Abstract
The orexin peptides and their two receptors are involved in multiple physiological processes, including energy homeostasis, arousal, stress and reward. Higher signaling of the orexin peptides at the orexin receptors (OXR) protects against obesity, but it is less clear how their activation in different brain regions contributes to this behavioral output. This review summarizes the evidence available for a role of central OXR in energy homeostasis and their contribution to obesity. A detailed analysis of anatomical, cellular and behavioral evidence shows that modulation of energy homeostasis by the OXR is largely dependent upon anatomical and cellular context. It also shows that obesity resistance provided by activation of the OXR is distributed across multiple brain sites with site-specific actions. We suggest that understanding the role of the OXR in the development of obesity requires considering both specific mechanisms within brain regions and interactions of orexinergic input between multiple sites.
Keywords: orexin, orexin receptors, hypothalamus, energy metabolism, diet-induced obesity, spontaneous physical activity
INTRODUCTION
The orexins are two closely related peptides, orexin-A (OXA, hypocretin 1) and orexin-B (OXB, hypocretin 2). In several mammalian species, the majority of the central nervous system orexin peptides are synthesized in neurons located in the lateral hypothalamus (LH) and perifornical area that project to multiple brain regions.1–7 The orexin peptides exert their biological effects through two G protein-coupled receptors, orexin receptor type-1 (OX1R, hypocretin receptor 1) and orexin receptor type-2 (OX2R, hypocretin receptor 2).8,9 Both orexin receptors (OXR) subtypes can bind to OXA and OXB, but with differential affinity; OX1R has a higher affinity for OXA, whereas OX2R has equal affinity for either orexin peptide.9,10
Following the discovery of the orexin/hypocretin peptides in 1998,8,9 research focused on their role in energy metabolism,11–14 as well as modulation of sleep and arousal.15–17 By early 2000, the role of the orexin peptides as modulators of acute food intake and arousal had been recognized.18 Development of the orexin neuron-deficient mice in 2001 defined the orexin peptides as positive modulators of energy expenditure and suggested a role for orexin peptides in development of obesity.19 Since then, a combination of evidence from pharmacological analysis and genetic models has established orexin peptides and their receptors as central regulators of energy expenditure, which influences energy balance. The aim of this review is to integrate evidence from cellular and behavioral experiments to examine our current understanding of the role of the OXR in energy metabolism and obesity.
Throughout this review, we use the term ’orexin system’ to describe neurons that produce orexin peptides (the orexinergic neurons), orexin-responsive neurons (i.e., those expressing OXR) and interactions between them that modulate animal behavior. It is important to remember that some orexinergic neurons can be regulated through the OX2R.20 Therefore, the categories of orexinergic neurons and orexin-responsive neurons are not mutually exclusive. We use this terminology to be explicit about the fact that behavioral and physiological effects associated with orexin peptides arise from combined action of orexin peptides at OXR-expressing neurons.
The data reviewed in this article suggests that both OXR subtypes can mediate increased energy expenditure and thus, contribute to obesity resistance mediated by the orexin system. However, we argue that the mechanisms by which each orexin receptor subtype acts are largely dependent upon their anatomical and cellular context. Furthermore, we suggest that the effects of the orexin system in energy metabolism result from parallel signaling to multiple brain regions. Thus, proper understanding of the function of the OXR in energy balance and obesity requires studying the anatomical and functional connections between sites that receive orexinergic innervation.
OXR BRAIN EXPRESSION IS PART OF A NETWORK
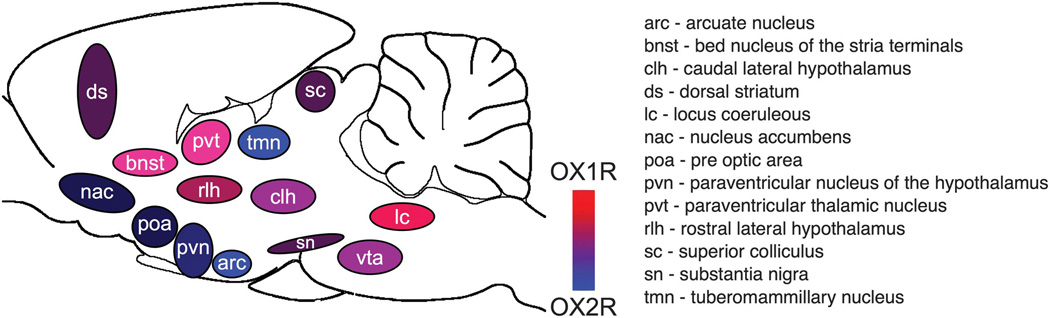
The orexin system is characterized by the wide expression of the OXR in brain, which matches the pattern of orexinergic neuronal projections.1–6 A number of anatomical studies in rat brain show an agreement between OXR mRNA and protein distribution.21–26 Across these studies, the overall conclusion is that there are overlapping, yet distinct, patterns of expression for OX1R and OX2R in brain (Figure 1). However, whether both OXR are co-expressed in the same neurons across different neuronal populations remains unknown.
Figure 1.
Expression of the OXR in rat brain. This figure illustrates the expression pattern of the OX1 and OX2 in rat brain in a subset of brain regions that express the OXR. The levels of OXR expression were taken from Johansson et al38
One important clue about the function of the orexin system comes from the fact that the OXR are expressed in different brain regions that control the same behaviors. For example, several brain regions involved in control of food intake receive orexinergic input and express the OXR, including the arcuate nucleus, ventromedial hypothalamic and the tuberomammillary nuclei.27,28 This expression pattern suggests the behavioral outcomes of the orexin system are due to simultaneous activation of the OXR in different brain regions. In direct support of this hypothesis, retrograde tracer studies have shown that orexin neurons have collateral projections within the central nervous system,29–31 trans-synaptically collateral central nervous system efferents32 or collateral efferents to both, to the central nervous system regions and brown adipose tissue.33
Together, the available anatomical evidence supports the hypothesis that the behavioral consequences of the activity of the orexin system are due to parallel signaling to multiple brain regions. The idea that modulation of energy homeostasis is a distributed process through a neuronal network is not without support in the literature.34,35 This framework suggests that understanding the function of the OXR requires studying them in a brain region-specific basis, as well as understanding the interactions between different brain regions that receive orexinergic input. Fundamental to this idea is whether there are common mechanisms that mediate the OXR function across different brain regions and/or neuronal populations. In the next section, we review cellular evidence to illustrate how the function and regulation of the OXR appears to be cellular type-specific.
OXR CAN INDUCE NEURONAL DEPOLARIZATION THROUGH MULTIPLE, CELL-SPECIFIC MECHANISMS
In vitro and in vivo studies suggest that both the OXR subtypes can couple to multiple G-proteins. Depending on the cell system for heterologous expression or tissue of choice, the OXR can couple with Gq, GI/O and GS subunits.36–47 Interestingly, in whole hypothalamic samples, food restriction has been demonstrated to increase coupling of either orexin receptor subtype to Gq, GS and GO, and decrease coupling to Gi. However, in the same study, the opposite effects were observed in adrenal cortex.48 Collectively, these data show that regulation of OXR signaling in response to energy status change is tissue-specific. These data also suggest that regulation of OXR signaling is cell type-specific.
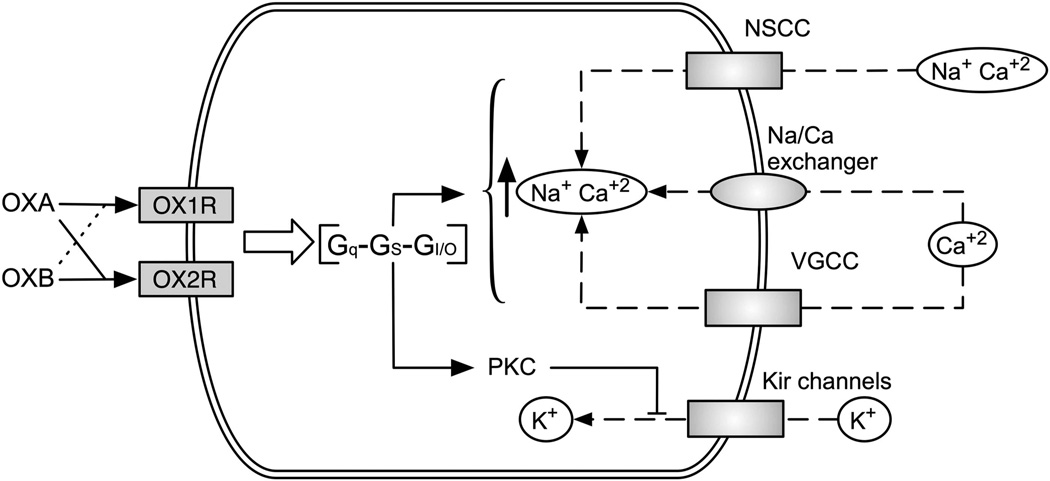
Activation of the OXR causes depolarization and increases neuronal firing by four possible mechanisms (Figure 2): (1) activation of non-specific cationic currents, (2) activation of the Na+/Ca2+ exchanger, (3) phosphorylation-dependent inhibition of inwardly rectifying K+ channels, and (4) increase in [Ca2+]i through activation of L- and N-type Ca2+ channels.43,49–59 The type of mechanism appears to be cell dependent. For example, in nucleus accumbens and nucleus of the solitary tract, OXR-mediated depolarization requires a simultaneous decrease in K+ conductance and increase in non-specific cationic currents.51,60 In GABAergic neurons from the arcuate nucleus, it occurs through a decrease in K+ conductance and activation of the Na+/Ca2+ exchanger.61 Finally, there are differences in the temporal profile of [Ca2+]i increase after OXR activation between neurons from the dorsal raphe and laterodorsal tegmentum areas.62,63
Figure 2.
Diversity of G-protein coupling and mechanisms of neuronal depolarization mediated by the OXR. The orexin peptides (OXA and OXB) differentially activate the OX1R and the OX2R: OX1R has a higher affinity for OXA, whereas OX2R has equal affinity for either orexin peptide. Activation of the OXR leads to an increase in neuronal activity, but the mechanisms are cell-specific. Depending on the particular cell population, the OXR couple to Gq, GI/O or GS proteins, and modulate different ion channels and exchangers to induce neuronal depolarization. Currently, modulation of non-selective cationic currents, voltage-gated calcium channels (VGCC), the Na/Ca exchanger and Kir channels by the OXR have been described.
In summary, both OXR subtypes can couple to multiple G-proteins and can thus induce neuronal depolarization through many mechanisms engaged in a cell-specific manner. In the next section, we review the literature about the role of OXR subtypes in control of spontaneous physical activity (SPA) and food intake. Together with cellular data discussed in this section, we interpret this evidence in favor of the idea that function of the OXR in energy metabolism is dependent on the anatomical and cellular context, and argue against generalization of the function of OXR.
ROLE OF THE OXR IN ENERGY METABOLISM AND OBESITY
Early pharmacological studies have demonstrated that intracerebro ventricular injections of either OXA or OXB increased food intake and locomotor activity.9,11,13,17,64 Although these data alone would suggest that both OXR subtypes would always mediate the same type of behaviors, the evidence discussed below supports the idea that the function of OXR subtypes is brain site-specific.
First, we summarize key evidence from mouse genetic models to review the general role of the orexin system in energy balance. Next, we discuss pharmacological and genetic data specific to the OXR and their function in energy metabolism, and its implications for obesity. Finally, we briefly review how the role of the orexin system in sleep might contribute to its influence on energy balance.
Role of the orexin system in energy metabolism and obesity
This section reviews key findings from genetic models to illustrate both the general role and important details of the function of the orexin system in control of energy metabolism.
In 2001, Hara et al.19 developed a mouse model that lacks orexin neurons (OX-AT3). These mice express the ataxia3 gene under the control of orexin promoter, which leads to loss of the orexin neurons postnatally due to expression of the toxic protein ataxin3.19 The OX-AT3 mice showed both hypophagia and lower levels of SPA, but the energetic consequences of lower activity were greater as they developed late-onset obesity when fed a regular diet.19,65 This result suggests that although activation of the orexin system can increase both SPA and food intake, its primary function is to promote an increase in energy expenditure. This hypothesis is supported by studies showing that chronic orexin intrathecal injections do not result in body weight gain.66,67 Further support comes from a mouse model, in which the pre-proorexin gene is under the control of the β-actin/cytomegalovirus (CAG) promoter (CAG-OX), leading to overexpression of the orexin peptides.68 Consistent with the role of orexin in promoting energy expenditure, the CAG-OX mice show resistance to obesity induced by consumption of a high-fat diet.69
It has been shown that genetic background and sex contribute to the role of the orexin system in energy balance. For example, the late spontaneous obesity observed in the OX-AT3 mice, but not the higher weight gain on a high-fat diet, was dependent on genetic background.70 Additional studies found that both female OX-AT3 and OX−/− mice (from both C57BL/6 and mixed genetic background) gained more body weight gain on a standard diet compared with male mice.71 Together, these studies suggest that activation of the orexin system increases energy expenditure, which results in protection against development of obesity. Recent evidence shows that orexin responsive neurons in raphe pallidus stimulate brown adipose tissue thermogenesis, which provides another mechanism by which elevated orexin system activity may elevate energy expenditure.72,73 The mechanisms by which genetic background, gender and diet modulate the activity of the orexin system remain unclear.
Evidence for a brain region-specific contribution of the OXR to locomotor activity and energy metabolism
Shortly after discovery of the orexins, a number of studies showed that intra-cerebro ventricular injections of either OXA or OXB increased energy expenditure, physical activity and food intake.25,64,74–76 However, the broad expression of the OXR subtypes21,23 and the redundancy of brain control mechanisms observed in energy metabolism35 suggest that intra-cerebro ventricular injections may not provide accurate information about the physiological role of the OXR subtypes.
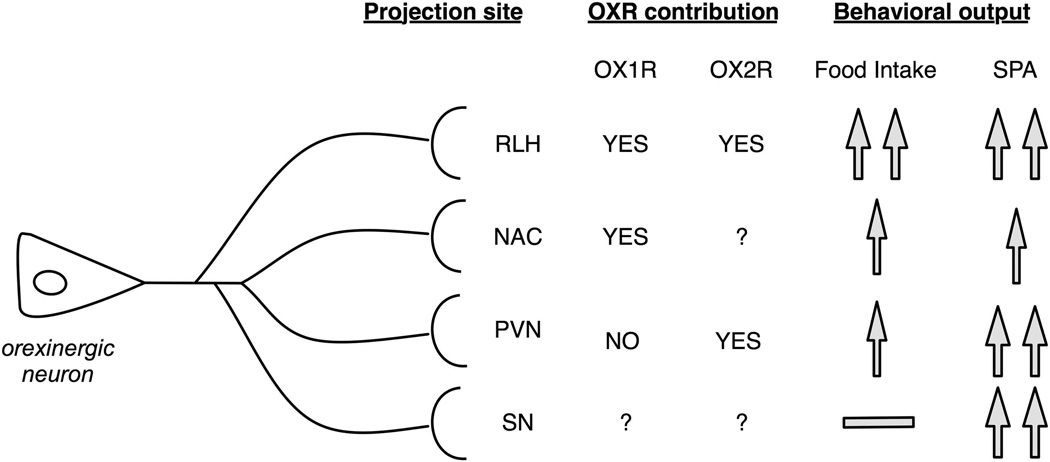
Injection of OXA in specific brain regions has shown that activation of the OXR can increase SPA and/or food intake with region-specific changes in the magnitude of the effects (Figure 3). The increase in food intake mediated by OXA injections provides a good example of brain regional specificity. Injection of OXA in the perifornical area, the rostral portion of LH (RLH), and the paraventricular nucleus of the hypothalamus (PVN) and nucleus accumbens (NAC), but not in the substantia nigra increases food intake.13,77–80 The orexin peptides modulate the activity of orexinergic neurons through activation of both OXR subtypes in glutamatergic interneurons81 and cell-intrinsic effects through the OX2R.20 It is likely that injection of OXA in the perifornical area is activating orexinergic neurons themselves and increasing orexin signaling through multiple brain sites, leading to the observed increase in food intake. OXA injected in the RLH increases food intake as well as locomotor activity,82 and most orexinergic neurons are located in the dorsal region of the LH, indicating this effect is not mediated by their activation.
Figure 3.
The behavioral effects of the orexin system in SPA and food intake involve multiple brain regions. This figure summarizes the overlap and distinct influence OXR have on food intake and SPA. Pharmacological studies have demonstrated that the contribution of either orexin receptor subtype on behavioral output is based on anatomical location. This would strongly suggest that although there appears to be an overlap in OXR distribution, the mechanisms that are activated could depend upon specific location and orexin innervation or efferent projections.
Figure 3 summarizes the results of site-specific injections of OXA in SPA in a subset of brain regions for which there is detailed pharmacological information regarding contributions of the OXR. Injection of OXA in PVN increases both SPA and energy expenditure, and these effects are blocked by pre-treatment with an OX1R antagonist.83 However, this same drug proves ineffective in blocking the increase in SPA resulting from OXA injection in the NAC.84 This example clearly shows that both OX1R and OX2R can modulate the same type of behavior through different brain regions. However, the magnitude of effect is different; the effects of OXA injection in SPA are similar in RLH, PVN and substantia nigra, followed by NAC. To date, there is no information about the relative contribution of the OXR subunits to effects of OXA in SPA and food intake through substantia nigra. The differences in magnitude across the brain regions could be caused by different mechanisms that mediate OXR-induced neuronal depolarization (see section ‘OXR can induce neuronal depolarization through multiple, cell-specific mechanisms’), differences in density of orexinergic innervation and/or destination of the efferent projections. Together, the data summarized in Figure 3 provides proof-of-concept that the effects of the orexin system in energy metabolism are distributed across multiple regions.
In summary, the evidence discussed is consistent with activation of both OXR subtypes increasing SPA, energy expenditure and food intake. It shows that multiple brain regions (including NAC, PVN, RLH and substantia nigra) contribute to the behavioral output of the orexin system. Furthermore, the specific mechanisms by which each orexin receptor subunit modulates changes in SPA seem to depend on its cellular and anatomical context. These data are consistent with the idea that the influence of the orexin system in animal behavior is mediated by parallel signaling to multiple brain regions, which is also supported by anatomical data (section ‘OXR brain expression is part of a network’).
If we consider the orexin system as a network, it becomes clear that the interactions between the simultaneous OXR activation in these brain sites are important for understanding how the orexin system drives SPA and increases energy expenditure. Currently, there is no information about interactions between the orexinergic inputs across multiple brain regions. Evaluation of anatomical connections between orexin-responsive neurons across different brain regions (i.e., using in situ hybridization coupled with retrograde tracer injections) will be an important first step towards understanding the interactions between brain regions that receive orexinergic input.
Evidence from obesity models and OXR knock-out mice
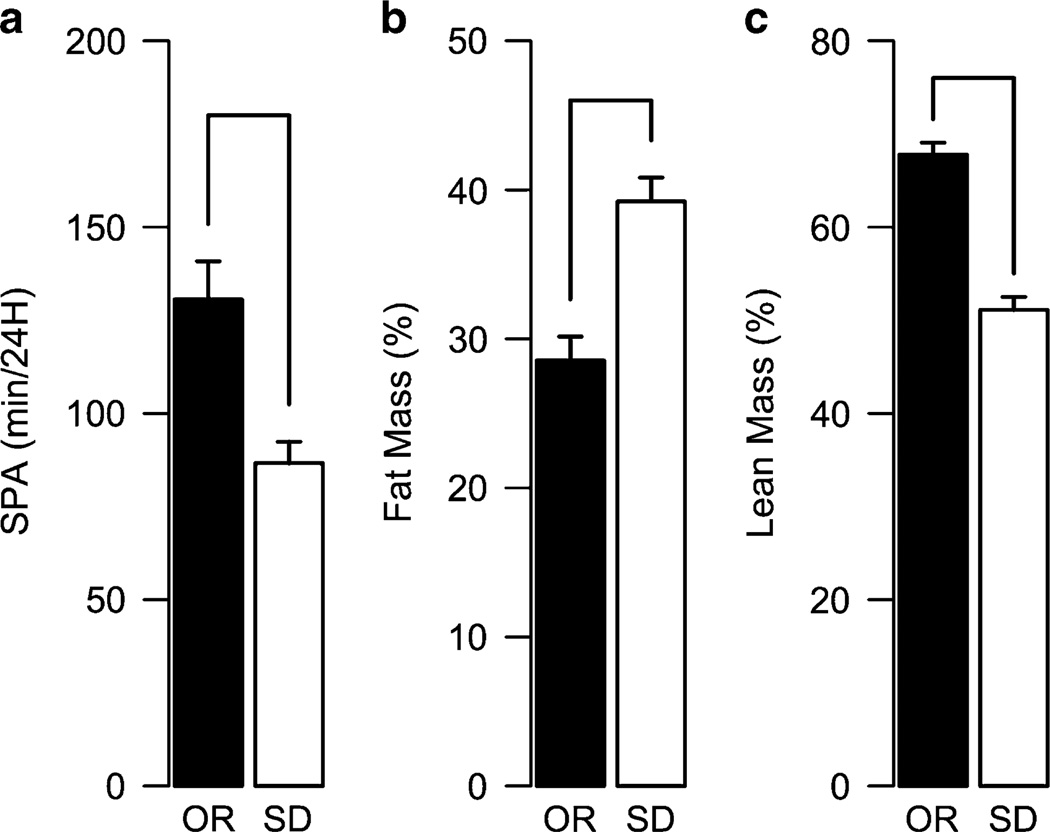
A polygenic obesity model, the obesity-prone and obesity-resistant (OR) rats has provided some insight in the role of the OXR in obesity. The obesity-prone and OR rats were derived by inbreeding from Sprague Dawley rats that show high or low body weight gain when fed a high-fat diet.85 The OR phenotype of the OR rats is associated with higher levels of SPA and increased OX1R and OX2R expression in the RLH.86 Long-term analysis of body composition of OR and Sprague Dawley rats showed that up to 18 months of age, the OR rats show lower fat mass and higher lean mass compared with Sprague Dawley rats (Figure 4).87 Together, these results agree with genetic models (section ‘Role of the orexin system in energy metabolism and obesity’) and support the view that higher orexinergic signaling provides resistance to the development of obesity.
Figure 4.
OR rats remain leaner throughout their lifetime. The OR rats were derived by selective breeding of Sprague Dawley (SD) rats that gain minimal body weight gain when fed a high-fat diet Levin et al85 The OR rats show higher expression of the OXR, higher orexin responsivity through RLH, and higher SPA compared with SD rats (a).86 These higher levels of SPA at an early age (1–2 months) are correlated with lower percent of fat mass (b) and higher percent of lean mass (c) at 18 months of age.87 These data illustrate how higher signaling through OXR allows resistance to obesity throughout the lifetime of these animals and supports the view that the overall effect of the activity of the orexin system is to promote negative energy balance. Data is from Teske et al86,87
As injection of OXA in multiple brain sites (with different levels of OXR subtypes expression) increases SPA (section ‘Role of the OXR in energy metabolism and obesity’), it is tempting to speculate that adaptations of the orexin system in other brain regions in addition to RLH may contribute to the OR phenotype of the OR rats. One possibility is that long-term potentiation due to activation of either OXR subtype88–90 can increase the gain of orexin signaling in OXR-expressing neurons, contributing to higher levels of SPA and energy expenditure in brain sites known to mediate the effects of the orexin system in SPA, such as RLH, NAC or PVN.
If injections of OXA and OXB can increase both food intake and SPA, one might expect that increased OXR expression would lead to hyperphagia and obesity instead of an OR phenotype. However, as discussed earlier, orexinergic activity has a net effect of increasing energy expenditure (section ‘Role of the orexin system in energy metabolism and obesity’). In summary, increased orexin responsivity in OR rats shows that activation of both the OXR subtypes can have a protective effect against obesity and suggests that at least in RLH, both OXR subtypes can drive the same behavioral response, which in this case is an increase in SPA.
The role of the OXR in obesity has been studied using genetic mouse models. When crossing the CAG-OX mice (section ‘Role of the orexin system in energy metabolism and obesity’) with the OX1R−/− and the OX2R−/− mice, only the OX1R−/−/CAG-OX mice were resistant to diet-induced obesity. This result was interpreted as evidence that signaling through OX2R mediates orexin system defense against diet-induced obesity.69 A key prediction based on this would be that OX2R−/− mice would have a higher sensitivity to diet-induced obesity, but there were no differences in body weight gain or fat mass between the wild type and either OX1R−/− or OXR2R−/− fed a low-fat or a high-fat diet,69 which precludes definitive conclusions regarding receptor subtype roles in obesity resistance.
Although the studies on the OX2R−/−/CAG-OX mice may implicate the OX2R in the resistance against diet-induced obesity, lack of higher sensitivity to high-fat diet in the OX2R−/− mice can be interpreted as evidence against an exclusive role of the OX2R in this phenomenon. For example, both OXR subtypes are expressed in PVN (Figure 1) and daily intra-PVN injections of OXA in male rats decreased body weight under a standard diet.66 Because OXA can activate both OX1R and OX2R, this suggests that both OXR subtypes are involved in the obesity-protective effects of orexin signaling, at least through PVN. However, to confirm this hypothesis, experiments using OXR antagonists in PVN are needed. These results highlight two important aspects of research into the function of the orexin system. First, that action of a particular OXR subtype depends on its anatomical and cellular context, and second, that a full understanding of the function of the orexin system will require use of multiple techniques that include pharmacological, genetic and optogenetic approaches.
In summary, the evidence reviewed in this section is most consistent with involvement of both OX1R and OX2R in energy balance. The data emphasize the multiplicity of factors important to defining the contribution of the orexin system in control of energy metabolism. Although evidence from genetic models is limited, our interpretation of the available evidence is that both OXR subtypes are involved in the physiological control of energy metabolism and resistance to diet-induced obesity.
Orexin system influence on sleep and arousal, and its relationship to energy balance
In addition to orexinergic control of energy expenditure by its effects on food intake and physical activity, the orexin system also controls arousal and behavioral state stabilization. In this section, we briefly discuss how orexin system modulation of arousal and behavioral state stabilization might contribute to its effect on energy expenditure. The inverse effect, how alterations in sleep and wake behavior affect energy metabolism, and the role of the orexin system in these processes, are beyond the scope of this section, and thus, we refer the reader to excellent reviews about this topic.91–94
It is well established that activation of the orexin system promotes wakefulness and its stability. The OX−/−, OX-ATX3 and double OX1R−/−; OX2R−/− mice display a phenotype similar to narcolepsy-cataplexy,16,19,95 which is characterized by frequent transitions from awake to sleep state during the animal’s active phase and by severe sleep fragmentation during the rest phase.96 Additionally, the orexin neurons show higher activation during the active (i.e., awake) phase of the light cycle,97 OXA cerebrospinal fluid levels peak during the active phase98 and the neuronal activity of orexin neurons is higher during awake episodes.99–101 Finally, optogenetic stimulation of orexin neurons directly increases the probability of transition from sleep to awake states and duration of awake periods.102 Together, this evidence suggests the orexin system has increased activity during the active/awake phase and contributes to behavioral state stability. These effects are mediated by orexinergic modulation of neuronal activity across regions with a known role in control of sleep. These include the dorsal raphe, locus coeruleus, tuberomammillary nucleus, medial preoptic area and laterodorsal tegmental nucleus/ pedunculopontine tegmental nucleus.12,59,103–110 Therefore, in parallel to its modulation of energy expenditure and SPA, control of sleep and arousal by the orexin system also appears to be distributed across multiple brain sites.
Shorter sleep time and sleep stability are associated with development of obesity in humans.111,112 Similarly, narcolepsy in humans and mouse models is associated with severe sleep/wake fragmentation, reduced energy expenditure and higher body mass index.18,19,70,96,113 As mentioned before, narcolepsy is characterized by inability to consolidate sleep or wake into long bouts, resulting in behavioral instability.114,115 It was suggested that sleep fragmentation at rest phase results in intrusion of sleep during active phase, and hence absence of consolidated wake episodes. This leads to reduced locomotor activity and energy expenditure resulting in weight gain.96 Two models of differential sensitivity to obesity based on the orexin system also show differences in their sleep quality; the OX-ATX3 mice, which develop spontaneous obesity, also show a higher number of wake--sleep transitions.19 The OR rats, which are resistant to diet-induced obesity compared with non-selectively bred Sprague Dawley rats, show better sleep quality, prolonged wakefulness and higher orexin signaling in brain regions involved in arousal. It is possible these differences directly contribute to their OR phenotype.116 This evidence suggests that positive modulation of arousal and behavioral state stabilization by the orexin system contributes to an OR phenotype.
CONCLUSION AND FUTURE PERSPECTIVES
During the last decade, different analyses of the orexin system suggest that it drives an increase in energy expenditure through maintenance and/or increase of SPA levels. The evidence suggests that the magnitude and OXR subtype contribution to the behavioral output is entirely dependent on its anatomical location. We suggest that the density of orexin innervation, differences in intracellular signaling pathways, mechanisms of neuronal depolarization and efferent projections contribute to the site-specific actions of the OXR.
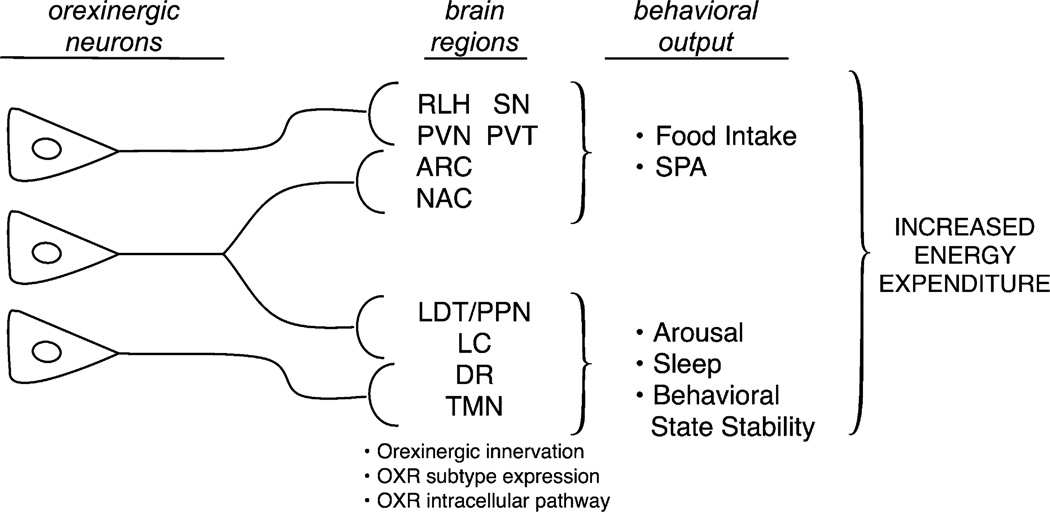
Figure 5 summarizes our current understanding of the role of the orexin system in energy metabolism. A key conclusion is that behavioral effects of the orexin system are mediated by parallel signaling through multiple brain regions, in which site-specific activation of either orexin receptor subtype results in an increase in either food intake and/or SPA. In addition, the orexin neurons also project to brain sites that increase arousal and behavioral wake stabilization. Orexin signaling throughout multiple brain sites affect changes in behavior (feeding, activity and arousal) and sympathetic nervous system activity, which together increase energy expenditure.
Figure 5.
The orexin system promotes negative energy balance through parallel signaling. This figure summarizes some of the key ideas discussed in this review. The orexin neurons projecting to either single or multiple brain regions can be (albeit somewhat arbitrarily) classified into regions involved in control of SPA, and food intake or control of sleep, arousal and behavioral stability. Within each brain site, there are at least three factors that contribute to the brain site-specific effects of the orexin neurons: (1) expression of the OXR, (2) density of orexinergic innervation and (3) intracellular pathway coupled by each OXR subtype. Our current interpretation of the evidence about the function of the orexin system suggest that it is the combined behavioral output from modulation of SPA, food intake, arousal and sleep that results in a negative energy balance. ARC, arcuate nucleus; PVN, paraventricular hypothalamic nucleus; PVT, paraventricular thalamic nucleus; NAC, nucleus accumbens; SN, substantia nigra; RLH, rostral LH; LC, locus coeruleus; LDT/PPN, laterodorsal tegmental nucleus/pedunculopontine tegmental nucleus; DR, dorsal raphe, TMN, tuberomammillary nucleus.
Brain site-specific action of OXR subtypes is a defining feature of the orexin system, and future studies of OXR in brain need to consider both regional specificity of their contribution to behavior, as well as interaction between brain sites receiving orexinergic input. We suggest that experiments with double site-specific OXA or OXB injections with and without specific OXR subtype antagonists will be useful in uncovering how parallel orexin signaling through different brain sites contributes to the increase in SPA and energy expenditure associated with activation of the orexin system.
Central to this discussion are the consequences of obesity on orexinergic signaling. A consistent point across the evidence reviewed here is that higher orexin signaling provides resistance to the development of obesity. It is possible that differences in synaptic strength, an increase in synthesis or release of orexin peptides or changes in expression of the OXR could contribute to this particular phenotype. Several studies have shown that development of obesity has a negative impact on neural plasticity across different neuronal populations.117–120 Therefore, it could be proposed that throughout development of obesity, there are changes in orexinergic signaling, perhaps reducing orexinergic signaling gain by changes in synaptic plasticity, which continue to change orexin system responsiveness and promote further development of obesity. As the function of the OXR subtypes in brain has regional specificity, this suggests that the mechanisms by which obesity affects orexinergic signaling and the associated changes will be brain region-specific.
In summary, the evidence related to OXR subtypes contribution to SPA and energy balance supports the idea of a system in which both OXR subtypes have a positive influence on SPA, but the mechanisms are dependent upon the anatomical and cellular context.
ACKNOWLEDGEMENTS
We thank Dr Joshua Nixon, Dr Jennifer Teske and Dr Vijaya Mavanji for valuable discussions and suggestions to this article. This study was supported by funding from the National Institute of Health, NIDDK, DK078985.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, et al. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci USA. 1999;96:748–753. doi: 10.1073/pnas.96.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iqbal J, Pompolo S, Sakurai T, Clarke IJ. Evidence that orexin-containing neurones provide direct input to gonadotropin-releasing hormone neurones in the ovine hypothalamus. J Neuroendocrinol. 2001;13:1033–1041. doi: 10.1046/j.1365-2826.2001.00719.x. [DOI] [PubMed] [Google Scholar]
- 3.Mintz EM, van den Pol AN, Casano AA, Albers HE. Distribution of hypocretin-(orexin) immunoreactivity in the central nervous system of Syrian hamsters (Mesocricetus auratus) J Chem Neuroanat. 2001;21:225–238. doi: 10.1016/s0891-0618(01)00111-9. [DOI] [PubMed] [Google Scholar]
- 4.Moore RY, Abrahamson EA, Van Den Pol A. The hypocretin neuron system: an arousal system in the human brain. Arch Ital Biol. 2001;139:195–205. [PubMed] [Google Scholar]
- 5.Nixon JP, Smale L. A comparative analysis of the distribution of immunoreactive orexin A and B in the brains of nocturnal and diurnal rodents. Behav Brain Funct. 2007;3:28. doi: 10.1186/1744-9081-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang JH, Sampogna S, Morales FR, Chase MH. Orexin (hypocretin)-like immunoreactivity in the cat hypothalamus: a light and electron microscopic study . Sleep. 2001;24:67–76. doi: 10.1093/sleep/24.1.67. [DOI] [PubMed] [Google Scholar]
- 8.de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 10.Ammoun S, Holmqvist T, Shariatmadari R, Oonk HB, Detheux M, Parmentier M, et al. Distinct recognition of OX1 and OX2 receptors by orexin peptides. J Pharmacol Exp Ther. 2003;305:507–514. doi: 10.1124/jpet.102.048025. [DOI] [PubMed] [Google Scholar]
- 11.Lubkin M, Stricker-Krongrad A. Independent feeding and metabolic actions of orexins in mice. Biochem Biophys Res Commun. 1998;253:241–245. doi: 10.1006/bbrc.1998.9750. [DOI] [PubMed] [Google Scholar]
- 12.Horvath TL, Diano S, van den Pol AN. Synaptic interaction between hypocretin (orexin) and neuropeptide Y cells in the rodent and primate hypothalamus: a novel circuit implicated in metabolic and endocrine regulations. J Neurosci. 1999;19:1072–1087. doi: 10.1523/JNEUROSCI.19-03-01072.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sweet DC, Levine AS, Billington CJ, Kotz CM. Feeding response to central orexins. Brain Res. 1999;821:535–538. doi: 10.1016/s0006-8993(99)01136-1. [DOI] [PubMed] [Google Scholar]
- 14.Haynes AC, Jackson B, Chapman H, Tadayyon M, Johns A, Porter RA, et al. A selective orexin-1 receptor antagonist reduces food consumption in male and female rats. Regul Pept. 2000;96:45–51. doi: 10.1016/s0167-0115(00)00199-3. [DOI] [PubMed] [Google Scholar]
- 15.Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 16.Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 17.Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, et al. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci USA. 1999;96:10911–10916. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willie JT, Chemelli RM, Sinton CM, Yanagisawa M. To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annu Rev Neurosci. 2001;24:429–458. doi: 10.1146/annurev.neuro.24.1.429. [DOI] [PubMed] [Google Scholar]
- 19.Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- 20.Yamanaka A, Tabuchi S, Tsunematsu T, Fukazawa Y, Tominaga M. Orexin directly excites orexin neurons through orexin 2 receptor. J Neurosci. 2010;30:12642–12652. doi: 10.1523/JNEUROSCI.2120-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cluderay JE, Harrison DC, Hervieu GJ. Protein distribution of the orexin-2 receptor in the rat central nervous system. Regul Pept. 2002;104:131–144. doi: 10.1016/s0167-0115(01)00357-3. [DOI] [PubMed] [Google Scholar]
- 22.Greco MA, Shiromani PJ. Hypocretin receptor protein and mRNA expression in the dorsolateral pons of rats. Brain Res Mol Brain Res. 2001;88:176–182. doi: 10.1016/s0169-328x(01)00039-0. [DOI] [PubMed] [Google Scholar]
- 23.Hervieu GJ, Cluderay JE, Harrison DC, Roberts JC, Leslie RA. Gene expression and protein distribution of the orexin-1 receptor in the rat brain and spinal cord. Neuroscience. 2001;103:777–797. doi: 10.1016/s0306-4522(01)00033-1. [DOI] [PubMed] [Google Scholar]
- 24.Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, et al. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- 25.Sunter D, Morgan I, Edwards CM, Dakin CL, Murphy KG, Gardiner J, et al. Orexins: effects on behavior and localisation of orexin receptor 2 messenger ribonucleic acid in the rat brainstem. Brain Res. 2001;907:27–34. doi: 10.1016/s0006-8993(01)02344-7. [DOI] [PubMed] [Google Scholar]
- 26.Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 1998;438:71–75. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- 27.Backberg M, Hervieu G, Wilson S, Meister B. Orexin receptor-1 (OX-R1) immunoreactivity in chemically identified neurons of the hypothalamus: focus on orexin targets involved in control of food and water intake. Eur J Neurosci. 2002;15:315–328. doi: 10.1046/j.0953-816x.2001.01859.x. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki R, Shimojima H, Funahashi H, Nakajo S, Yamada S, Guan JL, et al. Orexin-1 receptor immunoreactivity in chemically identified target neurons in the rat hypothalamus. Neurosci Lett. 2002;324:5–8. doi: 10.1016/s0304-3940(02)00140-4. [DOI] [PubMed] [Google Scholar]
- 29.Espana RA, Reis KM, Valentino RJ, Berridge CW. Organization of hypocretin/ orexin efferents to locus coeruleus and basal forebrain arousal-related structures. J Comp Neurol. 2005;481:160–178. doi: 10.1002/cne.20369. [DOI] [PubMed] [Google Scholar]
- 30.Ciriello J, McMurray JC, Babic T, de Oliveira CV. Collateral axonal projections from hypothalamic hypocretin neurons to cardiovascular sites in nucleus ambiguus and nucleus tractus solitarius. Brain Res. 2003;991:133–141. doi: 10.1016/j.brainres.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 31.Krout KE, Mettenleiter TC, Loewy AD. Single CNS neurons link both central motor and cardiosympathetic systems: a double-virus tracing study. Neuroscience. 2003;118:853–866. doi: 10.1016/s0306-4522(02)00997-1. [DOI] [PubMed] [Google Scholar]
- 32.Geerling JC, Mettenleiter TC, Loewy AD. Orexin neurons project to diverse sympathetic outflow systems. Neuroscience. 2003;122:541–550. doi: 10.1016/j.neuroscience.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 33.Oldfield BJ, Allen AM, Davern P, Giles ME, Owens NC. Lateral hypothalamic ‘command neurons’ with axonal projections to regions involved in both feeding and thermogenesis. Eur J Neurosci. 2007;25:2404–2412. doi: 10.1111/j.1460-9568.2007.05429.x. [DOI] [PubMed] [Google Scholar]
- 34.Olszewski PK, Cedernaes J, Olsson F, Levine AS, Schioth HB. Analysis of the network of feeding neuroregulators using the Allen Brain Atlas. Neurosci Biobehav Rev. 2008;32:945–956. doi: 10.1016/j.neubiorev.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berthoud HR, Morrison C. The brain, appetite, and obesity. Annu Rev Psychol. 2008;59:55–92. doi: 10.1146/annurev.psych.59.103006.093551. [DOI] [PubMed] [Google Scholar]
- 36.Ammoun S, Johansson L, Ekholm ME, Holmqvist T, Danis AS, Korhonen L, et al. OX1 orexin receptors activate extracellular signal-regulated kinase in Chinese hamster ovary cells via multiple mechanisms: the role of Ca2+ influx in OX1 receptor signaling. Mol Endocrinol. 2006;20:80–99. doi: 10.1210/me.2004-0389. [DOI] [PubMed] [Google Scholar]
- 37.Ekholm ME, Johansson L, Kukkonen JP. IP3-independent signalling of OX1 orexin/hypocretin receptors to Ca2+ influx and ERK. Biochem Biophys Res Commun. 2007;353:475–480. doi: 10.1016/j.bbrc.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 38.Johansson L, Ekholm ME, Kukkonen JP. Regulation of OX1 orexin/hypocretin receptor-coupling to phospholipase C by Ca2+ influx. Br J Pharmacol. 2007;150:97–104. doi: 10.1038/sj.bjp.0706959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johansson L, Ekholm ME, Kukkonen JP. Multiple phospholipase activation by OX(1) orexin/hypocretin receptors. Cell Mol Life Sci. 2008;65:1948–1956. doi: 10.1007/s00018-008-8206-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kukkonen JP, Akerman KE. Orexin receptors couple to Ca2+ channels different from store-operated Ca2+ channels. Neuroreport. 2001;12:2017–2020. doi: 10.1097/00001756-200107030-00046. [DOI] [PubMed] [Google Scholar]
- 41.Lund PE, Shariatmadari R, Uustare A, Detheux M, Parmentier M, Kukkonen JP, et al. The orexin OX1 receptor activates a novel Ca2+ influx pathway necessary for coupling to phospholipase C. J Biol Chem. 2000;275:30806–30812. doi: 10.1074/jbc.M002603200. [DOI] [PubMed] [Google Scholar]
- 42.Magga J, Bart G, Oker-Blom C, Kukkonen JP, Akerman KE, Nasman J. Agonist potency differentiates G protein activation and Ca2+ signalling by the orexin receptor type 1. Biochem Pharmacol. 2006;71:827–836. doi: 10.1016/j.bcp.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 43.Larsson KP, Peltonen HM, Bart G, Louhivuori LM, Penttonen A, Antikainen M, et al. Orexin-A-induced Ca2+ entry: evidence for involvement of trpc channels and protein kinase C regulation. J Biol Chem. 2005;280:1771–1781. doi: 10.1074/jbc.M406073200. [DOI] [PubMed] [Google Scholar]
- 44.Nasman J, Bart G, Larsson K, Louhivuori L, Peltonen H, Akerman KE. The orexin OX1 receptor regulates Ca2+ entry via diacylglycerol-activated channels in differentiated neuroblastoma cells. J Neurosci. 2006;26:10658–10666. doi: 10.1523/JNEUROSCI.2609-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peltonen HM, Magga JM, Bart G, Turunen PM, Antikainen MS, Kukkonen JP, et al. Involvement of TRPC3 channels in calcium oscillations mediated by OX orexin receptors. Biochem Biophys Res Commun. 2009;385:408–412. doi: 10.1016/j.bbrc.2009.05.077. [DOI] [PubMed] [Google Scholar]
- 46.Tang J, Chen J, Ramanjaneya M, Punn A, Conner AC, Randeva HS. The signalling profile of recombinant human orexin-2 receptor. Cell Signal. 2008;20:1651–1661. doi: 10.1016/j.cellsig.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 47.Holmqvist T, Johansson L, Ostman M, Ammoun S, Akerman KE, Kukkonen JP. OX1 orexin receptors couple to adenylyl cyclase regulation via multiple mechanisms. J Biol Chem. 2005;280:6570–6579. doi: 10.1074/jbc.M407397200. [DOI] [PubMed] [Google Scholar]
- 48.Karteris E, Machado RJ, Chen J, Zervou S, Hillhouse EW, Randeva HS. Food deprivation differentially modulates orexin receptor expression and signaling in rat hypothalamus and adrenal cortex. Am J Physiol Endocrinol Metab. 2005;288:E1089–E1100. doi: 10.1152/ajpendo.00351.2004. [DOI] [PubMed] [Google Scholar]
- 49.Hoang QV, Bajic D, Yanagisawa M, Nakajima S, Nakajima Y. Effects of orexin (hypocretin) on GIRK channels. J Neurophysiol. 2003;90:693–702. doi: 10.1152/jn.00001.2003. [DOI] [PubMed] [Google Scholar]
- 50.Hoang QV, Zhao P, Nakajima S, Nakajima Y. Orexin (hypocretin) effects on constitutively active inward rectifier K+ channels in cultured nucleus basalis neurons. J Neurophysiol. 2004;92:3183–3191. doi: 10.1152/jn.01222.2003. [DOI] [PubMed] [Google Scholar]
- 51.Mukai K, Kim J, Nakajima K, Oomura Y, Wayner MJ, Sasaki K. Electrophysiological effects of orexin/hypocretin on nucleus accumbens shell neurons in rats: an in vitro study. Peptides. 2009;30:1487–1496. doi: 10.1016/j.peptides.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 52.van den Pol AN, Ghosh PK, Liu RJ, Li Y, Aghajanian GK, Gao XB. Hypocretin (orexin) enhances neuron activity and cell synchrony in developing mouse GFP-expressing locus coeruleus. J Physiol. 2002;541(Pt 1):169–185. doi: 10.1113/jphysiol.2002.017426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Follwell MJ, Ferguson AV. Cellular mechanisms of orexin actions on paraventricular nucleus neurones in rat hypothalamus. J Physiol. 2002;545(Pt 3):855–867. doi: 10.1113/jphysiol.2002.030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van den Pol AN, Gao XB, Obrietan K, Kilduff TS, Belousov AB. Presynaptic and postsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretin/orexin. J Neurosci. 1998;18:7962–7971. doi: 10.1523/JNEUROSCI.18-19-07962.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Samson WK, Taylor MM, Follwell M, Ferguson AV. Orexin actions in hypothalamic paraventricular nucleus: physiological consequences and cellular correlates. Regul Pept. 2002;104:97–103. doi: 10.1016/s0167-0115(01)00353-6. [DOI] [PubMed] [Google Scholar]
- 56.Burlet S, Tyler CJ, Leonard CS. Direct and indirect excitation of laterodorsal tegmental neurons by Hypocretin/Orexin peptides: implications for wakefulness and narcolepsy. J Neurosci. 2002;22:2862–2872. doi: 10.1523/JNEUROSCI.22-07-02862.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bayer L, Eggermann E, Saint-Mleux B, Machard D, Jones BE, Muhlethaler M, et al. Selective action of orexin (hypocretin) on nonspecific thalamocortical projection neurons. J Neurosci. 2002;22:7835–7839. doi: 10.1523/JNEUROSCI.22-18-07835.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bisetti A, Cvetkovic V, Serafin M, Bayer L, Machard D, Jones BE, et al. Excitatory action of hypocretin/orexin on neurons of the central medial amygdala. Neuroscience. 2006;142:999–1004. doi: 10.1016/j.neuroscience.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 59.Liu RJ, van den Pol AN, Aghajanian GK. Hypocretins (orexins) regulate serotonin neurons in the dorsal raphe nucleus by excitatory direct and inhibitory indirect actions. J Neurosci. 2002;22:9453–9464. doi: 10.1523/JNEUROSCI.22-21-09453.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang B, Ferguson AV. Orexin-A depolarizes nucleus tractus solitarius neurons through effects on nonselective cationic and K+ conductances. J Neurophysiol. 2003;89:2167–2175. doi: 10.1152/jn.01088.2002. [DOI] [PubMed] [Google Scholar]
- 61.Burdakov D, Liss B, Ashcroft FM. Orexin excites GABAergic neurons of the arcuate nucleus by activating the sodium--calcium exchanger. J Neurosci. 2003;23:4951–4957. doi: 10.1523/JNEUROSCI.23-12-04951.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Uramura K, Funahashi H, Muroya S, Shioda S, Takigawa M, Yada T. Orexin-a activates phospholipase C- and protein kinase C-mediated Ca2+ signaling in dopamine neurons of the ventral tegmental area. Neuroreport. 2001;12:1885–1889. doi: 10.1097/00001756-200107030-00024. [DOI] [PubMed] [Google Scholar]
- 63.Kohlmeier KA, Inoue T, Leonard CS. Hypocretin/orexin peptide signaling in the ascending arousal system: elevation of intracellular calcium in the mouse dorsal raphe and laterodorsal tegmentum. J Neurophysiol. 2004;92:221–235. doi: 10.1152/jn.00076.2004. [DOI] [PubMed] [Google Scholar]
- 64.Jones DN, Gartlon J, Parker F, Taylor SG, Routledge C, Hemmati P, et al. Effects of centrally administered orexin-B and orexin-A: a role for orexin-1 receptors in orexin-B-induced hyperactivity. Psychopharmacology (Berl) 2001;153:210–218. doi: 10.1007/s002130000551. [DOI] [PubMed] [Google Scholar]
- 65.Akiyama M, Yuasa T, Hayasaka N, Horikawa K, Sakurai T, Shibata S. Reduced food anticipatory activity in genetically orexin (hypocretin) neuron-ablated mice. Eur J Neurosci. 2004;20:3054–3062. doi: 10.1111/j.1460-9568.2004.03749.x. [DOI] [PubMed] [Google Scholar]
- 66.Novak CM, Levine JA. Daily intraparaventricular orexin-A treatment induces weight loss in rats. Obesity (Silver Spring) 2009;17:1493–1498. doi: 10.1038/oby.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamanaka A, Sakurai T, Katsumoto T, Yanagisawa M, Goto K. Chronic intracerebroventricular administration of orexin-A to rats increases food intake in daytime, but has no effect on body weight. Brain Res. 1999;849:248–252. doi: 10.1016/s0006-8993(99)01905-8. [DOI] [PubMed] [Google Scholar]
- 68.Mieda M, Willie JT, Hara J, Sinton CM, Sakurai T, Yanagisawa M. Orexin peptides prevent cataplexy and improve wakefulness in an orexin neuron-ablated model of narcolepsy in mice. Proc Natl Acad Sci USA. 2004;101:4649–4654. doi: 10.1073/pnas.0400590101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Funato H, Tsai AL, Willie JT, Kisanuki Y, Williams SC, Sakurai T, et al. Enhanced orexin receptor-2 signaling prevents diet-induced obesity and improves leptin sensitivity. Cell Metab. 2009;9:64–76. doi: 10.1016/j.cmet.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hara J, Yanagisawa M, Sakurai T. Difference in obesity phenotype between orexin-knockout mice and orexin neuron-deficient mice with same genetic background and environmental conditions. Neurosci Lett. 2005;380:239–242. doi: 10.1016/j.neulet.2005.01.046. [DOI] [PubMed] [Google Scholar]
- 71.Fujiki N, Yoshida Y, Zhang S, Sakurai T, Yanagisawa M, Nishino S. Sex difference in body weight gain and leptin signaling in hypocretin/orexin deficient mouse models. Peptides. 2006;27:2326–2331. doi: 10.1016/j.peptides.2006.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tupone D, Madden CJ, Cano G, Morrison SF. An orexinergic projection from perifornical hypothalamus to raphe pallidus increases rat brown adipose tissue thermogenesis. J Neurosci. 2011;31:15944–15955. doi: 10.1523/JNEUROSCI.3909-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cerri M, Morrison SF. Activation of lateral hypothalamic neurons stimulates brown adipose tissue thermogenesis. Neuroscience. 2005;135:627–638. doi: 10.1016/j.neuroscience.2005.06.039. [DOI] [PubMed] [Google Scholar]
- 74.Espana RA, Baldo BA, Kelley AE, Berridge CW. Wake-promoting and sleep-suppressing actions of hypocretin (orexin): basal forebrain sites of action. Neuroscience. 2001;106:699–715. doi: 10.1016/s0306-4522(01)00319-0. [DOI] [PubMed] [Google Scholar]
- 75.Ida T, Nakahara K, Katayama T, Murakami N, Nakazato M. Effect of lateral cerebroventricular injection of the appetite-stimulating neuropeptide, orexin and neuropeptide Y, on the various behavioral activities of rats. Brain Res. 1999;821:526–529. doi: 10.1016/s0006-8993(99)01131-2. [DOI] [PubMed] [Google Scholar]
- 76.Matsuzaki I, Sakurai T, Kunii K, Nakamura T, Yanagisawa M, Goto K. Involvement of the serotonergic system in orexin-induced behavioral alterations in rats. Regul Pept. 2002;104:119–123. doi: 10.1016/s0167-0115(01)00355-x. [DOI] [PubMed] [Google Scholar]
- 77.Kotz CM, Teske JA, Levine JA, Wang C. Feeding and activity induced by orexin A in the lateral hypothalamus in rats. Regul Pept. 2002;104:27–32. doi: 10.1016/s0167-0115(01)00346-9. [DOI] [PubMed] [Google Scholar]
- 78.Dube MG, Kalra SP, Kalra PS. Food intake elicited by central administration of orexins/hypocretins: identification of hypothalamic sites of action. Brain Res. 1999;842:473–477. doi: 10.1016/s0006-8993(99)01824-7. [DOI] [PubMed] [Google Scholar]
- 79.Thorpe AJ, Teske JA, Kotz CM. Orexin A-induced feeding is augmented by caloric challenge. Am J Physiol Regul Integr Comp Physiol. 2005;289:R367–R372. doi: 10.1152/ajpregu.00737.2004. [DOI] [PubMed] [Google Scholar]
- 80.Kotz CM, Teske JA, Billington CJ. Neuroregulation of nonexercise activity thermogenesis and obesity resistance. Am J Physiol Regul Integr Comp Physiol. 2008;294:R699–R710. doi: 10.1152/ajpregu.00095.2007. [DOI] [PubMed] [Google Scholar]
- 81.Li Y, Gao XB, Sakurai T, van den Pol AN. Hypocretin/Orexin excites hypocretin neurons via a local glutamate neuron-A potential mechanism for orchestrating the hypothalamic arousal system. Neuron. 2002;36:1169–1181. doi: 10.1016/s0896-6273(02)01132-7. [DOI] [PubMed] [Google Scholar]
- 82.Thorpe AJ, Mullett MA, Wang C, Kotz CM. Peptides that regulate food intake: regional, metabolic, and circadian specificity of lateral hypothalamic orexin A feeding stimulation. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1409–R1417. doi: 10.1152/ajpregu.00344.2002. [DOI] [PubMed] [Google Scholar]
- 83.Kiwaki K, Kotz CM, Wang C, Lanningham-Foster L, Levine JA. Orexin A (hypocretin 1) injected into hypothalamic paraventricular nucleus and spontaneous physical activity in rats. Am J Physiol Endocrinol Metab. 2004;286:E551–E559. doi: 10.1152/ajpendo.00126.2003. [DOI] [PubMed] [Google Scholar]
- 84.Thorpe AJ, Kotz CM. Orexin A in the nucleus accumbens stimulates feeding and locomotor activity. Brain Res. 2005;1050:156–162. doi: 10.1016/j.brainres.2005.05.045. [DOI] [PubMed] [Google Scholar]
- 85.Levin BE, Dunn-Meynell AA, Balkan B, Keesey RE. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am J Physiol. 1997;273(2 Pt 2):R725–R730. doi: 10.1152/ajpregu.1997.273.2.R725. [DOI] [PubMed] [Google Scholar]
- 86.Teske JA, Levine AS, Kuskowski M, Levine JA, Kotz CM. Elevated hypothalamic orexin signaling, sensitivity to orexin A, and spontaneous physical activity in obesity-resistant rats. Am J Physiol Regul Integr Comp Physiol. 2006;291:R889–R899. doi: 10.1152/ajpregu.00536.2005. [DOI] [PubMed] [Google Scholar]
- 87.Teske JA, Billington CJ, Kuskowski MA, Kotz CM. Spontaneous physical activity protects against fat mass gain. Int J Obes. 2011 doi: 10.1038/ijo.2011.108. e-pub ahead of print 24 May 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Akbari E, Motamedi F, Davoodi FG, Noorbakhshnia M, Ghanbarian E. Orexin-1 receptor mediates long-term potentiation in the dentate gyrus area of freely moving rats. Behav Brain Res. 2010;216:375–380. doi: 10.1016/j.bbr.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 89.Walling SG, Nutt DJ, Lalies MD, Harley CW. Orexin-A infusion in the locus ceruleus triggers norepinephrine (NE) release and NE-induced long-term potentiation in the dentate gyrus. J Neurosci. 2004;24:7421–7426. doi: 10.1523/JNEUROSCI.1587-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wayner MJ, Armstrong DL, Phelix CF, Oomura Y. Orexin-A (Hypocretin-1) and leptin enhance LTP in the dentate gyrus of rats in vivo . Peptides. 2004;25:991–996. doi: 10.1016/j.peptides.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 91.Hanlon EC, Van Cauter E. Quantification of sleep behavior and of its impact on the cross-talk between the brain and peripheral metabolism. Proc Natl Acad Sci USA. 2011;108(Suppl 3):15609–15016. doi: 10.1073/pnas.1101338108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Van Cauter E, Knutson KL. Sleep and the epidemic of obesity in children and adults. Eur J Endocrinol. 2008;159(Suppl 1):S59–S66. doi: 10.1530/EJE-08-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sakurai T. Roles of orexin/hypocretin in regulation of sleep/wakefulness and energy homeostasis. Sleep Med Rev. 2005;9:231–241. doi: 10.1016/j.smrv.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 94.Tsujino N, Sakurai T. Orexin/Hypocretin: a neuropeptide at the interface of sleep, energy homeostasis, and reward system. Pharmacol Rev. 2009;61:162–176. doi: 10.1124/pr.109.001321. [DOI] [PubMed] [Google Scholar]
- 95.Kisanuki YY, Chemelli RM, Tokita S, Willie JT, Sinton CM, Yanagisawa M. Behavioral and polysomnographic characterization of orexin-1 receptor and orexin-2 receptor double knockout mice. Sleep. 2001;24:A22–A22. [Google Scholar]
- 96.Zhang S, Zeitzer JM, Sakurai T, Nishino S, Mignot E. Sleep/wake fragmentation disrupts metabolism in a mouse model of narcolepsy. J Physiol. 2007;581(Pt 2):649–663. doi: 10.1113/jphysiol.2007.129510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, et al. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21:1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kiyashchenko LI, Mileykovskiy BY, Maidment N, Lam HA, Wu MF, John J, et al. Release of hypocretin (orexin) during waking and sleep states. J Neurosci. 2002;22:5282–5286. doi: 10.1523/JNEUROSCI.22-13-05282.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–798. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25:6716–6720. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Takahashi K, Lin JS, Sakai K. Neuronal activity of orexin and non-orexin waking-active neurons during wake-sleep states in the mouse. Neuroscience. 2008;153:860–870. doi: 10.1016/j.neuroscience.2008.02.058. [DOI] [PubMed] [Google Scholar]
- 102.Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bourgin P, Huitron-Resendiz S, Spier AD, Fabre V, Morte B, Criado JR, et al. Hypocretin-1 modulates rapid eye movement sleep through activation of locus coeruleus neurons. J Neurosci. 2000;20:7760–7765. doi: 10.1523/JNEUROSCI.20-20-07760.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Huang ZL, Qu WM, Li WD, Mochizuki T, Eguchi N, Watanabe T, et al. Arousal effect of orexin A depends on activation of the histaminergic system. Proc Natl Acad Sci USA. 2001;98:9965–9970. doi: 10.1073/pnas.181330998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Methippara MM, Alam MN, Szymusiak R, McGinty D. Effects of lateral preoptic area application of orexin-A on sleep-wakefulness. Neuroreport. 2000;11:3423–3426. doi: 10.1097/00001756-200011090-00004. [DOI] [PubMed] [Google Scholar]
- 106.Xi MC, Morales FR, Chase MH. Effects on sleep and wakefulness of the injection of hypocretin-1 (orexin-A) into the laterodorsal tegmental nucleus of the cat. Brain Res. 2001;901:259–264. doi: 10.1016/s0006-8993(01)02317-4. [DOI] [PubMed] [Google Scholar]
- 107.Eriksson KS, Sergeeva O, Brown RE, Haas HL. Orexin/hypocretin excites the histaminergic neurons of the tuberomammillary nucleus. J Neurosci. 2001;21:9273–9279. doi: 10.1523/JNEUROSCI.21-23-09273.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brown RE, Sergeeva O, Eriksson KS, Haas HL. Orexin A excites serotonergic neurons in the dorsal raphe nucleus of the rat. Neuropharmacology. 2001;40:457–459. doi: 10.1016/s0028-3908(00)00178-7. [DOI] [PubMed] [Google Scholar]
- 109.Bayer L, Eggermann E, Serafin M, Saint-Mleux B, Machard D, Jones B, et al. Orexins (hypocretins) directly excite tuberomammillary neurons. Eur J Neurosci. 2001;14:1571–1575. doi: 10.1046/j.0953-816x.2001.01777.x. [DOI] [PubMed] [Google Scholar]
- 110.Eggermann E, Serafin M, Bayer L, Machard D, Saint-Mleux B, Jones BE, et al. Orexins/hypocretins excite basal forebrain cholinergic neurones. Neuroscience. 2001;108:177–181. doi: 10.1016/s0306-4522(01)00512-7. [DOI] [PubMed] [Google Scholar]
- 111.Beccuti G, Pannain S. Sleep and obesity. Curr Opin Clin Nutr Metab Care. 2011;14:402–412. doi: 10.1097/MCO.0b013e3283479109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol. 2009;5:253–261. doi: 10.1038/nrendo.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nishino S, Ripley B, Overeem S, Nevsimalova S, Lammers GJ, Vankova J, et al. Low cerebrospinal fluid hypocretin (Orexin) and altered energy homeostasis in human narcolepsy. Ann Neurol. 2001;50:381–388. doi: 10.1002/ana.1130. [DOI] [PubMed] [Google Scholar]
- 114.Nishino S, Mignot E. Article reviewed: plasma orexin-A is lower in patients with narcolepsy. Sleep Med. 2002;3:377–378. doi: 10.1016/s1389-9457(02)00078-3. [DOI] [PubMed] [Google Scholar]
- 115.Willie JT, Chemelli RM, Sinston CM, Tokita H, Williams SC, Kisanuki YY, et al. Distinct narcolepsy syndromes in Orexin receptor-2 and Orexin null mice: Molecular genetic dissection of non-REM and REM sleep regulatory processes. Neuron. 2003;38:715–730. doi: 10.1016/s0896-6273(03)00330-1. [DOI] [PubMed] [Google Scholar]
- 116.Mavanji V, Teske JA, Billington CJ, Kotz CM. Elevated sleep quality and orexin receptor mRNA in obesity-resistant rats. Int J Obes. 2010;34:1576–1588. doi: 10.1038/ijo.2010.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Horvath TL. The hardship of obesity: a soft-wired hypothalamus. Nat Neurosci. 2005;8:561–565. doi: 10.1038/nn1453. [DOI] [PubMed] [Google Scholar]
- 118.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 119.Stranahan AM, Mattson MP. Impact of energy intake and expenditure on neuronal plasticity. Neuromol Med. 2008;10:209–218. doi: 10.1007/s12017-008-8043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Noble EE, Billington CJ, Kotz CM, Wang C. The lighter side of BDNF. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1053–R1069. doi: 10.1152/ajpregu.00776.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]