SUMMARY
Genetic mediated physiological processes that rely on both pharmacological and nutritional principles hold great promise for the successful therapeutic targeting of reduced carbohydrate craving, body-friendly fat loss, healthy body recomposition, and overall wellness. By integrating an assembly of scientific knowledge on inheritable characteristics and environmental mediators of gene expression, we review the relationship of genes, hormones, neurotransmitters, and nutrients as they correct unwanted weight gain coupled with unhappiness. In contrast to a simple one-locus, one-mechanism focus on pharmaceuticals alone, we hypothesize that the use of nutrigenomic treatment targeting multi-physiological neurological, immunological, and metabolic pathways will enable clinicians to intercede in the process of lipogenesis by promoting lipolysis while attenuating aberrant glucose cravings. In turn, this approach will enhance wellness in a safe and predictable manner through the use of a Genetic Positioning System (GPS) Map. The GPS Map, while presently incomplete, ultimately will serve not only as a blueprint for personalized medicine in the treatment of obesity, but also for the development of strategies for reducing many harmful addictive behaviors and promoting optimal health by using substances compatible with the body’s immune system.
Introduction
Background
For the most part, conventional therapeutic interventions to treat a variety of health problems are biologically impositional, one-dimensional, targeted at single locus solutions, and employing single-mechanistic tactics. Anatomy, physiology, and metabolism are the result of a veritable symphony of biochemical transactions occurring simultaneously, interactively, and interdependently, following specific genetic instructions. All the functionally competent cells, tissues, and organs of the body are genetically programmed for survival. Biological impact of one-dimensional single locus phase-one interventions, such as with pharmacological agents, elicits a sequela of genetic, hormonal, immunological and biochemical responses, a sort of ripple effect, catalyzed by genetic survival instructions. This phase-one induced manifestation of survival responses can result in phase-two adverse reactions, rejection and retaliation against the impacting substance and its effects. Conventional treatments of conditions from obesity to addictions employ, among others, deprivation, inhibition, and/or stimulation tactics to achieve one-dimensional objectives without considering the genetically programmed systemic responses to and consequences of such interventions. The evidence, as examples, of the growing obesity epidemic and the alarming rates of relapse in addicts demonstrates the failure of these one-dimensional ‘body-un-friendly’ tactics at achieving sustainable success. Without finding ‘body-friendly’ strategies that work with and augment the body’s genetic programs and biological systems, the hope of achieving sustained success in a wide variety of chronic and compulsive behaviors and conditions is dim.
With the advent of nutrigenomics, a new more promising multi-dimensional paradigm in health management is emerging. The need for a multifaceted approach is exemplified with a simple illustration. Using only significant calorie reduction and deprivation to effect weight loss a most common tactic, the body is programmed to answer this stressful imposition by lowering the basal (or resting) metabolic rate (i.e. the rate of calorie burning) to conserve energy, increase fat storage depots, and increase cravings. This makes calorie deprivation alone a strong catalyst for these conservation- and survival-induced responses. Aside from basic survival instructions, because of the starvation-induced state, this deprivation affects energy homeostasis, the reward/pleasure cravings generated by the brain, hormonal responses due to related stress and energy deficits/production issues, and the immune system, which is programmed to ensure your survival. Combined, these responses guarantee long term failure of deprivation-based weight loss tactics.
Multiple biological pathways and related genetic polymorphisms need to be simultaneously addressed in order to achieve positive and sustainable outcomes. Our research has identified at least five of these important pathways, which seem to be related to energy management, stress responsiveness and management, the craving and reward system of the brain, neuro-endocrine system management and immune system management. The tendency to confine any of these pathways in a singular therapeutic compartment, unrelated to any of the other pathways, should be avoided. In this regard, we have developed a Genetic Positioning System (GPS) Map to enable more successful ‘charting’ of nutrigenomic therapeutic strategies.
Hypothesis
In regard to craving and reward management, based on neuro-chemical and genetic evidence, we suggested earlier that both prevention and treatment of multiple addictions, such as dependence to alcohol, nicotine, and glucose, should involve a biphasic approach. Thus, acute treatment should consist of preferential blocking of postsynaptic Nucleus Accumbens (NAc) dopamine receptors (D1–D5), whereas long-term activation of the mesolimbic dopaminergic system should involve activation and/or release of Dopamine (DA) at the NAc site. Failure to do so will result in abnormal mood, behavior and potential suicide ideation. Individuals possessing a paucity of serotonergic and/or dopaminergic receptors, and an increased rate of synaptic DA catabolism due to high catabolic genotype of the COMT gene, are predisposed to self-medicating any substance or behavior that will activate DA release, including glucose, alcohol, opiates, psychostimulants, nicotine, gambling, sex, and even excessive internet gaming. Acute utilization of these substances and/or stimulatory behaviors induces a feeling of well being. Unfortunately, sustained and prolonged abuse leads to a toxic “pseudo feeling” of well being resulting in tolerance, disease and/or discomfort. Thus, a reduced number of DA receptors, due to carrying the DRD2 A1 allelic genotype, results in excessive craving behavior; whereas a normal or sufficient amount of DA receptors results in low craving behavior. In terms of preventing substance abuse and or aberrant glucose ingestion, one goal would be to induce a proliferation of DA D2 receptors in genetically prone individuals. While in vivo experiments using a typical D2 receptor agonist induce down regulation, experiments in vitro have shown that constant stimulation of the DA receptor system via a known D2 agonist results in significant proliferation of D2 receptors in spite of genetic antecedents. In essence, D2 receptor stimulation signals negative feedback mechanisms in the mesolimbic system to induce mRNA expression causing proliferation of D2 receptors [1].
Proposal
The authors propose that D2 receptor stimulation can be accomplished via the use of Synaptamine™, a natural but therapeutic nutraceutical formulation that potentially induces DA release, causing the same induction of D2-directed mRNA and thus proliferation of D2 receptors in the human[2]. This proliferation of D2 receptors in turn will induce the attenuation of craving behavior. In fact, as mentioned earlier, this model has been proven in research showing DNA-directed compensatory overexpression (a form of gene therapy) of the DRD2 receptors, resulting in a significant reduction in alcohol craving behavior in alcohol preferring rodents. Utilizing natural dopaminergic repletion therapy to promote long term dopaminergic activation will ultimately lead to a common, safe and effective modality to treat Reward Deficiency Syndrome (RDS) behaviors including Substance Use Disorders (SUD), Attention Deficit Hyperactivity Disorder (ADHD), Obesity and other reward deficient aberrant behaviors [1]. This concept is further supported by the more comprehensive understanding of the role of dopamine in the NAc as a “wanting” messenger in the meso-limbic DA system. It is our opinion that the most complex disorder known as obesity can be treated with neurochemical pathway manipulation by nutrients, as defined in this context as Immunological Compatible Substances (pharmaceuticals may be considered in part to be non immunologically compatible and possibly foreign) using the Genetic Positioning System (GPS) Map.
Genes and obesity
While there is a plethora of research on the utilization of nutritional approaches to obesity related health consequences there is a paucity of research involving neurotransmitter manipulation involving the brain reward system coupled with genetic polymorphic identification and obesity. In fact, at the time of this writing there over 26,083 studies on the relationship between obesity and nutrition; there are 4258 studies on genes and obesity; there are 941 studies on brain neurotransmitters and obesity; there are 30 studies on the relationship between neurochemistry, obesity and genes; and other than from our laboratory, there are no known studies concerning the relationship of neurochemistry, obesity genes and nutrition. While there are literally thousands of genes involved in the expression of obesity, only 600 have been adequately associated with neurological and or metabolic pathways related to obesity and its sequelae. Li et al. [3] integrated 2343 items of evidence from peer-reviewed publications between 1976 and 2006 linking genes and chromosome regions to addiction by single-gene strategies, microarray, proteomics, or genetic studies. In these studies, Li et al. [3] identified 1500 human addiction-related genes and developed KARG (http://karg.cbi.pku.edu.cn), which is the first molecular database for addiction-related genes. Li et al. [3] then performed a meta-analysis of 396 genes that were supported by two or more independent items of evidence to identify 18 molecular pathways that were statistically significantly enriched, covering both upstream signaling events and downstream effects. Five molecular pathways significantly enriched for all four different types of addictive drugs were identified as common pathways which may underlie shared rewarding and addictive actions. These included two new ones, GnRH signaling pathway and gap junction. Li et al. connected the common pathways into a hypothetical common molecular network for addiction. They observed that fast and slow positive feedback loops were interlinked through CAMKII, which may provide clues to explain some of the irreversible features of addiction. Interestingly, the common thread involves dopaminergic and glutaminergic genes. The dopamine molecule promotes both “pleasure” and “stress coping abilities”. Wellness constitutes an enhanced state of pleasure with tranquility. A major link to overeating is uncontrollable stress and carbohydrate craving.
Role of dopamine agonists in proliferation of D2 receptors and glucose craving
Studies in vitro have shown that constant stimulation of DA receptors by agonists result in proliferation of Dopamine D2 receptors coupled to G proteins. Specifically it was shown [4,5] in trans-fected kidney cells and expressed in Spodoptera frugiperda insect cells that stimulation of DA receptors by the pure D2 receptor agonist Bromocriptine resulted in proliferation of D2 receptors over a 14 day period. In the same study it was shown that administration of a DA antagonist caused the proliferation of D2 antagonist receptors as well. These two independent effects suggest that environmental manipulation in spite of genetic antecedents will result in receptor proliferation. This can best be explained by the understanding that agonist activity involves the stimulation of the mRNA that is involved in transcription. Activation of the DRD2/mRNA results in a negative feedback that promotes an enhancement of mRNA directed D2 receptor proliferation. This fact becomes very important when coupled with the findings that an increase in substance seeking is due to a paucity of DA D2 receptors [6,7]. Therefore, if low D2 receptors equate to increased craving behavior then an increase in D2 receptors should result in attenuation of craving behavior. Our solution is to naturally promote greater DA release at the NAc, not via powerful DA agonists that could ultimately lead to DA down-regulation. As mentioned above, DA is known as the main neurotransmitter modulating the activation of the reward system of the brain. The DRD2 TaqlA polymorphism is associated with dopamine D2 receptor density which plays an important role in the context of reward. Persons carrying an A1 allele have a lower D2 receptor density and a higher risk to show substance abuse. In a recent study designed to investigate the influence of the DRD2 TaqlA polymorphism and the selective D2 receptor agonist bromocriptine on the activation of the reward system by means of functional magnetic resonance imaging (fMRI) supportive results were found. In a double-blind crossover study with 24 participants Kirsch et al. [8] found an increase of reward system activation from placebo to bromocriptine only in subjects carrying the Al allele. Furthermore, only Al carrier showed an increase of performance under bromocriptine. The results are interpreted as reflecting a specific sensitivity for dopamine agonists in persons carrying an Al allele and may complement actual data and theories of the development of addiction disorders postulating a higher genetic risk for substance abuse in carrier of the Al allele [8].
Whereas DA activation could occur with targeted pharmaceuticals such as Bromocriptine or other DA agonists [9],we prefer a more natural approach developed to augment the brain reward cascade; in essence, through the utilization of precursor amino-acids and simultaneous enkephalinase/COMT inhibition, which we suggest will systematically induce natural release of DA without side effects. While there other genes involved, the dopamine D2 receptor gene appears to be a major contributor [10].
DNA directed-customized anti-obesity approach
We hypothesized that genotyping certain known candidate genes would provide DNA-individualized customized nutraceuticals that may have significant influence on body re-composition by countering various genetic traits. It is well known that obesity and related symptoms significantly aggravates type 2 diabetes. Both obesity and diabetes are influenced by the interaction of both genes and environmental factors. Exploration of the current literature has identified a number of candidate genes to be associated with both of these two disorders and include amongst others the dopamine D2 receptor (DRD2), methylenetetrahydrofolate reductase (MTHFR), serotonin receptor (5-HT2a), Peroxisome Prolifera-tor-Activated Receptor gamma (PPAR-γ), and Leptin (OB) genes.
In light of these early hypothesis-generating studies, and a paucity of research, we set out to design a study to evaluate the process of DNA-customization of a nutritional solution for both wellness and weight management. For this study [11] we geno-typed 1058 subjects, and these subjects were administered a patented nutraceutical based on polymorphic outcomes. In a subset, simple t-tests comparing a number of parameters before and 80 days on the nutraceutical were performed. The significant results are as follows: weight loss (p < 0.008); sugar craving reduction (p < 0.008); appetite suppression (p < 0.004); snack reduction (p < 0.005); reduction of late night binging (p < 0.007); increased perception of over-eating (p < 0.02)]; increased energy (p < 0.004); enhanced quality of sleep (p < 0.02) and increased happiness (p < 0.02). Polymorphic correlates were obtained for a number of genes (PPAR gamma 2, MTHFR, 5-HT2a, and DRD2 genes) with positive clinical parameters tested in this study. Of all the outcomes and gene polymorphisms, only the DRD2 gene polymorphism (Al allele) had a significant Pearson correlation with days on treatment (r = 0.42, p = 0.045). This 2 fold increase is a very important genotype for compliance in treatment [12].
In another study [13], we systematically evaluated the impact of polymorphisms of these five candidate genes as important targets for the development of a DNA-customized nutraceutical LG839 [dl-phenylalanine, chromium, l-tyrosine other select amino-acids and adaptogens]) to combat obesity with special emphasis on body recomposition as measured by Body Mass Index (BMI). A total of 21 individuals were evaluated in a preliminary investigational study of LG839. Based on the results of buccal swab genotyping of each subject, an individualized customized nutraceutical formula was provided as a function of measured gene polymorphisms of the five gene candidates assessed. At the inception of the study and every two weeks subsequently, each subject completed a modified Blum-Downs OPAQuE Scale™ [Overweight Patient Assessment Questionnaire]. The alleles included the DRD2 Al; MTHFR C 677T; 5HT2a 1438G/A; PPAR-γProl2Ala and Leptin Ob1875 < 208bp. Pre- and post ad hoc analysis revealed a significant difference between the starting BMI and the BMI following an average of 41 days (28–70 d) of LG839 intake in the 21 individuals. The pre- BMI was 31.2 (weight/Ht2) compared to the post BMI of 30.4 (weight/Ht2) with a significance value of P < 0.034 (one tailed). Similarly the pre-weight in pounds (lb) was 183.52 compared to the post weight of 179 lb with a significance value of P < (0.047). We also found trends for reduction of late night snacking, carbohydrate craving reduction, reduction of stress, reduction of waist circumference. Moreover, in the 41 day period we found a trend in weight loss whereby 71.4% of subjects lost weight. Thus 15 out of 21 subjects lost weight with a z score of 2.4 and significance value of P < (0.02). In this group 53% lost on average over 2.5% of their starting weight. Further confirmation of these preliminary results (ongoing) warrants investigation and should ultimately provide novel DNA directed “omic” therapeutic targets of novel anti-obesity agents especially in diabetes and other related diseases.
Other preliminary findings using a Path Analysis also found important associations regarding anti-obesity related behaviors. In a one year cross sectional open trial study of 24 unscreened individuals utilization of oral Synaptamine variant resulted in the following benefits: stress reduction; sleep enhancement; increase in energy level; generalized well-being; reduction in cravings (sweets/carbs); improvement in mental focus/memory; improvement in blood sugar levels; reduction in food consumption; loss of inches around waist; loss of weight; reduction in blood pressure; improvement in workout performance; reduction in drug seeking behavior; reduction in hyperactivity; reduction in cholesterol levels [14].
The result of utilizing this natural dopaminergic activating approach over time should lead to neuronal DA release at the NAc, potentiating a proliferation of D2 receptors [4–7]. Moreover, support in humans is derived from anti-craving effects observed in numerous peer reviewed published clinical trials including randomized double-blind placebo controlled studies [15]. It is noteworthy that animal gene therapy utilizing cDNA vectors of the DRD2gene implanted into the NAc results in decrease alcohol craving behavior [16]. We are cognizant that the dopaminergic activation approach should be utilized to treat not only alcohol, cocaine and nicotine cravings, but glucose craving as well. Thus the coupling of genetic antecedents and nutrition may be a very viable alternative approach for the treatment of obesity.
In addition our laboratory developed a theoretical modeling study, in which we sought to evaluate health and economic implications of a nutrigenomic product for weight loss. We constructed a nutrigenomic economic model by linking (1) published study data related to the efficacy of a product and/or ingredients, (2) validated clinical assessments that have already been tied to health economics data, and (3) data involving condition prevalence and overall cost of illness. In this theoretical model, we demonstrate that a DNA-customized nutraceutical positively reduces the cost of illness at the macroeconomic and microeconomic level based upon a cost-effectiveness and cost-benefit analysis. From this proposed model, we have forecasted the prognostic health economic implications of a nutrigenomic intervention to demonstrate a theoretical model of nutrigenomic economics as it relates to obesity and overall wellness [17].
Genetic Positioning System (GPS) Map
Our 40 year journey through the meso-limbic system and dedicated scientific rigor provided insight into the addictive brain and the neurogenetic mechanisms involved in man’s quest for happiness. In brief, the site of the brain where one experiences feelings of well being is the meso-limbic system. This part of the brain has been termed the “reward center”. The chemical messages include serotonin, enkephalins, GABA and dopamine, glutamate, cannabinoid, and acetylcholine all working in concert to provide a net release of DA at the Nac (a region in the mesolimbic system). It is well known that genes control the synthesis, vesicular storage, metabolism, receptor formation and neurotransmitter catabolism. The polymorphic-versions of these genes have certain variations which could lead to an impairment of the neurochemical events involved in the neuronal release of DA. The cascade of these neuronal events has been termed “Brain Reward Cascade” [see Ref. [1] for updated description].
A breakdown of this cascade will ultimately lead to a dysregulation and dysfunction of DA. Since DA has been established as the “pleasure molecule” and the “anti-stress molecule,” any reduction in function could lead to reward deficiency and resultant aberrant substance seeking behavior and a lack of wellness.
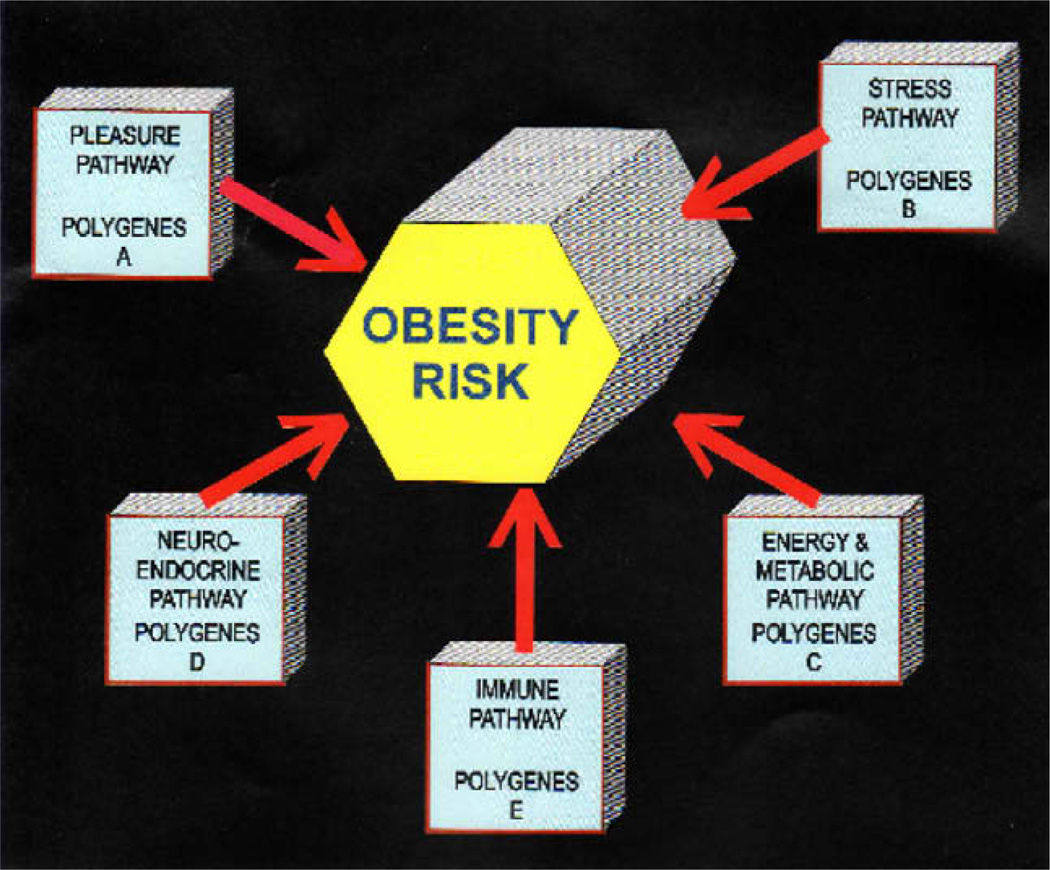
Homo sapiens physiology is motivationally programmed to drink, eat, have sex and desire pleasurable experiences. Impairment in the mechanisms involved in these natural processes lead to multiple impulsive, compulsive and addictive behaviors governed by genetic polymorphic antecedents we now term GPS. While there are a plethora of genetic variations at the level of mesolimbic activity, polymorphisms of the serotonergic-2A receptor (5-HTT2a); dopamine D2 receptor (DRD2) and the Catechol-o-methyl –transferase (COMT) genes predispose individuals to excessive cravings and resultant aberrant behaviors. This map is certainly incomplete and in reality should include over 600 genes. It is our proposal, however, that when it is complete it will serve a “blue-print” for the future development of personalized medicine in the treatment of obesity. Currently our recent data supports the view that multiple pathways are involved in the metabolic syndrome expression in any individual and includes the following pathways: energy production and regulation; stress management; reward cravings of the brain, neuro-endocrine system and metabolism; and immune system (including inflammation regulation) (see Table 1 and Fig. 1).
Table 1.
Genetic Positioning Map (GPS): number of scientific studies validating specific genes analyzed in proposed panel for nutrigenomic application. As of July 31, 2008.
| Gene | General | Reward & appetite regulation |
Stress | Metabolism & energy |
Neuro- endocrine |
Immune system |
|---|---|---|---|---|---|---|
| Leptin OB | 3618 | 57 | 104 | 981 | 3 | 108 |
| Serotonergic 2A receptor Gene | 289 | 13 | 8 | 2 | 6 | 4 |
| Phenylethanolamine-N-Methyltransferase | 247 | 3 | 38 | 1 | 6 | 5 |
| Dopamine D2receptor | 1999 | 334 | 31 | 8 | 73 | 23 |
| Tumor necrosis factors (or the TNF-family | 593 | -0- | 37 | 443 | -0- | 160 |
| Steroid sulfatase (STS) gene | 156 | -0- | -0- | 2 | -0- | 15 |
| peroxisome proliferator-activated receptor γ (PPARγ) | 2878 | -0- | 100 | 87 | 1 | 303 |
| Carbohydrate responsive element-binding protein (ChREBP) gene | 27 | -0- | -0- | 24 | 27 | -0- |
| FTO Gene | 107 | -0- | -0- | 10* | -0- | -0- |
| Monoamine Oxidase | 882 | 47 | 61 | 7 | 18 | 15 |
| ADAM 8-ADAM metallopeptidase domain 8, also known as ADAM8 | 13 | -0- | -0- | 1 | 1 | -0- |
| Corticotrophin-Releasing Factor (CRF) | 612 | -0- | 243 | 16 | 202 | 221 |
| Vitamin D gene | 5443 | -0- | 64 | 82 | 28 | 223 |
Sources: Blum et al. Gene Therapy Mol Biol 2008;12:301–312.
Fig. 1.
With regard to polymorphic associations depicted in the GPS schematic the following list of genes (not complete) provides target information that assists in the netraceutical and pharmaceutical customization: Pleasure Pathway A genes (leptin ob, serotonergic 2A receptor, Phenylethanoalamine-N-methyltransferase, dopamine D2 receptor, and monoamine oxidase); Stress Pathway B genes (leptin OB, serotonergic 2A receptor, Phenylethanoalamine-N-methyltransferase, dopamine D2 receptor, Tumor necrosis factor family, peroxisome proliferator-activated receptor, monoamine oxidase, cortocotropin-releasing factor and vitamin D); Energy Pathway C (leptin OB, serotonergic 2A receptor, phenylethanolamine-N-methyltransferase, dopamine D2 receptor, Tumor necrosis factor family, peroxisome proliferator-activated receptor, monoamine oxidase, cortocotropin-releasing factor, carbohydrate responsive element binding protein, FTO, ADAM metallopeptidase domain 8 and vitamin D); Neuroendocrine Pathway D (leptin OB, serotonergic 2A receptor, phenylethanolamine-N-methyltransferase, dopamine D2 receptor, peroxisome proliferator-activated receptor, carbohydrate responsive element binding protein, monoamine oxidase, cortocotropin-releasing factor, ADAM metallopeptidase domain 8 and vitamin D; Immune Pathway E (leptin OB, serotonergic 2A receptor, phenylethanolamine-N-methyltransferase, dopamine D2 receptor, peroxisome proliferator-activated receptor, monoamine oxidase, cortocotropin-releasing factor, Tumor necrosis factor family, ADAM metallopeptidase domain 8 and vitamin D).
Importance of RDS in obesity
An umbrella term to describe common genetic antecedents of multiple impulsive, compulsive and addictive behaviors is Reward Deficiency Syndrome (RDS). Individuals possessing a paucity of serotonergic and/or dopaminergic receptors and an increased rate of synaptic DA catabolism, due to high catabolic genotype of the COMT gene, are predisposed to self-medicating any substance or behavior that will activate DA release including alcohol, opiates, psychostimulants, nicotine, glucose, gambling, sex, and even excessive internet gaming, among others [18].
Obesity-related medical conditions are the second leading cause of death in the U.S. Classified as a chronic disease in 1985, the understanding of obesity and its causes and effects has been further elucidated through additional research into the genetic and biologic factors influencing this miserable and potentially deadly disease. What used to be understood as primarily a behavioral problem of overeating and under-exercising has only contributed to continued increases in the rates of obesity despite increases in dieting, exercise and the understanding of genes [19]. Successful strategies to induce sustainable fat loss and manage obesity effectively have been elusive. For the most part, the tactics employed have not been multi-faceted, multi-system approaches, but have been characterized by one-dimensional metabolic approaches (e.g. cannabinoid (CB1) receptor blockade; serotonin receptor stimulation; fat blockers, carb/starch blockers; central nervous system stimulation; etc.) targeted at achieving weight loss as measured by linear criteria (i.e. scale weight, Body Mass Index (BMI), percent body fat, etc). Phase 1 success is almost unanimously followed by Phase 2 failure, termed “Yo-Yo Diet” rebound weight gain.
Recent evidence indicates a much more complex and multidimensional syndrome, characterized by the simultaneous breakdown of many facets of metabolism exacerbated or limited by the predispositions of inherited genetic traits and interactions [20]. There is significant evidence to substantiate the existence of RDS as a new paradigm shift in the understanding of obesity [21]. Specifically, there are genetic links to the various roles of cate-cholaminergic-influenced pathways in aberrant substance seeking behavior, in particular cravings for carbohydrates [22]. We propose that these various neurological factors involved in the etiology of obesity, regulated by genetic predispositions, are a subtype of RDS. The treatment of obesity and or metabolic syndrome genomic mechanisms may pave the way for novel prescription pharmaceuticals as well as nutritional and/or nutraceutical therapies. There is growing evidence to support the augmentation of precursor amino acid therapy and enkephalinase and COMT inhibition leading to enhanced levels of neurotransmitters: serotonin, enkephalins, GABA and dopamine/norepinephrine [23]. Utilizing the combination of nutraceuticals directed at replenishing the nutrigenomic needs of multiple pathways, including brain reward/metabolic targets, mechanistically mimicking the brain reward cascade as well as fat regulation and cell repair (DRD2, 5-HTT2a. PPAR-Gamma, MTHFR and Leptin genes) will provide significant anti-obesity benefits [24,25].
Our laboratory recently presented evidence to support the significant benefits of a DNA-directed personalized weight management solution [1]. We are proposing potential mechanisms herein, along with the rationale for utilizing this multifaceted approach to attenuate the pleiotropic defaults in obesity as well as other addictions including alcohol, cocaine and nicotine. In this regard, preliminary testing for the first time seems to support a combination of neurotransmitter precursor amino acids, enkephalinase inhibition, and catecholamine 0-methyl-transferase (C.O.M.T.) inhibition therapy. Components of a nutrigenomic formula are modified based on the identification of specific gene polymorphisms resulting from genomic testing (Gene Profile) and the determination of correct dosage levels to promote successful and sustainable results in improved body recomposition [26].
In summary, the impact of biomics technology and the DNA directed nutraceutical targeting of the brain reward circuitry may provide a customized approach to prevent and treat high risk individuals who are carriers of a genetic predisposition to obesity and related RDS behaviors. While over 600 genes have been associated with obesity, we believe that selective candidate genes could provide useful information. Thus, we present the necessity of exploiting systems biology and “omics” [27].
A false utilization of dopaminergic blockade
Acute utilization of glucose induces a feeling of well being. But, unfortunately sustained and prolonged abuse leads to a toxic pseudo feeling of well being resulting in development of tolerance, regulatory hormone resistance (i.e. insulin and leptin), disease and/or discomfort. Thus, low DA receptors due to carrying the DRD2 Al allelic genotype results in excessive cravings and consequential behavior, whereas normal or high DA receptors results in low craving-induced behavior. In terms of preventing substance abuse, or excessive glucose craving, one goal would be to induce a proliferation of DA D2 receptors in genetically prone individuals. While blockade of Dopamine receptors may lead to reduction of craving in the short term, inevitably this approach leads to emotional upset and even suicide ideation in the long term.
Finally, utilizing the long term dopaminergic activation approach will ultimately lead to a common safe and effective modality to treat RDS behaviors including Substance Use Disorders (SUD), Attention Deficit Hyperactivity Disorder (ADHD), and Obesity among other reward deficient aberrant behaviors. Support for the impulsive nature of individuals possessing dopaminergic gene variants is supported by a recent article suggesting that variants in the COMT gene predicts impulsive choice behavior and may shed light on treatment targets. We can’t ignore the importance of neurochemical mechanisms involved in drug induced relapse behavior as suggested by Crombag et al. [28]. These investigators have found using a drug relapse model, previously shown to induce relapse by re-exposing rats to heroin-associated contexts, after extinction of drug-reinforced responding in different contexts, reinstates heroin seeking. This effect is attenuated by inhibition of glutamate transmission in the ventral tegmental area and medial accumbens shell, components of the mesolimbic dopamine system. This process enhances DA net release in the N. accumbens. This fits well with Li’s [3] KARG addiction network map.
Customizing anti-obesity agents: nutrigenomics targeting
In the future, our goal as scientists should be to analyze before we act, test before we recommend usage and/or prescribe, so that we can better understand the genetic factors influencing the obesity confronted by an individual. By understanding the evolutionary contributory factors to their outward presentation, we can better provide the obese individual with a customized program to bring their body composition into balance involving behavioral and biological changes and treatments. This simple doctrine relates back to the understanding of the “thrifty gene” concept [29]. In this regard, we further hypothesize that using a multi-variant nutrigenomic index for the purposes of customizing or adjusting the formulation of nutritional supplements will result in an improved and novel approach to the diagnosis, stratification, prognosis, and treatment of RDS induced obesity and related behaviors. This multi-variant genetic index, or Geneprofile™ (a published and patented process, LifeGen, Inc. La Jolla, California) [30] is derived by analyzing genotype and/or phenotype through measuring multiple genetic mutations of single nucleotide polymorphisms, gene expression, or other forms of genetic and phenotypic measurements. This process will provide the opportunity for DNA-customized nutrition covering multiple genes involved in RDS, as well as the metabolism, efficacy and/or toxicity associated with specific vitamins, minerals, herbal supplements, homeopathic ingredients and other ingredients in the nutritional and/or dietary supplement regimen. We feel strongly that utilization of this genomic approach independent of whether we are administering a pharmaceutical and or a nutraceutical genomic resolution will result in enhanced positive clinical outcomes “dopamine sensitivity”.
Should we make laws against obesity?
With this knowledge, is it feasible that the legal profession should make laws against obesity? This question has been part of a theme issue in the Australian and New Zealand Health Policy journal [31]. Only time will be able to predict the best policy in the ongoing fight against the war on obesity utilizing “optimal default” as one tactic [32]. However, now we must deal with the reality that it will not go away unless we incorporate new scientifically validated concepts whereby at least one tactic should embrace a nutrigenomic approach. In this regard, we are developing the Genetic Positioning System (GPS) Map. Our well-being is counting on it.
Conclusion
In support of our hypothesis that we can treat obesity via neurochemical manipulation by utilizing nutrients governed by targeting certain gene polymorphisms seems quite feasible based on our current knowledge.
Just as obesity affects multiple systems of the body resulting in a plethora of co-morbidities, its treatment must consider the contributing factors and health of multiple systems. Existing weight loss tactics for the most part have failed to provide successful means to achieve sustainable healthy body composition and weight loss accordingly due to a myopic view of the cause of obesity. Emerging technologies that customize nutritional/nutraceutical needs of an individual based on genetic traits, merit further investigation, as preliminary data appears very promising recognizing polygenic inheritance in dyslipidemia [33].
It is common knowledge that factors like exercise or extreme exertion, disease, drug intake, poor diet, etc., alter nutritional needs. Along this line, research has shown that genetic polymorphisms also alter nutritional needs to maintain healthy homeostasis. This is particularly true for phenotypes exhibiting polymorphisms predisposing aberrant pleasure seeking (and addictive) behavior, excessive cravings and obesity, and related Metabolic Syndrome X disorders. There is significant evidence to substantiate the existence of RDS as a new paradigm shift in the understanding of obesity. Moreover, we and others have established a role of catecholaminergic pathways in aberrant substance seeking behavior, in particular cravings for carbohydrates.
In previous multiple publications from our laboratory we exposed the theoretical model that the genetic basis for generalized craving behavior involves polygenic inheritance as well multiple physiological pathways. There is growing evidence to support the augmentation of precursor amino acid therapy and enkephalinase and COMT inhibition leading to enhanced levels of neurotransmitters: serotonin, enkephalins, GABA and dopamine/norepinephrine [1]. Utilization of this combination in a published nutraceutical complex, including Synaptamine (SG8839), Super CitriMax DNA-Strength, Passion flower and a proprietary oxygen coordinated chromium nicotinate have been evaluated in an open label trial. Based on a number of case reports, it seems possible that this unique combination of ingredients may have a generalized anti-stress and anti-craving effect resulting in an inhibition of carbohydrate bingeing, inducing fat loss and enhanced body image/composition. One such natural ingredient IGOB131 may play an important multifaceted role in the control of adipogenesis and have further implications in in-vivo anti obesity effects by targeting the PPAR gamma gene, a known contributory factor to obesity in humans [34].
Accordingly, this is the first time the components of this formula have been combined at the clinically tested dosage levels indicated, simultaneously addressing multiple pathways to promote successful and sustainable loss of fat and improvement in body composition. While there are a number of important pathways identified on the road toward unwarranted weight gain, weight and obesity can be independent of each other. And, it is clear from the path analysis concerning the effects of Synaptamine™ complex that these same pathways are interactive and multi-directional, resulting in a net enhancement of body composition.
Additionally, there are a number of studies cited above which relate not only to the serotonergic and leptinergic pathways, weight loss and weight regain, but the dopaminergic and other endorphinergic genes (i.e. POMC) are associated with obesity in both animals and humans. There is also the work of Nora Volkow and others on D2 receptor density, utilizing neuroimaging to determine low D2 receptors in obese humans [35]. There is evidence as cited above showing the relationship between body type and the D2 receptors. There are studies as reviewed by Noble [36] to show the relationship of dopamine receptors and hyperphagia, body mass index, and carbohydrate bingeing. There is also evidence showing associations with the D2 A1 allele as well as the leptin receptor gene (which has links to dopaminergic fibers in the mesolimbic system) and carbohydrate bingeing, obesity, and body mass index as well as energy expenditure.
In essence, similar to pharmaceutical counter parts such as Meridia and Acomplia, neuronutrient amino-acid based compositions of this type will cause the synthesis of the brain reward neurotransmitters like serotonin and catecholamines and through its effect on the natural opioids will by virtue of inhibiting GABA cause a significant release of dopamine at the Nucleus accumbens. This constant release of possibly therapeutic amounts of dopamine (anti-stress and pleasure substance) occupies dopamine D2 receptors, especially in carriers of the A1 allele (low D2 receptors and high glucose craving), and over time (possibly 6–8 weeks) effects RNA transcription leading to a proliferation of D2 receptors, thereby, reducing craving for carbohydrates. Interestingly, among many other genes the dopamine D2 receptor gene is now part of the Human Obesity Gene Map [37]. Understanding the evolutionary concepts related to eating behavior and genetic antecedents such as the “thrifty gene” (inter-relationships of fasting/starvation and fat production) enabled us to develop these newer genomic concepts as they relate to obesity and eating behavior. We contend that if as scientists we test these theories proposed and obtain positive results the potential of stopping the obesity epidemic as a genetic pleiotropic disease may be achievable someday.
Finally recent support on the role of dopaminergic gene polymorphisms (i.e. D2 A1 allele) and obesity was reported by Stice et al. [38]. Stice and colleagues in 2008 using functional MRI found that subjects may overeat to compensate for a blunted or hypo-functioning dorsal striatum, especially in subjects carrying the dopamine D2 A1 variant (DRD2A1 allele). Additionally it was recently found that obesity–prone rats were found to have an attenuated central dopamine system, implying that lowered dopamine would reduce the hedonistic response associated with feeding and induce compensatory hyperphagia and weight gain [39]. Certainly we are cognizant that multiple gene polymorphisms play a role in obesity and related behaviors and it is these polygenes’ linked to known physiological pathways that provide promise in the successful amelioration of the current obesity epidemic. The contribution to the variance for any gene polymorphism is rather small and therefore any gene map by necessity must consist of multiple genes. Comings and associates showed in one study that the contribution to the obesity risk variance in young obese females for the leptin Ob and DRD2 genes was 22% [40]. In terms of well-being, known genetic antecedents may also contribute to personality which can also impact the treatment response of any anti-obesity treatment and must be taken into account [41]. It is our opinion that the literature to date tends to disagree with the null hypothesis that we cannot treat obesity neurochemical pathway manipulation by nutrients using a Genetic Positioning System (GPS) Map. Nevertheless, we must caution against any real interpretation of this theoretical proposed model until the entire GPS map is developed. While this may be a long time in coming at least we have a start. Translational research will impact millions struggling with sugar addiction [42–49].
Acknowledgements
The authors would like to thank the staff at Path Research & Medical Foundation, New York as well financial support. The writing of this review was supported in part by funds from the US Department of Health and Human Services, NIAAA (R01-AA07112 and K05-AA00219) and the Medical Research Service of the US Department of Veterans Affairs to Marlene Oscar-Berman.
Footnotes
Conflict of interest
Kenneth Blum, B. William Downs, Roger L. Waite own stock in LifeGen, Inc. the exclusive distributors of patents related to Synaptamine complex™ and other variants. There are no other conflicts of interest.
References
- 1.Blum K, Chen AL, Chen TJ, Braverman ER, Blum SH, Cassel K, et al. Activation instead of blocking mesolimbic dopaminergic reward circuitry is a preferred modality in the long term treatment of reward deficiency syndrome (RDS): a commentary. Theor Biol Med Model. 2008;5:24. doi: 10.1186/1742-4682-5-24. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blum K, Chen TJH, Downs BW, Meshkin B, Blum SH, Martinez-Pons M, et al. Synaptamine (Sg8839): an amino-acid enkephalinase inhibition nutraceutical improves recovery of alcoholics, a subtype of Reward Deficiency Syndrome (RDS) Trends Appl Sci Res. 2007;2:132–138. [Google Scholar]
- 3.Li CY, Mao X, Wei L. Genes and (common) pathways underlying drug addiction. PLoS Comput Biol. 2008;4(1):e2. doi: 10.1371/journal.pcbi.0040002. [Epub 2007 Nov 20] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boundy VA, Pacheco MA, Guan W, Molinoff PB. Agonists and antagonists differentially regulate the high affinity state of the D2L receptor in human embryonic kidney 293 cells. Mol Pharmacol. 1995;48:956–964. [PubMed] [Google Scholar]
- 5.Boundy VA, Lu L, Molinoff PB. Differential coupling of rat D2 dopamine receptor isoforms expressed in Spodoptera frugiperda insect cells. J Pharmacol Exp Ther. 1996;276:784–794. [PubMed] [Google Scholar]
- 6.Noble EP, Blum K, Ritchie T, Montgomery A, Sheridan PJ. Allelic association of the D2 dopamine receptor gene with receptor-binding characteristics in alcoholism. Arch Gen Psychiatry. 1991;48:648–654. doi: 10.1001/archpsyc.1991.01810310066012. [DOI] [PubMed] [Google Scholar]
- 7.Ferré S, Quiroz C, Woods AS, Cunha R, Popoli P, Ciruela F, et al. An update on adenosine A2A–dopamine D2 receptor interactions: implications for the function of G protein-coupled receptors. Curr Pharm Des. 2008;14:1468–1474. doi: 10.2174/138161208784480108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirsch P, Reuter M, Mier D, Lonsdorf T, Stark R, Gallhofer B, et al. Imaging gene-substance interactions: the effect of the DRD2 Taql A polymorphism and the dopamine agonist bromocriptine on the brain activation during the anticipation of reward. Neurosci Lett. 2006;405:196–201. doi: 10.1016/j.neulet.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 9.Lawford BR, Young RM, Rowell JA, Qualichefski J, Fletcher BH, Syndulko K, et al. Bromocriptine in the treatment of alcoholics with the D2 dopamine receptor Al allele. Nat Med. 1995;1:337–341. doi: 10.1038/nm0495-337. [DOI] [PubMed] [Google Scholar]
- 10.Blum K, Chen TJ, Meshkin B, Waite RL, Downs BW, Blum SH, et al. Manipulation of catechol-O-methyl-transferase (COMT) activity to influence the attenuation of substance seeking behavior, a subtype of Reward Deficiency Syndrome (RDS), is dependent upon gene polymorphisms: a hypothesis. Med Hypotheses. 2007;69:1054–1060. doi: 10.1016/j.mehy.2006.12.062. [DOI] [PubMed] [Google Scholar]
- 11.Blum K, Chen AL, Chen TJ, Rhoades P, Prihoda TJ, Downs BW, et al. LG839: anti-obesity effects and polymorphic gene correlates of reward deficiency syndrome. Adv Ther. 2008;25:894–913. doi: 10.1007/s12325-008-0093-z. [DOI] [PubMed] [Google Scholar]
- 12.Blum K, Chen ALC, Chen TLC, Rhoades P, Prihoda P, Downs BW, et al. Dopamine D2 Receptor Taq A1 allele predicts treatment compliance of LG839 in a subset analysis of a pilot study in The Netherlands. Gene Therapy Mol Biol. 2008;12:129–140. [Google Scholar]
- 13.Blum K, Chen TJH, Williams L, Chen ALC, Downs BW, Waite RL, et al. A short term pilot open label study to evaluate efficacy and safety of LG839, a customized DNA directed nutraceutical in obesity: exploring nutrigenomics. Gene Ther Mol Biol. 2008:112. Chen. [Google Scholar]
- 14.Blum K, Chen TJH, Meshkin B, Downs BW, Gordon CA, Blum SH, et al. Reward Deficiency Syndrome in obesity: a preliminary cross-sectional trial with a genotrim variant. Adv Ther. 2006;23:1040–1051. doi: 10.1007/BF02850224. [DOI] [PubMed] [Google Scholar]
- 15.Chen TJ, Blum K, Payte JT, Schoolfield J, Hopper D, Stanford M. Narcotic antagonists in drug dependence: pilot study showing enhancement of compliance with SYN-10, amino-acid precursors and enkephalinase inhibition therapy. Med Hypotheses. 2004;63:538–548. doi: 10.1016/j.mehy.2004.02.051. [DOI] [PubMed] [Google Scholar]
- 16.Thanos PK, Volkow ND, Freimuth P, Umegaki H, Ikari H, Roth G, et al. Overexpression of dopamine D2 receptors reduces alcohol self-administration. J Neurochem. 2001;78:1094–1103. doi: 10.1046/j.1471-4159.2001.00492.x. [DOI] [PubMed] [Google Scholar]
- 17.Meshkin B, Chen TJH, Chen ALC, Prihoda TJH, Morrisette H, Braverman ER, et al. Health economics of nutrigenomics in weight management. Gene Ther Mol Biol. 2008;12:25–30. [Google Scholar]
- 18.Comings DE, Blum K. Reward deficiency syndrome: genetic aspects of behavioral disorders. Prog Brain Res. 2000;126:325–341. doi: 10.1016/S0079-6123(00)26022-6. [DOI] [PubMed] [Google Scholar]
- 19.Chen ALC, Blum K, Chen TJH, Rhinking J, Waite RL, Downs BW, et al. The impact of biomics technology and DNA directed anti-obesity targeting of the brain reward circuitry. Gene Therapy Mol Biol. 2008;12:45–68. [Google Scholar]
- 20.Bowirrat A, Oscar-Berman M. Relationship between dopaminergic neurotransmission, alcoholism, and Reward Deficiency Syndrome. Am J Med Genet B Neuropsychiatr Genet. 2005;132B(1):29–37. doi: 10.1002/ajmg.b.30080. [DOI] [PubMed] [Google Scholar]
- 21.Epstein LH, Temple JL, Neaderhiser BJ, Salis RJ, Erbe RW. Food reinforcement, the dopamine D2 receptor genotype, and energy intake in obese and nonobese humans. Behav Neurosci. 2007;121:877–889. doi: 10.1037/0735-7044.121.5.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maes HH, Neale Mc, Eaves IJ. Genetic and environmental factors in relative body weight and human adiposity. Behav Gen. 1997;27:325–351. doi: 10.1023/a:1025635913927. [DOI] [PubMed] [Google Scholar]
- 23.Blum K, Braverman ER, Holder JM, Lubar JF, Monastra VJ, Miller D, et al. Reward deficiency syndrome: a biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. J Psychoactive Drugs. 2000;32(Suppl.):1–100. doi: 10.1080/02791072.2000.10736099. [DOI] [PubMed] [Google Scholar]
- 24.Chen TJH, Blum K, Kaats G, Braverman E, Pullin D, Downs BW, et al. Reviewing the role of putative candidate genes in “Neurobesigenics” a clinical subtype of Reward Deficiency Syndrome (RDS) Gene Ther Mol Biol. 2007;11:61–74. [Google Scholar]
- 25.Blum K, Cull JG, Chen TJH, Garcia-Swan S, Holder JM, Wood RC, et al. Clinical evidence for effectiveness of PhenCal in maintaining weight loss in an open-label, controlled, 2 year study. Curr Ther Res. 1997;58:745–763. [Google Scholar]
- 26.Blum K, Meshkin B, Downs BW. DNA based customized nutraceutical ““gene therapy” utilizing a genoscore: a hypothesized paradigm shift of a novel approach to the diagnosis, stratification, prognosis and treatment of inflammatory processes in the human. Med Hypotheses. 2006;66:1008–1018. doi: 10.1016/j.mehy.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 27.Corthésy-Theulaz I, den Dunnen JT, Ferré P, Geurts JM, Müller M, Van Belzen N. Nutrigenomics: the impact of biomics technology on nutrition research. Ann Nutr Metab. 2005;49:355–365. doi: 10.1159/000088315. [DOI] [PubMed] [Google Scholar]
- 28.Crombag HS, Bossert JM, Koya E, Shaham Y. Context-induced relapse to drug seeking: a review. Philos Trans R Soc Lond B Biol Sci. 2008;36:3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prentice AM, Rayco-Solon P, Moore SE. Insights from the developing world: thrifty genotypes and thrifty phenotypes. Proc Nutr Soc. 2005;64:153–161. doi: 10.1079/pns2005421. [DOI] [PubMed] [Google Scholar]
- 30.Blum K, Chen TJH, Blum SH, Downs BW, Braverman ER, Mengucci JF, et al. Nutrigenomics and pharmacogenomics: a scientific wonderland. Biol Social Life (Social Sci Inform) 2006;45:35–52. [Google Scholar]
- 31.Martin R. The role of law in the control of obesity in England: looking at the contribution of law to a healthy food culture. Aust New Zealand Health Policy. 2008;5:21. doi: 10.1186/1743-8462-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brownell KD, Warner KE. The perils of ignoring history: m big tobacco played dirty and millions died: how similar is big Food? The Milbank Quarterly. 2009;87:259–294. doi: 10.1111/j.1468-0009.2009.00555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kathiresan S, Willer CJ, Peloso GM, Demissie S, Musunuru K, Schadt EE, et al. Common variants at 30 loci contribute to polygenic dyslipidemia. Nat Genet. 2009;41(1):56–65. doi: 10.1038/ng.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oben JE, Ngondi JL, Blum K. Inhibition of Irvingia gabonensis seed extract (OB131) on adipogenesis as mediated via down regulation of the PPARgamma and Leptin genes and up-regulation of the adiponectin gene. Lipids Health Dis. 2008;7:4450. doi: 10.1186/1476-511X-7-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Volkow ND, Wang GJ, Telang F, Fowler JS, Thanos PK, Logan J, et al. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage. 2008;42:1537–1543. doi: 10.1016/j.neuroimage.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barnard ND, Noble EP, Ritchie T, Cohen J, Jenkins DJ, Turner-McGrievy G, et al. D2 dopamine receptor Taq1A polymorphism, body weight, and dietary intake in type 2 diabetes. Nutrition. 2009;25:58–65. doi: 10.1016/j.nut.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bray MS, Hagberg JM, Pérusse L, Rankinen T, Roth SM, Wolfarth B, et al. The human gene map for performance and health-related fitness phenotypes: the 2006–2007 update. Med Sci Sports Exerc. 2009;41:35–73. doi: 10.1249/mss.0b013e3181844179. [DOI] [PubMed] [Google Scholar]
- 38.Stice E, Spoor S, Bohan C, Small DM. Relation between Obesity and blunted striatal response to food is moderated by Taq A1 allele. Science. 2008;322:449–452. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geiger BM, Behr GG, Frank LE, Caldera-Siu AD, Beinfeld MC, Kokkotou EG, et al. Evidence for defective mesolimbic dopamine exocytosis in obesity-prone rats. FASEB J. 2008;22(8):2740–2746. doi: 10.1096/fj.08-110759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Comings DE, Gade R, MacMurray JP, Muhleman D, Peters WR. Genetic variants of the human obesity (OB) gene: association with psychiatric symptoms and body mass index in young women, and interaction with the dopamine D2 receptor (DRD2) gene. Mol Psychiatry. 1996;1:325–335. [PubMed] [Google Scholar]
- 41.Ponce G, Jimenez-Arriero MA, Rubio G, Hoenicka J, Ampuero I, Ramos JA, et al. The A1 allele of the DRD2 gene (TaqI A polymorphisms) is associated with antisocial personality in a sample of alcohol-dependent patients. Eur Psychiatry. 2003;18:356–360. doi: 10.1016/j.eurpsy.2003.06.006. [DOI] [PubMed] [Google Scholar]
- 42.Davis CA, Levitan RD, Reid C, Carter JC, Kaplan AS, Patte AS, et al. Dopamine for “Wanting” and Opioids for “Liking”: A comparison of Obese adults with and without Binge “Eating”. Obesity. 2009 doi: 10.1038/oby.2009.52. in press. [DOI] [PubMed] [Google Scholar]
- 43.Rada P, Avena NM, Hoebel BG. Daily bingeing on sugar repeatedly releases dopamine in the accumbens. Neuroscience. 2005;134:737–744. doi: 10.1016/j.neuroscience.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 44.Chang GO, Gaysinskaya V, Karatayev O, Leibowitz SF. Maternal high-fat diet and fetal programming: increased proliferation of Hypothalmic pepetide-producing neurons that increase risk for overeating and obesity. J Neurosci. 2008;28:12107–12119. doi: 10.1523/JNEUROSCI.2642-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blass EM. Biological and Environmental determinants of childhood obesity. Nutr Clin Care. 2003;6:13–19. [PubMed] [Google Scholar]
- 46.Hodgkins CC, CaHill KS, Seraphine AE, Frost-Pineda K, Gold MS. Adolescent drug addiction treatment and weight gain. J Addict Dis. 2004:55–65. doi: 10.1300/J069v23n03_05. [DOI] [PubMed] [Google Scholar]
- 47.Grimm JW, Osincup D, Wells B, Manaois M, Fyall A, Buse C, et al. Environmental enrichment attenuates cue-induced reinforcement of sucrose seeking in rats. Behav Pharmacol. 2008;19:777–785. doi: 10.1097/FBP.0b013e32831c3b18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lenoir M, Serre F, Cantin L, Ahmed SH. Intense sweetness surpasses cocaine reward. Plos One. 2007;2(8):e698. doi: 10.1371/journal.pone.0000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gearhardt AN, Corbin WR, Brownell KD. Preliminary validation of the Yale Food Addiction Scale. Appetite. 2009;52:439–446. doi: 10.1016/j.appet.2008.12.003. [DOI] [PubMed] [Google Scholar]