Abstract
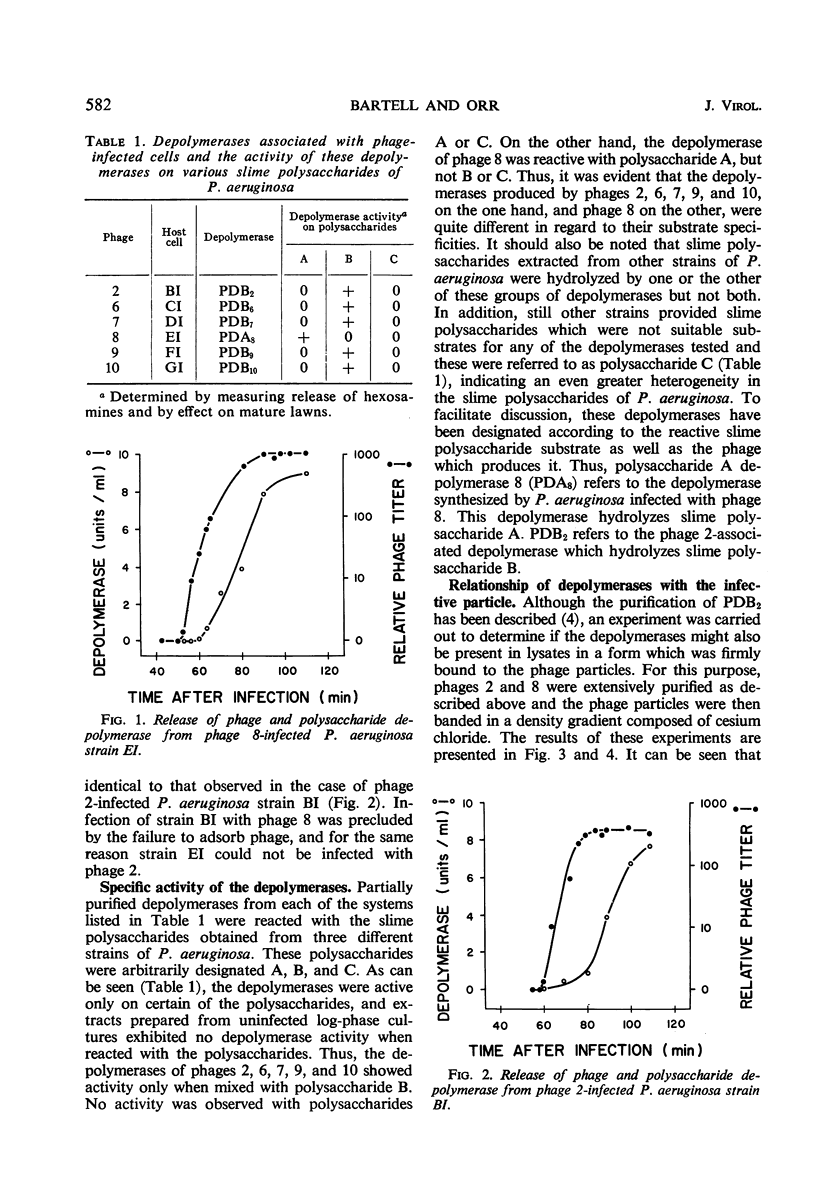
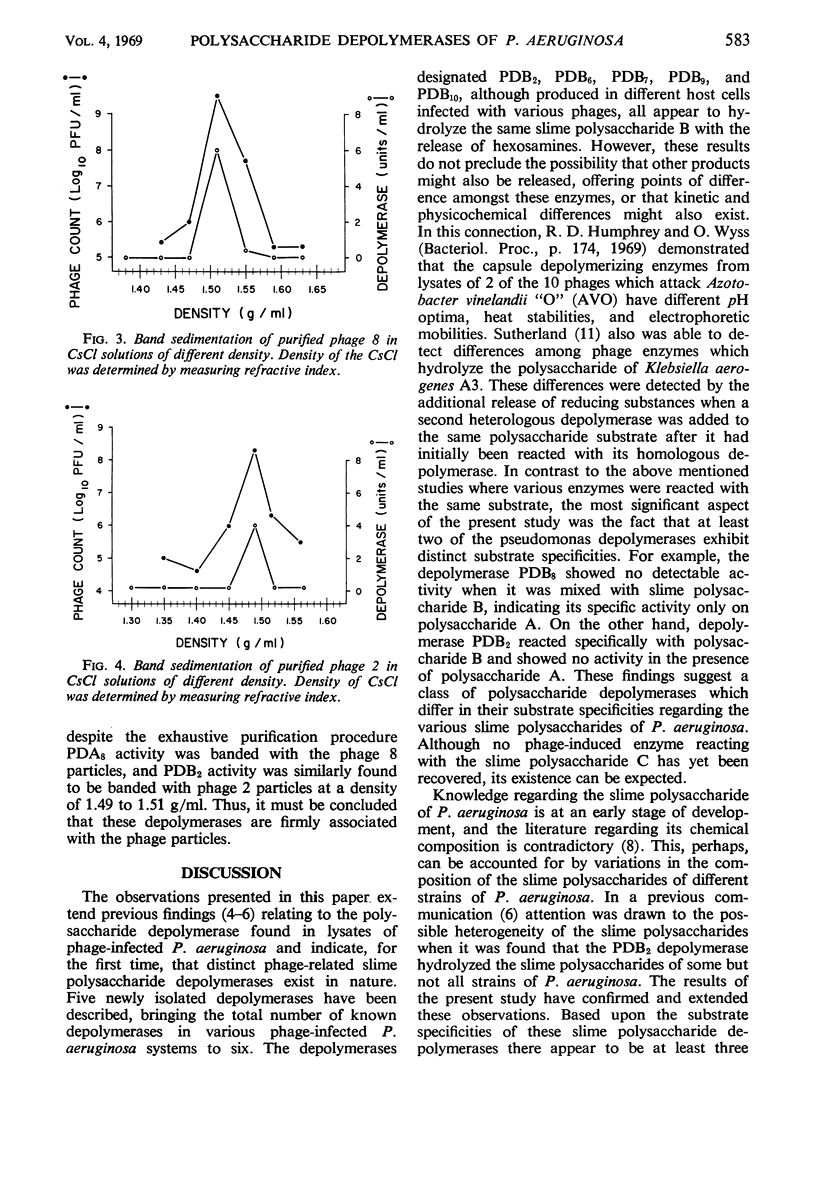
Five new polysaccharide depolymerases were isolated from cultures of Pseudomonas aeruginosa infected with phages 6, 7, 8, 9, and 10. The production of enzyme paralleled the release of phage. Depolymerase associated with phage 8 was active on slime polysaccharide A, whereas depolymerases associated with phages 6, 7, 9, and 10, like pseudomonas phage 2, hydrolyzed slime polysaccharide B. None of the depolymerases was active on slime polysaccharide C. Despite exhaustive purification, depolymerase activity was found to band with the phage particles at a density of 1.49 to 1.51 g/ml in a density gradient composed to cesium chloride. These results suggest that the depolymerases are firmly bound to the phage particles.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAMS M. H., PARK B. H. An enzyme produced by a phage-host cell system. II. The properties of the polysaccharide depolymerase. Virology. 1956 Dec;2(6):719–736. doi: 10.1016/0042-6822(56)90054-x. [DOI] [PubMed] [Google Scholar]
- Alms T. H., Bass J. A. Immunization against Pseudomonas aeruginosa. I. Induction of protection by an alcohol-precipitated fraction from the slime layer. J Infect Dis. 1967 Jun;117(3):249–256. doi: 10.1093/infdis/117.3.249. [DOI] [PubMed] [Google Scholar]
- BOAS N. F. Method for the determination of hexosamines in tissues. J Biol Chem. 1953 Oct;204(2):553–563. [PubMed] [Google Scholar]
- Bartell P. F., Lam G. K., Orr T. E. Purification and properties of polysaccharide depolymerase associated with phage-infected Pseudomonas aeruginosa. J Biol Chem. 1968 May 10;243(9):2077–2080. [PubMed] [Google Scholar]
- Bartell P. F., Orr T. E., Lam G. K. Polysaccharide depolymerase associated with bacteriophage infection. J Bacteriol. 1966 Jul;92(1):56–62. doi: 10.1128/jb.92.1.56-62.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartell P. F., Orr T. E. Origin of polysaccharide depolymerase associated with bacteriophage infection. J Virol. 1969 Mar;3(3):290–296. doi: 10.1128/jvi.3.3.290-296.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. R., Foster J. H., Clamp J. R. Composition of Pseudomonas aeruginosa slime. Biochem J. 1969 May;112(4):521–525. doi: 10.1042/bj1120521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU P. V., ABE Y., BATES J. L. The roles of various fractions of Pseudomonas aeruginosa in its pathogenesis. J Infect Dis. 1961 Mar-Apr;108:218–228. doi: 10.1093/infdis/108.2.218. [DOI] [PubMed] [Google Scholar]
- RONDLE C. J., MORGAN W. T. The determination of glucosamine and galactosamine. Biochem J. 1955 Dec;61(4):586–589. doi: 10.1042/bj0610586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland I. W. Phage-induced fucosidases hydrolysing the exopolysaccharide of Klebsiella arogenes type 54 [A3(S1)]. Biochem J. 1967 Jul;104(1):278–285. doi: 10.1042/bj1040278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland I. W., Wilkinson J. F. The exopolysaccharide of Klebsiella aerogens A3 (S1) (type 54). The isolation of O-acetylated octasaccharide, tetrasaccharide and trisaccharide. Biochem J. 1968 Dec;110(4):749–754. doi: 10.1042/bj1100749. [DOI] [PMC free article] [PubMed] [Google Scholar]


