Abstract
Background
Between the ages 45 and 65 years, incident stroke is 2 to 3 times more common in blacks than in whites, a difference not explained by traditional stroke risk factors.
Methods
Stroke risk was assessed in 27 748 black and white participants recruited between 2003 and 2007, who were followed up through 2011, in the REasons for Geographic And Racial Differences in Stroke (REGARDS) study. Racial differences in the impact of systolic blood pressure (SBP) was assessed using proportional hazards models. Racial differences in stroke risk were assessed in strata defined by age (<65 years, 65–74 years, and ≥75 years) and SBP (<120 mm Hg, 120–139 mm Hg, and 140–159 mm Hg).
Results
Over 4.5 years of follow-up, 715 incident strokes occurred. A 10–mm Hg difference in SBP was associated with an 8% (95% CI, 0%-16%) increase in stroke risk for whites, but a 24% (95% CI, 14%-35%) increase for blacks (P value for interaction, .02). For participants aged 45 to 64 years (where disparities are greatest), the black to white hazard ratio was 0.87 (95% CI, 0.48–1.57) for normotensive participants, 1.38 (95% CI, 0.94–2.02) for those with prehypertension, and 2.38 (95% CI, 1.19–4.72) for those with stage 1 hypertension.
Conclusions
These findings suggest racial differences in the impact of elevated blood pressure on stroke risk. When these racial differences are coupled with the previously documented higher prevalence of hypertension and poorer control of hypertension in blacks, they may account for much of the racial disparity in stroke risk.
Similar to racial differences in stroke mortality, the difference between blacks and whites in stroke incidence is substantially larger between the ages of 45 and 65 years.1,2 Moreover, “traditional stroke risk factors” explain only approximately half of this excess.2
Factors potentially contributing to the unexplained racial disparity in incident stroke risk include the following: (1) racial differences in the impact of risk factors, (2) residual confounding from incomplete characterization of the attributes of these risk factors, (3) nontraditional risk factors (not included in the Framingham Stroke Risk Function3), and (4) measurement error in predictive factors.2 Herein, we use data from the REasons for Geographic And Racial Differences in Stroke (REGARDS) study to focus on the first of these possibilities, differences in the impact of the traditional risk factors between races. This report addresses the possibility that an elevated systolic blood pressure (SBP) level is associated with a greater increase in stroke risk in blacks than in whites, with particular emphasis on relatively young adults (age 45–65 years), where the disparity in stroke between blacks and whites is greatest.
METHODS
The REGARDS study is a population-based longitudinal cohort study of 30 239 black and white individuals 45 years and older, recruited between 2003 and 2007 and followed up through 2011. The study oversampled blacks (42%) and residents of the southeastern “Stroke Belt” (Alabama, Arkansas, Georgia, Louisiana, Mississippi, North Carolina, South Carolina, and Tennessee) (56%). The traditional stroke risk factors were assessed through a combination of telephone interview and a physical examination conducted in the participant’s home that included the collection of glucose level and blood pressure (BP) measurements. Because the racial disparities in stroke risk are larger for blacks than for other minority race/ethnic groups,4 the REGARDS study was designed to focus on the stroke disparity between blacks and whites. Study design details5 and event identification and adjudication1 are available elsewhere.
Of the 30 239 REGARDS study participants, 56 (0.2%) were excluded owing to data anomalies, and follow-up was not available for 547 participants (1.8%). Of the remaining 29 636 participants, 1888 (6.4%) reported a prevalent stroke at baseline and were excluded, resulting in a final analysis dataset of 27 748 participants.
Age strata were created to reflect a decreasing stroke mortality risk for whites compared with blacks, where the risk for blacks younger than 65 years was 2 to 3 times higher than that for whites younger than 65 years, but the racial disparity decreases and is completely absent by age 85 years.6–8 A number of recent publications have shown a very similar pattern in stroke incidence, with large disparities below age 65 years and smaller disparities above that age.1,2,9,10 Because of this age-by-race interaction in the risk of incident stroke, participants were stratified into approximate tertiles by age (ages 45–64, 65–74, and ≥75 years). Likewise, following SBP categories suggested by “The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure” (JNC7),11 participants were classified as normotensive (SBP <120 mm Hg), prehypertension (120–139 mm Hg), stage 1 hypertension (140–159 mm Hg), and stage 2 hypertension (≥160 mm Hg). Because very few white participants had stage 2 hypertension (eg, only 1.6% of those aged 45–64 years; Table 1), those with stage 2 hypertension were omitted from subsequent analysis; hence, we jointly considered stroke risk in 9 age-hypertension strata (3 age strata × 3 SBP strata).
Table 1.
Description of the Study Population by Age and SBP Strata
| Age Strata/Parameter | SBP Strata
|
|||||||
|---|---|---|---|---|---|---|---|---|
| <120 mm Hg
|
120–139 mm Hg
|
140–159 mm Hg
|
≥160 mm Hg
|
|||||
| White | Black | White | Black | White | Black | White | Black | |
| 45–59 y | ||||||||
| Participants, No. | 3548 | 1717 | 3633 | 3033 | 821 | 1076 | 127 | 329 |
| Male, % | 39 | 31 | 52 | 38 | 57 | 41 | 54 | 42 |
| SBP, mean, mm Hg | 109 | 111 | 127 | 129 | 146 | 146 | 170 | 170 |
| Using antihypertensive medications, % | 23 | 44 | 40 | 59 | 54 | 69 | 61 | 72 |
| Diabetes, % | 9 | 21 | 15 | 27 | 21 | 35 | 25 | 35 |
| Atrial fibrillation, % | 6 | 7 | 6 | 7 | 7 | 8 | 9 | 9 |
| LVH, % | 3 | 7 | 6 | 12 | 9 | 16 | 16 | 25 |
| Heart disease, % | 10 | 9 | 13 | 10 | 15 | 15 | 17 | 16 |
| Current smoker, % | 16 | 21 | 15 | 20 | 21 | 23 | 20 | 31 |
| Stroke events, No. (%) | 40 (1.1) | 17 (1.0) | 59 (1.6) | 64 (2.1) | 13 (1.6) | 36 (3.3) | 3 (2.4) | 14 (4.3) |
| 60–69 y | ||||||||
| Participants, No. | 1632 | 756 | 2723 | 1736 | 879 | 739 | 175 | 239 |
| Male, % | 48 | 34 | 53 | 39 | 58 | 40 | 59 | 41 |
| SBP, mean, mm Hg | 111 | 111 | 128 | 129 | 146 | 146 | 169 | 171 |
| Using antihypertensive medications, % | 36 | 57 | 50 | 66 | 59 | 76 | 71 | 77 |
| Diabetic, % | 14 | 28 | 18 | 32 | 22 | 38 | 29 | 40 |
| Atrial fibrillation, % | 10 | 9 | 9 | 6 | 10 | 8 | 13 | 9 |
| LVH, % | 4 | 12 | 8 | 16 | 9 | 19 | 13 | 22 |
| Heart disease, % | 20 | 15 | 22 | 17 | 24 | 17 | 30 | 22 |
| Current smoker, % | 11 | 15 | 9 | 13 | 12 | 14 | 15 | 19 |
| Stoke events, No. (%) | 46 (2.8) | 25 (3.3) | 100 (3.7) | 70 (4.0) | 39 (4.4) | 44 (6.0) | 9 (5.1) | 15 (6.3) |
| ≥70 y | ||||||||
| Participants, No. | 781 | 283 | 1510 | 772 | 553 | 396 | 158 | 132 |
| Male, % | 49 | 38 | 54 | 38 | 52 | 42 | 54 | 30 |
| SBP, mean, mm Hg | 111 | 111 | 128 | 129 | 147 | 147 | 171 | 174 |
| Using antihypertensive medications, % | 42 | 63 | 52 | 67 | 61 | 70 | 65 | 77 |
| Diabetes, % | 14 | 27 | 15 | 33 | 16 | 31 | 17 | 34 |
| Atrial fibrillation, % | 18 | 9 | 16 | 7 | 14 | 11 | 10 | 7 |
| LVH, % | 6 | 18 | 9 | 18 | 13 | 19 | 17 | 34 |
| Heart disease, % | 31 | 20 | 31 | 20 | 31 | 21 | 34 | 25 |
| Current smoker, % | 6 | 5 | 5 | 8 | 5 | 7 | 8 | 6 |
| Stroke events, No. (%) | 39 (5.0) | 14 (4.9) | 98 (6.5) | 46 (6.0) | 34 (6.1) | 38 (9.6) | 20 (12.7) | 8 (6.1) |
Abbreviations: LVH, left ventricular hypertrophy; SBP, systolic blood pressure.
The analysis approach was to first assess the potential of a differential impact of hypertension on stroke risk using race-by-SBP interaction terms in proportional hazards models, initially after adjustment for demographic factors (age, race, age-by-race interaction, and sex) plus the use of antihypertensive medications and subsequently after adjustment for stroke risk factors (diabetes, atrial fibrillation, left ventricular hypertrophy, heart disease, and current cigarette smoking). The potential for effect modification between SBP, race, and age were assessed by the addition of interaction terms (a priori, P = .10 was considered significant for interaction terms). In addition, the magnitude of differences between blacks and whites in each of the 9 strata was determined using proportional hazards analysis to adjust for only sex plus antihypertensive use and then to subsequently adjust for the aforementioned risk factors. Although the REGARDS study has a wealth of risk factors that could be used, we have intentionally limited the covariate adjustment to the major traditional stroke risk factors defined by inclusion in the Framingham Stroke Risk Function.3 These set of risk factors were confirmed as the major stroke risk factors by an independent risk assessment analysis in the Cardiovascular Health Study (CHS).12
The race of participants was determined by self-report. As recommended by the JNC7,11 SBP was defined as the mean of 2 seated measures taken after a 5-minute rest. Use of antihypertensive medications was defined by self-report. Diabetes was defined as a fasting glucose level of 126 mg/dL or greater (to convert to millimoles per liter, multiply by 0.0555) (or non-fasting glucose level of ≥200 mg/dL if the participant was not fasting) or self-reported use of diabetic medications. Atrial fibrillation was defined by self-report of physician diagnosis or electrocardiogram evidence, and left ventricular hypertrophy was defined by the Sokolow-Lyon criteria.13 Current cigarette smoking was defined by self-report. History of heart disease was defined as a self-reported myocardial infarction, electrocardiogram evidence of myocardial infarction, or self-reported coronary artery bypass, angioplasty, or stent.
Because the disparity in stroke mortality has traditionally been defined as a difference in all strokes (ie, infarcts plus hemorrhages), we have chosen a similar definition for the stroke outcome for this study. Suspected stroke events reported on semiannual telephone contacts and associated medical records were retrieved and adjudicated by a physician committee. A suspected stroke was triggered for adjudication if the patient or proxy reported a stroke or transient ischemic attack event, was hospitalized for stroke symptoms, or was hospitalized for unknown reasons. For proxy-reported deaths, an interview was conducted with an informed proxy.
Because some medical records could not be retrieved and records remained in the adjudication process at the time of analysis (approximately 10% each), multiple imputation techniques were used to reduce the potential bias arising from undocumented stroke events. Ten imputation data sets were created using the approach of Rubin.14 Details of the multiple imputation approach used in the REGARDS study cohort, including sensitivity analysis for differences in estimates, are provided elsewhere.15
RESULTS
Over a mean (SD) of 5.6 (2.0) years of follow-up, 715 adjudicated stroke events occurred among the 27 780 participants. Only 15% of the participants voluntarily withdrew from follow-up (<3% per year), and these participants were censored at their last observed contact. The characteristics of the population within the age-by-SBP strata are described in Table 1 (those with SBP ≥160 mm Hg were excluded from subsequent analyses). With the exception of current cigarette smoking, risk factor prevalence was generally higher for older participants and for participants with higher BP levels. Across age and SBP level, black participants were more likely to be using antihypertensive medications and to have a higher prevalence of diabetes and left ventricular hypertrophy but had a lower prevalence for atrial fibrillation, smoking, and heart disease.
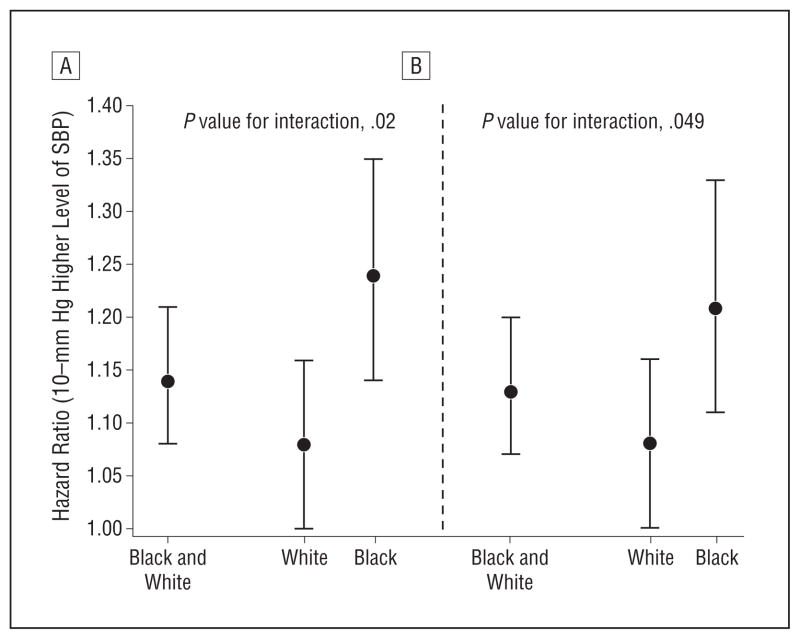
Figure 1 shows the estimated impact of a 10–mm Hg higher level of SBP both overall (pooled black and white participants) and separately by race groups. Overall, there was a 14% increased risk of stroke associated with a 10–mm Hg higher SBP (hazard ratio [HR], 1.14; 95% CI, 1.08–1.21); however, there was evidence of racial differences in this association (P value for interaction, .02) with an 8% increase in whites (HR, 1.08; 95% CI, 1.00–1.16) and a 24% increase in blacks (HR, 1.24; 95% CI, 1.14–1.35). Adjustment for risk factors (Figure 1B) reduced the estimated impact of SBP differences, but the same pattern of racial differences persisted (P value for interaction, .049).
Figure 1.
Differential racial susceptibility to 10–mm Hg systolic blood pressure difference. A, After adjustment for demographic factors plus use of antihypertensive medications. B, After further adjustment for risk factors (diabetes, atrial fibrillation, left ventricular hypertrophy, heart disease, and current cigarette smoking). Estimates are provided where races have been pooled (black and white) and for race-specific estimates. Error bars indicate 95% confidence intervals.
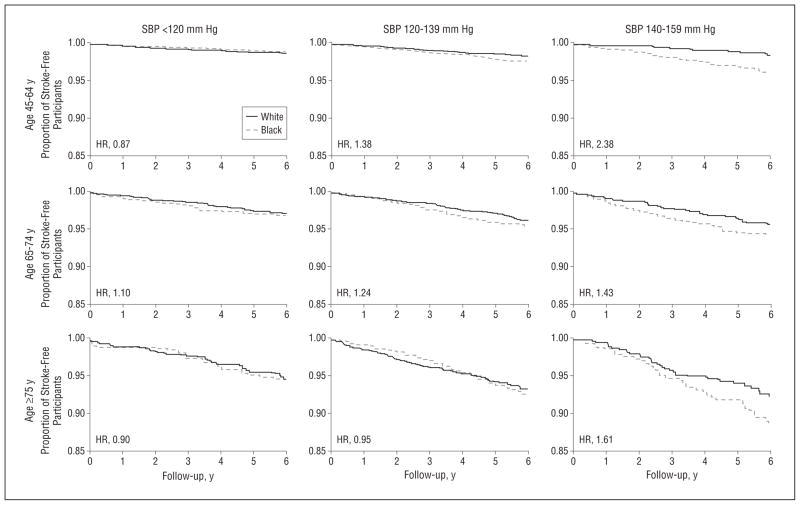
The proportions of participants remaining stroke-free within the 9 SBP-age strata are shown in Figure 2, with estimated black to white HRs given in Table 2. In the youngest age stratum, there were relatively few strokes and little racial difference in stroke for those with an SBP lower than 120 mm Hg; however, whites tended to be at greater stroke risk (black to white HR, 0.87; 95% CI, 0.48–1.57). Among prehypertensive subjects (SBP, 120–139 mm Hg), whites had a higher stroke risk than normotensive subjects, but black participants had a risk 38% higher than their white counterparts (HR, 1.38; 95% CI, 0.94–2.02). For participants with stage 1 hypertension, black participants were at 2.38 times the stroke risk of their white counterparts (HR, 2.38; 95% CI, 1.19–4.72). Among these young participants, the test for trend suggested an increasing magnitude of disparity in stroke risk with higher BP levels (P = .03). Adjustment for additional risk factors only marginally mediated this pattern (Table 2), and further adjustment for income and education as measures of socioeconomic status did not meaningfully affect the results (data not shown).
Figure 2.
Proportion of white and black stroke-free participants, shown with age and systolic blood pressure (SBP) strata and hazard ratio (HR) after adjustment for sex and use of antihypertensive medications. See also Table 2 for HRs and 95% confidence intervals in the risk factor adjusted model (ie, after additional adjustment for diabetes, atrial fibrillation, left ventricular hypertrophy, heart disease, and current cigarette smoking).
Table 2.
Black to White Hazard Ratios (HRs) Within Age and SBP Strata
| Age Strata, y | Black to White HR (95% CI)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| After Adjustment for Sex and Hypertensive Medications
|
After Adjustment for Sex, Hypertensive Medications, and Risk Factorsa
|
|||||||
| SBP <120 mm Hg | SBP 120–139 mm Hg | SBP 140–159 mm Hg | P Value for Trend | SBP <120 mm Hg | SBP 120–139 mm Hg | SBP 140–159 mm Hg | P Value for Trend | |
| 45–64 | 0.87 (0.48–1.57) | 1.38 (0.94–2.02) | 2.38 (1.19–4.72) | .03 | 0.93 (0.51–1.71) | 1.33 (0.89–1.98) | 2.14 (1.07–4.30) | .09 |
| 65–74 | 1.10 (0.64–1.88) | 1.24 (0.89–1.71) | 1.43 (0.90–2.26) | .43 | 1.06 (0.60–1.86) | 1.13 (0.81–1.59) | 1.27 (0.80–2.04) | .61 |
| ≥75 | 0.90 (0.45–1.81) | 0.95 (0.65–1.40) | 1.61 (0.99–2.62) | .16 | 0.94 (0.47–1.90) | 0.81 (0.53–1.24) | 1.47 (0.89–2.42) | .22 |
| P value for trend | .86 | .19 | .50 | .93 | .13 | .56 | ||
Risk factors include diabetes, atrial fibrillation, left ventricular hypertrophy, heart disease, and current cigarette smoking.
The overall risk of stroke was higher for those aged 65 to 74 years. In Figure 2, for those with SBP lower than 120 mm Hg, there was little racial difference in stroke risk (HR, 1.10; 95% CI, 0.64–1.88). No significant differences in black to white HRs for stroke risk were detected (P value for trend, .43) at higher levels of SBP (120–139 mm Hg: HR, 1.24 [95% CI, 0.89–1.71]; and 140–159 mm Hg: HR, 1.43 [95% CI, 0.90–2.26]). Adjustment for additional risk factors did not meaningfully change these patterns.
Stroke risk was highest in the oldest age strata (≥75 years) (Figure 2). In those with an SBP lower than 120 mm Hg, the stroke risk among blacks was marginally lower (HR, 0.90; 95% CI, 0.45–1.81), and there was little evidence of a change in the black-white disparity for those with prehypertension (HR, 0.95; 95% CI, 0.65–1.40). However, there was modest evidence of higher risk in blacks for those with stage 1 hypertension (HR, 1.61; 95% CI, 0.99–2.62). There was also modest evidence that the stroke risk for blacks compared with whites changed with increasing levels of BP (P value for trend, .16). Adjustment for additional risk factors did not substantially modify the pattern of racial disparity (Table 2).
COMMENT
We document a differential impact of SBP on stroke risk for blacks compared with whites, a difference that was not sub-stantially modified by adjustment for other traditional stroke risk factors. This differential impact of high BP is the third pathway through which BP could contribute to racial disparities in stroke risk. The 3 pathways are as follows:
Pathway 1
It has long been accepted that the “prevalence of hypertension in blacks in the United States is among the highest in the world, and it is increasing.”16(p102) This finding was confirmed in the REGARDS study population, where 71% of blacks compared with 51% of whites were hypertensive.17
Pathway 2
There is increasing appreciation that while blacks are more likely than whites to be aware of their hypertension and are also more likely to be treated for their hypertension, they are remarkably less likely to have their BP controlled. For example, in the National Health and Nutrition Examination Survey 1999–2002, awareness of hypertension was 77.7% for blacks compared with 70.4% for whites, and hypertension was treated in 68.2% of blacks and 60.4% of whites but was controlled in 48.9% of blacks compared with 59.7% of whites.18 In the REGARDS study, we found a 1.45-times greater odds (95% CI, 1.25–1.71) that blacks would be aware of their hypertension, a 1.56-times greater odds (95% CI, 1.34–1.83) that their hypertension would be treated, but a 0.67-times lesser odds (95% CI, 0.60–0.74) that they would have controlled BP.19 In the REGARDS study, the achieved BP of blacks compared with whites was approximately 5 mm Hg higher.17
Pathway 3
Herein, we estimate that the impact of higher BP levels on stroke is 3 times greater for blacks than for whites, that is, for the same 10–mm Hg difference in SBP, there was a 24% increase in risk for blacks but only an 8% increase for whites.
Thus, hypertension is then a “triple curse” for stroke risk in blacks, and this triple curse is particularly potent for those aged 45 to 64 years, where the magnitude of the black-white disparity in stroke increases substantially at higher SBP levels.
We were struck that in the REGARDS study, regardless of age, there was no evidence of a racial disparity in the risk of incident stroke among normotensive individuals (Figure 1 and Table 2). This observation in normotensive individuals suggests the theoretical potential of eliminating all 3 pathways through which BP contributes to racial disparities (higher prevalence, poorer control, and larger impact in blacks), whereas, when controlled, there appears to be no racial disparity in stroke incidence. This is particularly remarkable for those aged 45 to 64 years, where the overall risk of stroke across all SBP levels is between 2 and 3 times greater in blacks than in whites.1,2 However, this observation in the REGARDS study is conditional on the participant having an SBP less than 120 mm Hg, and only 28% (1717 of 6155) of blacks in this age range (45–64 years) meet this criteria compared with 44% (3548 of 8129) of whites. In the 45- to 64-year age group, much of the excess stroke risk was among those with stage 1 hypertension (SBP levels between 140 and 159 mm Hg), which included 1076 blacks (18%) and only 821 whites (10%).
Our data suggest that the striking racial disparity in stroke risk between ages 45 to 64 years may be substantially reduced by improved BP control, and justify a clinical trial or other observational studies for confirmation. Theoretically, moving all black and white individuals from stage 1 hypertension to prehypertension would reduce the black to white HR from 2.38 to 1.38, reducing the disparity in stroke by 72% [(2.38–1.38)/(2.38–1.00) = 0.72], thereby nearly eliminating the racial disparity. Additional reductions in the racial disparity could be further realized if the BP could be reduced to levels where we observed blacks to have stroke risk nonsignificantly lower than whites. As we have previously noted, this might be best achieved with primordial prevention of hypertension at ages even younger than 45 years; however, this assumes that the reduction of BP to a lower level is associated with a reduction in risk similar to the level of others already at that level.2
Compared with younger individuals, we also observed similar patterns of increasing disparities in stroke for those aged 65 to 74 years; however, the differences were not as dramatic. The smaller black to white HRs at older ages is perhaps not surprising, since the overall magnitude of black-white racial disparities in the age strata is substantially smaller.1,2
Although the goal of reducing BP to levels lower than 120 mm Hg for blacks is a lofty (and perhaps unachievable) goal, the theoretical economic and public health implications are staggering and can be estimated with a number of speculative assumptions. Considering only those between the ages of 45 and 64 years, according to the Centers for Disease Control and Prevention’s WONDER (Wide-ranging Online Data for Epidemiologic Research), in 2007, there were 4537 stroke deaths among the 8 674 534 US blacks (a crude rate of 52.3 per 100 000) and 11 577 stroke deaths among the 63 787 911 US whites (a crude rate of 18.1 per 100 000).20 According to the Atherosclerosis Risk in Communities Study, 30-day case fatality following stroke in this age range is 12.5% for blacks,21 implying a crude estimate of 36 296 (4537 divided by 0.125) strokes in the US black population. It would take a 65.3% reduction in stroke mortality for blacks to die at the same rate as whites, a difference that these data suggest could be achieved with improved BP control. If this reduction were applied to the estimated 36 296 stroke events in blacks, the number of strokes would be reduced by 23 701 events. It is estimated that the cost of a stroke is approximately $140 000,22 so that BP control could result in an annual savings of $3.3 billion dollars. As previously stated herein, these calculations require substantial assumptions; however, they provide a “ball park” estimate of the potential impact of improved BP control for blacks between ages 45 and 64 years.
This report has the following strengths and weaknesses. The REGARDS study is one of few studies with a sufficient number of both black and white participants to allow for the study of differences in the impact of risk factors between blacks and whites. To date, we have accrued 715 incident stroke events among those stroke free at baseline. While this number compares favorably with other studies1 and allows age-SBP-race stratification as performed herein, a larger number of stroke events that are currently being accrued will continue to improve the precision of the estimates. There are many reports documenting a wide array of psychosocial, biomarker, and interview and physical measures as stroke risk factors. We relied on the major stroke risk publications3,11 to guide the selection of risk factors for the adjustment in our statistical models; however, stroke risk factors not considered in the models may result in an underestimation of the degree racial differences could be explained. The study is also limited to a single measure of exposures at baseline and as such cannot adjust for either changes in the risk factors or for measurement error affecting results (ie, regression dilution bias).
In conclusion, it has long been known that the prevalence of hypertension is higher in blacks and that control of hypertension in blacks is relatively poor. In this study we show that blacks, compared with whites, also appear to be more susceptible to stroke, given the same level of elevated BP. Blacks have more hypertension and are less likely to have it controlled, and when it is not controlled they are at greater risk for incident stroke (relative to whites with the same BP levels). Our findings raise the potential that appropriate BP control, particularly for those aged 45 to 64 years, may play a major role in reducing the longstanding racial disparity in stroke risk in the United States.
Acknowledgments
Funding/Support: The research reported in this article was supported by cooperative agreement NS 041588 from the National Institute of Neurological Disorders and Stroke.
Footnotes
Conflict of Interest Disclosures: None reported.
Author Contributions: Study concept and design: G. Howard, Lackland, Kleindorfer, Moy, Safford, Cushman, and V. J. Howard. Acquisition of data: G. Howard, Kleindorfer, Safford, Cushman, and V. J. Howard. Analysis and interpretation of data: G. Howard, Lackland, Kleindorfer, Kissela, Moy, Judd, Safford, and Glasser. Drafting of the manuscript: G. Howard, Lackland, Kleindorfer, Judd, and Glasser. Critical revision of the manuscript for important intellectual content: Lackland, Kissela, Moy, Judd, Safford, Cushman, Glasser, and V. J. Howard. Statistical analysis: G. Howard, Lackland, and Safford. Obtained funding: G. Howard, Kissela, Cushman, and V. J. Howard. Administrative, technical, and material support: Kleindorfer, Kissela, Judd, Safford, and V. J. Howard. Study supervision: G. Howard and Safford.
References
- 1.Howard VJ, Kleindorfer DO, Judd SE, et al. Disparities in stroke incidence contributing to disparities in stroke mortality. Ann Neurol. 2011;69(4):619–627. doi: 10.1002/ana.22385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howard G, Cushman M, Kissela BM, et al. REasons for Geographic And Racial Differences in Stroke (REGARDS) Investigators. Traditional risk factors as the underlying cause of racial disparities in stroke: lessons from the half-full (empty?) glass. Stroke. 2011;42(12):3369–3375. doi: 10.1161/STROKEAHA.111.625277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22(3):312–318. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 4.Howard G, Howard VJ REasons for Geographic And Racial Differences in Stroke (REGARDS) Investigators. Ethnic disparities in stroke: the scope of the problem. Ethn Dis. 2001;11(4):761–768. [PubMed] [Google Scholar]
- 5.Howard VJ, Cushman M, Pulley LV, et al. The Reasons for Geographic and Racial Differences in Stroke Study: objectives and design. Neuroepidemiology. 2005;25(3):135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 6.Howard G, Anderson R, Sorlie P, Andrews V, Backlund E, Burke GL. Ethnic differences in stroke mortality between non-Hispanic whites, Hispanic whites, and blacks: the National Longitudinal Mortality Study. Stroke. 1994;25(11):2120–2125. doi: 10.1161/01.str.25.11.2120. [DOI] [PubMed] [Google Scholar]
- 7.Cooper ES. Cardiovascular diseases and stroke in African Americans: a call for action. J Natl Med Assoc. 1993;85(2):97–100. [PMC free article] [PubMed] [Google Scholar]
- 8.Gillum RF. Stroke mortality in blacks: disturbing trends. Stroke. 1999;30(8):1711–1715. doi: 10.1161/01.str.30.8.1711. [DOI] [PubMed] [Google Scholar]
- 9.Kleindorfer DO, Khoury J, Moomaw CJ, et al. Stroke incidence is decreasing in whites but not in blacks: a population-based estimate of temporal trends in stroke incidence from the Greater Cincinnati/Northern Kentucky Stroke Study. Stroke. 2010;41(7):1326–1331. doi: 10.1161/STROKEAHA.109.575043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kleindorfer D, Broderick J, Khoury J, et al. The unchanging incidence and case-fatality of stroke in the 1990s: a population-based study. Stroke. 2006;37(10):2473–2478. doi: 10.1161/01.STR.0000242766.65550.92. [DOI] [PubMed] [Google Scholar]
- 11.Chobanian AV, Bakris GL, Black HR, et al. National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 12.Manolio TA, Kronmal RA, Burke GL, O’Leary DH, Price TR. Short-term predictors of incident stroke in older adults: the Cardiovascular Health Study. Stroke. 1996;27(9):1479–1486. doi: 10.1161/01.str.27.9.1479. [DOI] [PubMed] [Google Scholar]
- 13.Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J. 1949;37(2):161–186. doi: 10.1016/0002-8703(49)90562-1. [DOI] [PubMed] [Google Scholar]
- 14.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: J Wiley & Sons; 1987. [Google Scholar]
- 15.Howard G, McClure LA, Moy CS, et al. Imputation of incident events in longitudinal cohort studies. Am J Epidemiol. 2011;174(6):718–726. doi: 10.1093/aje/kwr155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roger VL, Go AS, Lloyd-Jones DM, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation. 2011;123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cushman M, Cantrell RA, McClure LA, et al. Estimated 10-year stroke risk by region and race in the United States: geographic and racial differences in stroke risk. Ann Neurol. 2008;64(5):507–513. doi: 10.1002/ana.21493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hertz RP, Unger AN, Cornell JA, Saunders E. Racial disparities in hypertension prevalence, awareness, and management. Arch Intern Med. 2005;165(18):2098–2104. doi: 10.1001/archinte.165.18.2098. [DOI] [PubMed] [Google Scholar]
- 19.Howard G, Prineas R, Moy C, et al. Racial and geographic differences in awareness, treatment, and control of hypertension: the REasons for Geographic And Racial Differences in Stroke study. Stroke. 2006;37(5):1171–1178. doi: 10.1161/01.STR.0000217222.09978.ce. [DOI] [PubMed] [Google Scholar]
- 20. (Centers for Disease Control and Prevention, National Center for Health Statistics).Compressed Mortality File 1999–2007. [Accessed June 8, 2011];CDC WONDER On-line Database, compiled from Compressed Mortality File 1999–2007 Series 20 No. 2M. 2010 http://wonder.cdc.gov/cmf-icd10.html.
- 21.Rosamond WD, Folsom AR, Chambless LE, et al. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999;30(4):736–743. doi: 10.1161/01.str.30.4.736. [DOI] [PubMed] [Google Scholar]
- 22.Di Carlo A. Human and economic burden of stroke. Age Ageing. 2009;38(1):4–5. doi: 10.1093/ageing/afn282. [DOI] [PubMed] [Google Scholar]