall 96-well plate, and ATP was quantified (Promega BacTiter-Glo™ Microbial Cell Viability Assay, Promega, Madison, WI). 100-fold dilutions of obtained solutions were also plated on THB-blood agar plates and surviving CFUs were enumerated after 7 days anaerobic incubation at 37°C. To assess that all bacteria were removed from the substrates, following the removal of PBS after sonication we added 50 μL of pre-reduced oxygen PBS to the disc + 50 μL of BacTiter-Glo reagent and transferred the solution to a well plate and measured the luminescence again for assessing lack of bacteria activity. After being challenged with bacteria for 8-days, each type of surface was prepared for Scanning Electron Microscopy (SEM, TM3000, Hitachi, Japan). Bacteria were fixed with 2% glutaraldehyde in 0.1 M sodium cacodylate buffer for 1 hour, followed by dehydration in increasing ethanol concentrations, critical-point drying (Samdri-780A, Tousimis), and platinum coating (DV-502/VCR, Denton).
2.9 Cytocompatibility tests
Human gingival fibroblasts (HGF) and MC3T3-E1 murine osteoblasts (OB) were seeded on titanium-discs (2×103 cells/ml). After 24, 48, 72, and 120-hours, substrates were washed with PBS and fixed with 4% paraformaldehyde for 10-minutes at room temperature. The cell numbers in each well were determined by staining cell nuclei with 300 nM DAPI (Invitrogen, Carlsbad, CA) in PBS for 10-minutes at room temperature, washing with PBS followed by fluorescence microscopy (Eclipse E800, Nikon, Japan) at 10x magnification. Cell nuclei were counted with ImageJ software (NIH, Bethesda, MD, USA) from three representative images per sample and averaged over replicated samples.
2.10 Statistical analysis
Statistically-significant differences between groups for material characterizations and antimicrobial tests were assessed with single-factor ANOVA with post-hoc multiple comparisons tests (p<0.05) (SPSS Statistics, IBM). Tukey-Kramer or Dunnett’s T3 post-hoc tests were used, for equal variances assumed or not-assumed, respectively. For cytocompatibility tests a two-way ANOVA with Bonferroni post-hoc test (Prism, GraphPad Software) was used. At least three replicates were used per experiment.
3. RESULTS
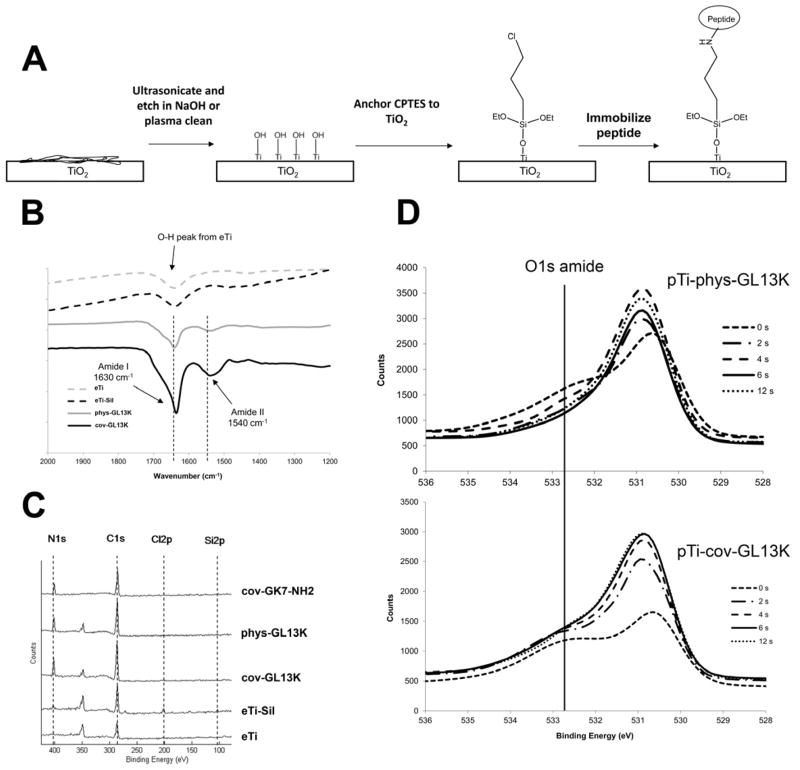
DRIFTS and XPS were used to characterize the presence of the immobilized GL13K peptide on Ti surfaces. DRIFTS spectra show amide I and II peaks at 1630 and 1540 cm−1, respectively (Fig. 1B), which indicate the presence of the peptide on silanized (cov-GL13K) and non-silanized (phys-GL13K) surfaces. XPS data (Table 1 and Figure 1C) show an increase in nitrogen-N1s signal following treatment with the GL13K and GK7 peptides. The increase in C% and N% in the peptide-modified samples proves the immobilization of the peptides on the surface of titanium. The N/Ti ratios (Table 1) for all the peptide-functionalized surfaces suggest a higher quantity of peptide was retained (higher N/Ti ratio) on the cov-GL13K surfaces than on the surfaces with physisorbed peptide, phys-GL13K. This is in accordance with the higher absorption signal on the DRIFT spectra for the cov-GL13K samples than for the phys-GL13K samples. Thus, both covalent attachment from the CPTES silane-linker and physisorption on eTi contributed to immobilization of the GL13K peptide on the Ti surface. Figure 1D shows the oxygen-O1s amide signal exponentially decreased with Ar+ sputtering time, suggesting the peptide coating is thicker and denser when GL13K is covalently attached to Ti surfaces. This is supported by the higher time constant, τ, of the O1s attenuation curve (Figure S1) for the covalently-anchored coatings, τpTi-cov-GL13K = 4.1 seconds, compared to the physisorbed coatings, τpTi-phys-GL13K = 2.1 seconds. These results are consistent with the aforementioned higher absorption DRIFT signal and N/Ti ratio for the surfaces with covalently-anchored peptides than for the ones with physisorbed peptides.
Figure 1.
Surface chemical characterizations. A) Schematic of chemical surface modification methodology using a silane linker, 3-chloropropyltriethoxysilane (CPTES), to immobilize peptides GL13K and GLK7-NH2 to etched Ti. B) Diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) of eTi-samples, showing amide peaks from the peptides on the Ti-surface. C) XPS survey scans (80–420 eV) of coated-Ti demonstrate nitrogen-N1s signal on the peptide-coated surfaces, but not the non-coated eTi. D) High-resolution XPS spectra of O1s peak with Ar+ sputtering time (0–12s) show depth profiling evidence of a thicker peptide coating on covalently-anchored cov-GL13K compared to physisorbed phys-GL13K.
Table 1.
X-ray Photoelectron Spectroscopy atomic composition.
| Sample | %C | %N | %O | %Si | %Ti | %Cl | %Bal. | N/Ti |
|---|---|---|---|---|---|---|---|---|
| eTi | 22.7 | 56.0 | 17.1 | 4.2 | 0 | |||
| eTi-Sil | 22.3 | 1.1 | 54.2 | 1.3 | 17.1 | 1.4 | 2.6 | 0.06 |
| phys-GL13K | 41.7 | 8.4 | 38.0 | 9.7 | 2.3 | 0.87 | ||
| cov-GL13K | 45.5 | 8.9 | 34.9 | 8.8 | 1.9 | 1.01 | ||
| cov-GK7-NH2 | 33.0 | 6.5 | 43.0 | 0.6 | 13.2 | 3.8 | 0.49 |
Etching Ti with NaOH is known to alter surface topography [1]. SEM images in Figure 2A show how the etching created a nano-rough surface (eTi, Ra = 68 nm by AFM, Figure S2). This nano-roughness influences surface area for peptide immobilization, adhesion of bacteria, and surface properties.
Figure 2.
Physicochemical surface properties. A) Scanning electron microscopy of etched Ti (eTi) samples at low and high magnifications. B) Advancing contact angle, θc results with 3 μl de-ionized water sessile drop before and after ultrasonication in de-ionized water for 2 hours. Data are shown as mean ±SEM (N=3), *different from nonsonicated sample, p<0.05. C) Thermochemical stability of the surface determined by advancing contact angle θc. The samples were immersed in PBS (pH 7.4, 37°C) for 0, 5, and 8 days right after ultrasonication. Data are shown as mean ±SEM (N=3). No statistically significant differences before and after thermochemical challenge were observed, except after 8 days for the cov-GK7-NH2 surface, *different from day 0, p<0.05.
θc were determined to assess wettability of peptide-coated Ti (Figure 2B). The GL13K-coatings on eTi-surfaces were highly hydrophobic, with a statistically-significant increase in θc to 125±1.35° (p<0.01) on both CPTES-anchored and physisorbed GL13K surfaces compared to etched controls. θc increased upon ultrasonication, possible due to removal of unbound silane molecules. The θc observed with physisorbed (phys-GL13K) and covalently-anchored (cov-GL13K) samples did not differ significantly (p>0.05). Figure 2C shows no significant decrease in θc after 8-day incubation at 37°C, indicating that the GL13K-modified surfaces were thermochemically stable. The θc for control peptide-coated surfaces cov-GK7-NH2 decreased following ultrasonication and 8-day incubation at 37°C (p<0.05), suggesting higher loss of GK7-NH2 peptide than in the case of cov-GL13K surfaces. The process for immobilizing the peptides did not control the orientation of the covalent anchoring for any of the peptides and thus, the differences in peptide retention can be attributable to the fact that GK7-NH2 peptides have less number of reactive amines (less immobilization sites) than GL13K peptides. However, the contact angle of cov-GK7-NH2 surfaces was still statistically-significantly higher (p<0.05) than uncoated surfaces (eTi-Sil) before and after ultrasonication and incubation (Figures 2B and 2C).
To test the antibacterial properties of the GL13K-coated surfaces, biofilms of P. gingivalis were cultured on the coated Ti-discs and live cells were quantitated by ATP content and CFU (Figure 3A). The cov-GL13K coated disks exhibited significantly fewer live cells than discs coated with either the control-peptide, cov-GK7-NH2, or uncoated eTi (p<0.01). This difference was not due to differences in bacterial adhesion, since SEM revealed that the number of bacteria on Ti-substrates was roughly the same on GL13K-modified and unmodified eTi surfaces (Figure 3B).
Figure 3.
Characterization of biofilm formation. A) P. gingivalis ATCC 33277 viability on GL13K-modified surfaces after culture for 8 days under anaerobic conditions at 37°C. Bacterial ATP content (shaded bars) and CFUs (open bars) of P. gingivalis. Data are expressed as mean ±SEM of 5 samples. *different from eTi and cov-GK7-NH2 controls, p<0.05. B) Scanning electron microscopy of P. gingivalis adhered to Ti surfaces after incubation for 8 days. Final magnification is indicated by the scale bars.
To test the biocompatibility of GL13K coated Ti-discs, the proliferation of HGF fibroblast and osteoblast cells was tested. There was no decrease in cell numbers between cultures incubated for 1,3,5-days on GL13K-coated eTi and uncoated-controls (Figure 4). No statistically significant differences were found, except for HGF-cells at day-3.
Figure 4.
Proliferation of HGF and OB cells after 5 days on GL13K-modified and eTi controls characterized by fluorescence microscopy. A) Cell nuclei detected by fluorescence microscopy after 1 day and 5 days culture of HGF and OB cells. Representative images are shown. B) Cell counts of HGF after 1, 3, and 5 days. Data are expressed as mean ± SEM of 5 samples, *different from non-coated eTi at the same time of incubation, p<0.05.
4. DISCUSSION
In this report, we demonstrate the successful immobilization of the bio-inspired antimicrobial peptide GL13K on Ti-surfaces. The GL13K peptide coatings were stable on eTi as their high hydrophobicity was retained after mechanical and physicochemical challenges. The mechanical stability of the GL13K coatings following ultrasonication suggests that they will not detach during surgical placement or while bearing in vivo fluid flow forces. Thermochemical stability is important for the long-term retention of peptide on the Ti-surface [23] and antimicrobial effectiveness. The hydrophobic nature of the GL13K peptide protects the silane from hydrolysis, thus slowing the decrease in θc over 8-days of incubation in PBS.
The robust coating was achieved by both covalent and non-covalent interactions, because the silanized and non-silanized Ti-surfaces with GL13K showed very similar results. Etching forms a nano-rough amorphous titanate [24] with vast number of polar hydroxyl-groups. This produces a very hydrophilic and negatively-charged surface [24]. GL13K is highly cationic and thus, strongly attracted to and stabilized on the surface by electrostatic forces. However, our results suggest more peptide was retained on the silanized compared to non-silanized plasma-treated surfaces. Nano-rough topography of eTi was not conducive to XPS depth-profiling characterization, but a similar result is expected on those surfaces because of the equivalent reactivity (high hydrophilicity) of the activated etched and plasma-treated Ti.
The observed reduction of viable bacteria on GL13K-modified eTi is attributed to a bactericidal effect of the surface. The results suggest that bacterial surface adhesion is not GL13K-dependent because the GL13K-coating did not affect P. gingivalis cell adhesion to eTi surfaces compared to controls. The antimicrobial effect can be explained by electrostatic interference of the bacterial membrane by positively-charged lysine residues in the GL13K peptide facilitated by specific interaction with hydrophobic fimbriae of P. gingivalis [25] and the low surface-energy (θc>100°) of GL13K-modified eTi surfaces. Since soluble GL13K is not effective against P. gingivalis (Hirt, unpublished observation), the antimicrobial activity of GL13K-modified surfaces is likely due to immobilized peptide rather than release of peptide into the solution. We hypothesize that surface immobilization protects GL13K from degradation by gingipain-proteases secreted by P. gingivalis. Consistent with this interpretation, GL13K was not significantly released from coated eTi-surfaces when immersed in saliva or serum for 1 week, as analyzed by surface fluorescence signal of fluorescently-labeled GL13K-peptide coatings (Figure S4). Thus, sustained resistance to hydrolytic and enzymatic degradation of the coatings may be expected once the coatings are implanted and exposed to a biological environment, although in vivo experiments are required to further investigate this claim.
The surface’s effect on oral tissues must be considered in addition to its antimicrobial activity, because implant surface properties can affect mucosal attachment [26, 27]. The peri-mucosal seal is important for osseointegration and resisting peri-implantitis. A hydrophilic surface positively supports soft tissue attachment to the implant [28, 29], which poses a concern for the highly hydrophobic GL13K-modified eTi surfaces. However, it has also been shown that a nanoporous TiO2-surface, like the eTi surface, offers good soft tissue attachment and high marginal bone around implants [30]. The GL13K eTi-surface has a unique balance of physicochemical properties, with low surface-energy, but significant nanoroughness. GL13K-modifed surfaces promoted increase in cell number of HGF and OB cells with time, warranting further investigation of the coating’s in vivo biocompatibility. An ideal surface will support both osseointegration and peri-mucosal seal. Clinically, the rough osseointegrating implant body surface, which would significantly benefit from biofilm resistance, is exposed when peri-implant bone height is lost [31]. The implant body needs to support soft-tissue attachment in addition to the coronal surface so bone level can be maintained to prevent disease progression and late failures.
5. CONCLUSIONS
We successfully functionalized titanium with GL13K, a novel bio-inspired coating. We demonstrated with a series of in vitro experiments that the coating is highly stable, cytocompatible with HGF and OB, and antimicrobial against P. gingivalis, a putative pathogen of peri-implantitis. Our surface chemistry provides a basis for development of multifunctional bioactive surfaces to reduce patient morbidities and improve long-term clinical efficacy of titanium dental implants [32].
Supplementary Material
Acknowledgments
The authors thank Drs. R. Chen, E. Jensen, B. Luo, and R. Hegde, and Mr. Y. Maazouz for their technical assistance. Funding: 3M Non-tenured Faculty Award (CA), UMN-SOD Summer Fellowship (KVH), and PHS grant R01DE017989 from the NIDCR (SUG). Parts of this work were carried out in the Characterization Facility, University of Minnesota, which receives partial support from NSF through the MRSEC program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kyle V. Holmberg, Email: holmb103@umn.edu.
Mahsa Abdolhosseini, Email: mahsaabd@dental.upenn.edu.
Yuping Li, Email: lixx1191@umn.edu.
Xi Chen, Email: chenx838@umn.edu.
Sven-Ulrik Gorr, Email: sugorr@umn.edu.
Conrado Aparicio, Email: apari003@umn.edu.
References
- 1.Norowski PA, Bumgardner JD. Biomaterial and Antibiotic Strategies for Peri-implantitis. J Biomed Mater Res Part B. 2009;88B:530–43. doi: 10.1002/jbm.b.31152. [DOI] [PubMed] [Google Scholar]
- 2.Mombelli A. Microbiology and antimicrobial therapy of peri-implantitis. Periodontol 2000. 2002;28:177–89. doi: 10.1034/j.1600-0757.2002.280107.x. [DOI] [PubMed] [Google Scholar]
- 3.Berglundh T, Persson L, Klinge B. A systematic review of the incidence of biological and technical complications in implant dentistry reported in prospective longitudinal studies of at least 5 years. Journal of Clinical Periodontology. 2002;29:197–212. doi: 10.1034/j.1600-051x.29.s3.12.x. [DOI] [PubMed] [Google Scholar]
- 4.Mombelli A, Muller N, Cionca N. The epidemiology of peri-implantitis. Clinical Oral Implants Research. 2012;23:67–76. doi: 10.1111/j.1600-0501.2012.02541.x. [DOI] [PubMed] [Google Scholar]
- 5.Renvert S, Roos-Jansåker A-M, Lindahl C, Renvert H, Rutger Persson G. Infection at titanium implants with or without a clinical diagnosis of inflammation. Clinical Oral Implants Research. 2007;18:509–16. doi: 10.1111/j.1600-0501.2007.01378.x. [DOI] [PubMed] [Google Scholar]
- 6.Leonhardt Å, Renvert S, Dahlén G. Microbial findings at failing implants. Clinical Oral Implants Research. 1999;10:339–45. doi: 10.1034/j.1600-0501.1999.100501.x. [DOI] [PubMed] [Google Scholar]
- 7.Yoshinari M, Oda Y, Kato T, Okuda K, Hirayama A. Influence of surface modifications to titanium on oral bacterial adhesion in vitro. J Biomed Mater Res. 2000;52:388–94. doi: 10.1002/1097-4636(200011)52:2<388::aid-jbm20>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 8.Andrian E, Grenier D, Rouabhia M. Porphyromonas gingivalis-Epithelial Cell Interactions in Periodontitis. J Dent Res. 2006;85:392–403. doi: 10.1177/154405910608500502. [DOI] [PubMed] [Google Scholar]
- 9.Kazemzadeh-Narbat M, Kindrachuk J, Duan K, Jenssen H, Hancock REW, Wang R. Antimicrobial peptides on calcium phosphate-coated titanium for the prevention of implant-associated infections. Biomaterials. 2010;31:9519–26. doi: 10.1016/j.biomaterials.2010.08.035. [DOI] [PubMed] [Google Scholar]
- 10.Yoshinari M, Kato T, Matsuzaka K, Hayakawa T, Shiba K. Prevention of biofilm formation on titanium surfaces modified with conjugated molecules comprised of antimicrobial and titanium-binding peptides. Biofouling. 2010;26:103–10. doi: 10.1080/08927010903216572. [DOI] [PubMed] [Google Scholar]
- 11.Jenssen H, Hamill P, Hancock REW. Peptide Antimicrobial Agents. Clin Microbiol Rev. 2006;19:491–511. doi: 10.1128/CMR.00056-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hilpert K, Elliott M, Jenssen H, Kindrachuk J, Fjell CD, Körner J, et al. Screening and Characterization of Surface-Tethered Cationic Peptides for Antimicrobial Activity. Chemistry & Biology. 2009;16:58–69. doi: 10.1016/j.chembiol.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Gorr SU, Sotsky JB, Shelar AP, Demuth DR. Design of bacteria-agglutinating peptides derived from parotid secretory protein, a member of the bactericidal/permeability increasing-like protein family. Peptides. 2008;29:2118–27. doi: 10.1016/j.peptides.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 14.Gorr SU, Abdolhosseini M, Shelar A, Sotsky J. Dual host-defence functions of SPLUNC2/PSP and synthetic peptides derived from the protein. Biochem Soc Trans. 2011;39:1028–32. doi: 10.1042/BST0391028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdolhosseini M, Nandula SR, Song J, Hirt H, Gorr S-U. Lysine substitutions convert a bacterial-agglutinating peptide into a bactericidal peptide that retains anti-lipopolysaccharide activity and low hemolytic activity. Peptides. 2012;35:231–8. doi: 10.1016/j.peptides.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green JBFT, Nordhaus MA. Review of immobilized antimicrobial agents and methods for testing. Biointerphases. 2011;6:CL2–43. doi: 10.1116/1.3645195. [DOI] [PubMed] [Google Scholar]
- 17.Costa F, Carvalho IF, Montelaro RC, Gomes P, Martins MCL. Covalent immobilization of antimicrobial peptides (AMPs) onto biomaterial surfaces. Acta Biomater. 2011;7:1431–40. doi: 10.1016/j.actbio.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Zhao LZ, Chu PK, Zhang YM, Wu ZF. Antibacterial Coatings on Titanium Implants. J Biomed Mater Res Part B. 2009;91B:470–80. doi: 10.1002/jbm.b.31463. [DOI] [PubMed] [Google Scholar]
- 19.Bumgardner JD, Adatrow P, Haggard WO, Norowski PA. Emerging antibacterial biomaterial strategies for the prevention of peri-implant inflammatory diseases. Int J Oral Maxillofac Implants. 2011;26:553–60. [PubMed] [Google Scholar]
- 20.Graziani F, Figuero E, Herrera D. Systematic review of quality of reporting, outcome measurements and methods to study efficacy of preventive and therapeutic approaches to peri-implant diseases. Journal of Clinical Periodontology. 2012;39:224–44. doi: 10.1111/j.1600-051X.2011.01832.x. [DOI] [PubMed] [Google Scholar]
- 21.Hallstrom H, Persson GR, Lindgren S, Olofsson M, Renvert S. Systemic antibiotics and debridement of peri-implant mucositis. A randomized clinical trial. Journal of Clinical Periodontology. 2012;39:574–81. doi: 10.1111/j.1600-051X.2012.01884.x. [DOI] [PubMed] [Google Scholar]
- 22.Esposito M, Grusovin Maria G, Worthington Helen V. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd; 2012. Interventions for replacing missing teeth: treatment of peri-implantitis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dubruel P, Vanderleyden E, Bergada M, De Paepe I, Chen H, Kuypers S, et al. Comparative study of silanisation reactions for the biofunctionalisation of Ti-surfaces. Surf Sci. 2006;600:2562–71. [Google Scholar]
- 24.Aparicio C, Manero JM, Conde F, Pegueroles M, Planell JA, Vallet-Regi M, et al. Acceleration of apatite nucleation on microrough bioactive titanium for bone-replacing implants. J Biomed Mater Res Part A. 2007;82A:521–9. doi: 10.1002/jbm.a.31164. [DOI] [PubMed] [Google Scholar]
- 25.Chen R, Willcox MDP, Cole N, Ho KKK, Rasul R, Denman JA, et al. Characterization of chemoselective surface attachment of the cationic peptide melimine and its effects on antimicrobial activity. Acta Biomater. 2012;8:4371–9. doi: 10.1016/j.actbio.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 26.Abrahamsson I, Berglundh T, Glantz PO, Lindhe J. The mucosal attachment at different abutments. Journal of Clinical Periodontology. 1998;25:721–7. doi: 10.1111/j.1600-051x.1998.tb02513.x. [DOI] [PubMed] [Google Scholar]
- 27.Myshin HL, Wiens JP. Factors affecting soft tissue around dental implants: A review of the literature. The Journal of Prosthetic Dentistry. 2005;94:440–4. doi: 10.1016/j.prosdent.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 28.An N, Rausch-fan X, Wieland M, Matejka M, Andrukhov O, Schedle A. Initial attachment, subsequent cell proliferation/viability and gene expression of epithelial cells related to attachment and wound healing in response to different titanium surfaces. Dental Materials. 2012;28:1207–14. doi: 10.1016/j.dental.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 29.Kloss FR, Steinmüller-Nethl D, Stigler RG, Ennemoser T, Rasse M, Hächl O. In vivo investigation on connective tissue healing to polished surfaces with different surface wettability. Clinical Oral Implants Research. 2011;22:699–705. doi: 10.1111/j.1600-0501.2010.02038.x. [DOI] [PubMed] [Google Scholar]
- 30.Rossi S, Tirri T, Paldan H, Kuntsi-Vaattovaara H, Tulamo R, Närhi T. Peri-implant tissue response to TiO2 surface modified implants. Clinical Oral Implants Research. 2008;19:348–55. doi: 10.1111/j.1600-0501.2007.01478.x. [DOI] [PubMed] [Google Scholar]
- 31.Neoh KG, Hu XF, Zheng D, Kang ET. Balancing osteoblast functions and bacterial adhesion on functionalized titanium surfaces. Biomaterials. 2012;33:2813–22. doi: 10.1016/j.biomaterials.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 32.Chen X, Sevilla P, Aparicio C. Surface biofunctionalization by covalent co-immobilization of oligopeptides. Colloids and Surfaces B: Biointerfaces. 2013;107:189–97. doi: 10.1016/j.colsurfb.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.