Abstract
Metals are essential cellular components selected by nature to function in several indispensable biochemical processes for living organisms. Metals are endowed with unique characteristics that include redox activity, variable coordination modes, and reactivity towards organic substrates. Due to their reactivity, metals are tightly regulated under normal conditions and aberrant metal ion concentrations are associated with various pathological disorders, including cancer. For these reasons, coordination complexes, either as drugs or prodrugs, become very attractive probes as potential anticancer agents. The use of metals and their salts for medicinal purposes, from iatrochemistry to modern day, has been present throughout human history. The discovery of cisplatin, cis-[PtII(NH3)2Cl2], was a defining moment which triggered the interest in platinum(II)- and other metal-containing complexes as potential novel anticancer drugs. Other interests in this field address concerns for uptake, toxicity, and resistance to metallodrugs. This review article highlights selected metals that have gained considerable interest in both the development and the treatment of cancer. For example, copper is enriched in various human cancer tissues and is a co-factor essential for tumor angiogenesis processes. However the use of copper-binding ligands to target tumor copper could provide a novel strategy for cancer selective treatment. The use of nonessential metals as probes to target molecular pathways as anticancer agents is also emphasized. Finally, based on the interface between molecular biology and bioinorganic chemistry the design of coordination complexes for cancer treatment is reviewed and design strategies and mechanisms of action are discussed.
Keywords: Cancer, metal, zinc, copper, platinum, metal complex, DNA, proteasome
1. INTRODUCTION
“Everything is poisonous, and nothing is harmless. The dose (amount) alone defines whether something isn't poison”i Paracelsus, 1493-1541.
The current toolbox of active anticancer agents is broad in scope, and targets multiple cellular and biological properties across several tumor types. Over the last fifty years, the development of anticancer drugs moved away from conventional cytotoxicity and towards the rational design of selective agents that act on specific cellular targets [1, 2]. However, significant challenges remain, and the interface between structural biology and chemistry may provide the most productive means for discovering and improving upon novel anticancer agents [3].
In nature, many biological systems make extensive use of metal ions, such as zinc and copper, which play critical roles in the normal functioning of organisms [4]. Transition metals such as copper, iron, and manganese, among others, are involved in multiple biological processes, from electron transfer to catalysis to structural roles, and are frequently associated with active sites of proteins and enzymes [4]. However, dysregulation of some of these essential metals during normal biochemical processing has been implicated in the development of various pathological disorders, such as cancer [5]. These cellular functions only require the “trace metals” in miniscule but tightly regulated amounts. By comparison, other metals such as arsenic, cadmium, chromium, and nickel are less beneficial since they produce a wide range of toxic side-effects, including carcinogenesis [4, 6].
Many metal-containing compounds have been utilized throughout history to treat a wide variety of disorders [7]. In medicinal chemistry —traditionally dominated by organic chemistry— metal complexes have gained favor as diagnostic tools and anticancer agents [8]. Research in anticancer agents was stimulated by the accidental discovery of cisplatin, cis-[PtII(NH3)2Cl2] (Fig. (1)). However, its clinical use is restricted due to dose-dependent toxicity and resistance coupled with a narrow spectrum of activity [9, 10]. These limitations have triggered a search for platinum-based compounds that show lower toxicity, higher selectivity, and a broader spectrum of activity [11, 12]. The complexes known as carboplatin and oxaliplatin, among others seen in Fig. 1 arose from this search. Nevertheless, in addition to numerous platinum analogs, other metal complexes containing metal ions such as zinc(II), copper(II), gold, and copper chelating agents have received considerable attention as potential anticancer agents [13-16]. Additionally, the investigation of ruthenium-containing compounds in clinical trials attests to the rich potential of utilizing non-platinum metal-based compounds in the treatment of cancer [17, 18]. Furthermore, metals that take advantage of their unique physiochemical properties have been utilized as powerful tools in cancer diagnosis [19]. This review discusses the role of selected metals in biological processes in cells as they pertain to malignancy and to highlight the medicinal applications of the metals and their complexes in the design and development of metallodrugs for the treatment of cancer. This article also places considerable emphasis on their cellular targets and mechanisms of action.
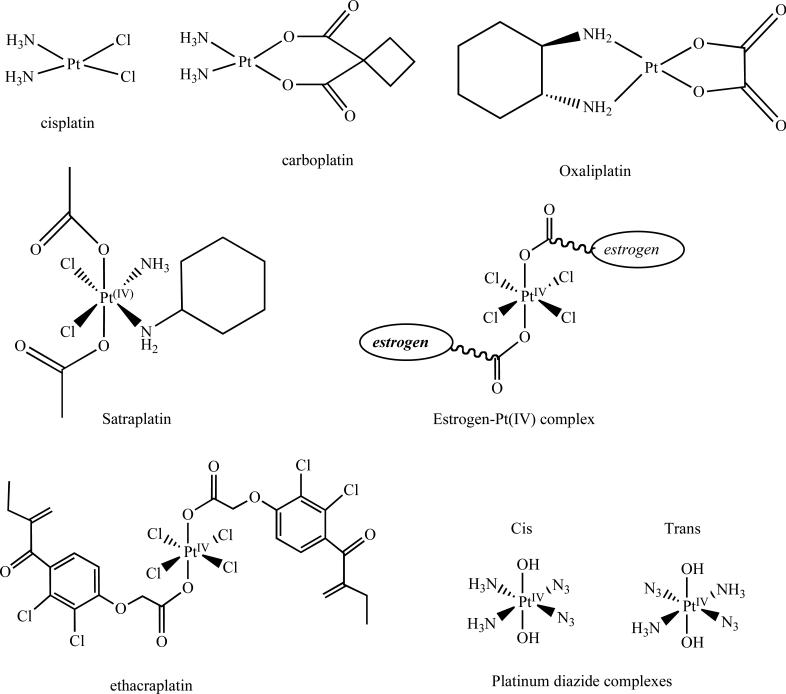
Fig. (1).
Chemical structures of platinum(II) complexes, Cisplatin, Carboplatin, and Oxaliplatin. The platinum(IV) complex Satraplatin has been used to overcome concerns of low bioavailability. Platinum(IV)-complexes have been designed with appended moieties (estrogen and the GHT inhibitor ethacraplatin) to optimize the anticancer effects of cisplatin. Platinum(IV) diazide complexes have been designed that are activated upon irradiation.
2. UNIQUE PROPERTIES OF METAL IONS AND METAL-BASED COMPLEXES
The field of medicinal inorganic chemistry encompasses, but is not limited to, the administration (or removal) of a metal ion into (or from) a biological system for either therapeutic or diagnostic purposes [20]. An important property of metals is that they form positively charged ions in aqueous solution that can bind to negatively charged biological molecules. Thus, the charge can be fine-tuned depending on the coordination environment involved, leading to the generation of a species that can be cationic, anionic, or neutral [4, 21]. Additionally, metal ions with high electron affinity can significantly polarize groups that are coordinated to them, fostering the generation of hydrolysis reactions [21].
In recent years, the field of medicinal inorganic chemistry has received considerable attention in the design of anticancer agents [22, 23]. Although metals have been used throughout human history in treating various pathological disorders, it has only been since the landmark discovery of cisplatin (Fig. (1)) in the 1960's that the full impact of metal-based compounds in the treatment of cancer has been fully realized. Because the presence of metals under cellular conditions is a tightly regulated process, appropriate administrations of metal-containing drugs must be carefully defined to achieve an optimal therapeutic response [24, 25]. Otherwise, both excess and deficiency of metals may result in undesirable toxicity.
Metal-containing compounds offer many advantages over conventional carbon-based compounds in the development of new medicinal compounds. These advantages are due to their ability to coordinate ligands in a three dimensional configuration, thus allowing functionalization of groups that can be tailored to defined molecular targets [26, 27]. Metal-based complexes offer a rich environment to build upon a variety of distinct molecular structures that confer a wide spectrum of coordination numbers and geometries, as well as kinetic properties, that cannot be realized with conventional carbon-based compounds [8, 28, 29]. The partially filled d orbitals in transition metals impart interesting electronic properties that can act as suitable probes in the design of anticancer agents [30]. The oxidation state of a metal is also an important consideration in the design of coordination compounds, given that it allows the participation in biological redox chemistry and plays an influential role in optimal dose and bioavailability of the agent administered [4, 6]. Furthermore, the ability to undergo ligand exchanged reactions offers a myriad of opportunities for metals to interact and coordinate to biological molecules, as demonstrated by the widely used drug cisplatin [26]. Furthermore, when designing metal-based therapeutics, one is not restricted solely by metals selected by nature and can take advantage of the unique properties of nonessential metals, including other 1st and 2nd row transition metals and metals that can impart additional utility not found naturally [21]. Most notable is the design of radiopharmaceuticals that exploit the radioactive properties of metals that are commonly used in the diagnosis of cancer and other medicinal applications [21].
3. PLATINUM-BASED ANALOGS IN CANCER THERAPY
The discovery of cisplatin (Fig. (1)) more than four decades ago represented a significant achievement in cancer therapy and stimulated efforts to investigate other platinum and non platinum metal-containing compounds for their potential use in the treatment of cancer [29, 31]. Cisplatin has been widely employed to treat a variety of tumors including ovarian, cervical, head and neck, non-small cell lung carcinoma, and testicular cancers, and is commonly used in combination regimens [32, 33]. However, its widespread clinical use has been hampered by increased toxicity, and the appearance of intrinsic and acquired resistance [34, 35]. In an effort to address these shortcomings, 2nd and 3rd generation platinum analogs, namely carboplatin and oxaliplatin (Fig. (1)), have been designed and clinically approved that maintain a more manageable toxicity profile [36]. Carboplatin is effective in the treatment of ovarian carcinoma, lung, and head and neck cancers [37], while oxaliplatin is clinically approved for the treatment of colorectal cancer, which is resistant to cisplatin [38].
A key factor underlying the antitumor effect of platinum-based compounds is related to its ligand exchange kinetics [39]. Although the platinum-ligand bond exhibits similar thermodynamic durability (less than 100 kJ/mol) and is much weaker than typical coordination bonds, such as C-C, C-N, or C-O single and double bonds (between 250 and 500 kJ/mol) [40], the ligand exchange behavior is rather slow. This gives them a high kinetic stability and allows much slower ligand exchange reactions on the order of minutes to days rather than seconds [39, 41]. Additionally, in the case of Pt(II) compounds, ligands oriented in the trans position are more rapidly substituted than those in the cis position [42]. These important insights are believed to play a significant role in the antitumor efficacy of platinum(II) compounds. Cisplatin (Fig. (1)) governed by a square planar complex is kinetically inert, so it does not easily expand its coordination number and undergoes ligand substitution reactions [9, 43]. Cisplatin is taken up through cells by either passive or active transport [32, 44], where its chloride ions are replaced with water molecules before reacting with DNA, forming coordinative bonds to nitrogen atoms of DNA, specifically 1,2-intrastrand d(GpG) cross links [32, 45]. The high concentration of chloride ions present blood plasma (100 mM) renders cisplatin stable toward hydrolysis. However, the significantly lower intracellular chloride concentrations (4-23 mM) facilitate rapid hydrolysis (T1/2 2 h) to the activated cationic species capable of binding DNA [46-48]. On the other hand, carboplatin (Fig. (1)) has a more favorable pharmacokinetic profile, which is primarily predicated on its slower rate of conversion to its reactive species [49]. Studies indicate that the reaction proceeds via ring opening in carboplatin and subsequent binding to DNA bases [39, 50]. Due to a more favorable toxicity profile, several-fold higher doses of carboplatin are required compared to cisplatin, and the rate of adduct formation is ten-fold slower [33, 49]. However, carboplatin appears to have a similar spectrum of activity as cisplatin based on its similar mechanism of action, and thus problems persists when used against many cisplatin-resitant tumors [26]. The Replacement of the chloride group of cisplatin by cyclobutanedicarboxylate ligand of carboplatin (Fig. (1)) confers good aqueous solubility and greater stability because it forms a six-membered ring with the platinum atom [11]. This in turn leads to diminishing side effects, while retaining a similar level of clinical activity and cross resistance to cisplatin [51].
Upon binding of platinum-based compounds, various signal transduction pathways are activated, which interfere with different cellular processes including transcription and DNA replication, thereby triggering apoptotic cell death [52]. The antitumor activity of cisplatin and carboplatin is derived from the formation of identical 1,2-intrastrand DNA cross-links [53, 54]. In contrast to cisplatin and carboplatin, the bulky diaminocyclohexane (DACH) carrier ligand of oxaliplatin (Fig. (1)) is thought to confer less cross-resistance and a more favorable toxicity profile [51]. From a molecular standpoint, mismatch repair (MMR) recognition proteins do not recognize the formation of oxaliplatin-induced DNA adducts, due to the nature of the 1,2 intrastrand cross links [33]. This has been suggested as a critical determinant in its retention of antitumor activity across cisplatin-resistant cells [55, 56]. Oxaliplatin was approved in 2002 by the FDA for the treatment of advanced colon cancer often in combination with chemotherapy [38].
Over the last several decades, investigation into platinum-based drugs has been dominated by the clinical use of cisplatin, carboplatin, and more recently, oxaliplatin [57]. However, due to persistent problems including undesirable toxicity and resistance, investigators have been pursuing innovative strategies in designing the next generation of platinum drugs [58, 59]. The underlying knowledge gained from platnum(II) compounds, including cellular processing and resistance mechanisms, coupled with a further understanding of the mechanisms of action could help translate the next generation of analogs into clinical practice.
Poor solubility and low bioavailability of clinically approved platinum compounds are two important considerations that prompted the search for novel platinum-based coordination compounds with emphasis on oral administration [60]. Whereas platinum(II) chemistry is based on ligand exchange reactions, the octahedral gemometry of platinum(IV) presents two extra ligand sites and their high kinetic inertness lowers reactivity, which can minimize off target effects [61]. The preponderance of evidence suggests that platinum(IV) complexes are reduced in vivo to platinum(II), which is the species responsible for its activation, and can be considered as a pro-drug [62, 63]. The most prominent example from this group is satraplatin, an octhahedral platinum(IV) complex that is administered orally and is currently in advanced clinical stages for treatment of hormone refractory prostate cancer (Fig. (1)) [64]. Platinum(IV) complexes display advantages owing to their greater stability and bioreductive activation, thus allowing a greater proportion of drug to reach its biological target. The two axial acetate groups play a pivotal role in increasing its lipophilic character and providing a more bioavailable agent [60, 65]. Upon metabolic activation, staraplatin becomes structurally similar to cisplatin except for the replacement of one of its amine groups with a cyclohexylamine group [65]. The anti-tumor activity of satraplatin has been shown to operate in a similar manner as cisplatin, as evidenced by formation of intra and interstrand DNA cross links [60, 66].
The reduction and intracellular modification of these complexes offer many opportunities to modify bioactive ligands as tumor targeting moieties to better facilitate the delivery of platinum to its intended site of action [67]. Based on the observation that estrogen receptor (ER)-positive breast cancer cells treated with estrogen are sensitized to cisplatin [68], Barnes et al. formulated a Pt(IV)-estrogen (Fig. (1)) compound to sensitize ER(+)-breast cancer cells and overcome cisplatin resistance [69]. The intracellular reduction of the compound led to the release of one equivalent cisplatin and two equivalents of estrodiol. Its mechanism entails upregulation of the high mobility group protein (HMGB1), a protein which is important in blocking platinum-DNA adduct repair [67, 69]. Another design strategy afforded the use of ethacrynic acid, a known inhibitor of glutathione S-transferase [70], which has been found to be overexpressed in many cisplatin-resistant tumors, also appended to a platinum(IV) center (ethacraplatin; Fig. (1)). Intracellular reduction results in the simultaneous effect of blocking GST by ethacrynic acid while exerting the cytotoxic effect of platinum(II) [70]. Another strategy pursued relies on photo-activation of platinum(IV) complexes rather than the traditional mode of intracellular reduction [71, 72]. This can provide advantages in tumor selectivity by eliciting activation of the complex at the site of the tumor, therefore potentially mitigating the collateral damage to normal tissue [48]. These platinum(IV) complexes were designed as dihydroxy in the trans position and are appended with two azide ligands, either positioned cis or trans. (Fig. (1)) [71, 72]. These prodrugs are relatively nontoxic under darkened conditions, but activation of these complexes by irradiation results in growth inhibition of human bladder cancer cells while avoiding toxicity to human skin cells. It is also notable to mention that the cis-azide complex does not exhibit cross resistance to cisplatin and different platinum-DNA adducts appear responsible for their cytoxicity [67, 71, 72].
Significant progress has been made in the field of platinum-containing anticancer agents, particularly with respect to design strategies and mechanistic understanding of their pharmacological effects. Building upon our current understanding of platinum coordination compounds, including structure-activity-relationship (SAR), tumor uptake and resistance mechanisms can help facilitate the clinical introduction of the next generation of platinum-based anticancer agents.
4. ZINC, APOPTOSIS, AND CANCER
Zinc is an indispensable trace element that plays a critical role in a wide range of cellular processes including cell proliferation, differentiation, and defense against free radicals [73, 74]. Zinc acts as a key structural component in many proteins and enzymes, including transcription factors, cellular signaling proteins, and DNA repair enzymes [75, 76].
It has also been well established that zinc plays a critical role in the regulation of apoptosis in mammalian cells [77-79]. However the precise role of zinc in modulating this response appears to be cell specific, highly complex, and lacking firm conclusion [79]. In many cell types, including prostate epithelial, glial cells, ovarian epithelial cells, and others, zinc has been reported to induce apoptosis [79]. However, in breast cells, lung epithelial cells, renal cells, macrophages, and Hela cells, zinc exhibits antiapoptotic effects [79]. This has been supported by evidence showing that in some cells, exposure to low levels of zinc induces apoptosis, whereas exposure to high zinc levels inhibits apoptosis [80]. These seemingly contradictory results have been the subject of intense investigation and currently remain unanswered.
Given the indispensible role of Zn in a myriad of biochemical processes, it is not surprising that altered levels of Zn are associated with systemic abnormalities, including the development of cancer [81]. Although the levels of zinc have been found to be compromised in cancer patients as compared to normal subjects, the relationship between tumor development and Zn levels appears to lack discernable conclusions and is dependent on tumor type [79, 82, 83]. Zinc levels have been found to be reduced in patients afflicted with cancer of the liver, gallbladder, digestive tract, or prostate [84-86], whereas breast cancer patients showed decreased and elevated Zn levels in serum and malignant tissues [84, 87, 88]. Emerging evidence suggests that expression levels of Zn transporters are associated with cancer progression [81, 89]. Compelling evidence has been provided that links the zinc uptake transporter ZIP4 with the development of pancreatic cancer [79, 90]. Both in vitro and in vivo evidence points to an increase in ZIP4 expression is associated with increased zinc uptake, increased cell proliferation, and increased tumor growth [90]. Additionally, ZIP1 has been reported to act as a tumor suppressor of prostate cancer [91], while ZIP6 has been implicated in breast cancer progression and metastasis [92, 93]. Studies have also shown positive results in establishing a molecular link between STAT3, ZIP6 and Snail during EMT (Epithelial Mesenchymal Transition), which may be a contributing factor in tumor cell invasion and metastasis [94]. It was recently shown that the zinc transporter ZIP10 is implicated in the migration and metastasis of breast cancer cells, and consequently this invasive behavior could be inhibited by knock down of ZIP10 expression [81]. These studies were correlated with findings from clinical samples showing that breast cancers with lymph node metastases expressed significantly higher levels of ZIP10 than those without lymph node metastases [81, 95]. Thus, altered Zn transporter expression may play a key role in the development of cancer by disrupting normal intracellular distribution and function. In addition to the critical role that zinc plays in biological systems, the unique properties of zinc have allowed it to gain favor as potential anticancer agents (discussed in sections 5.1 and 7.3).
5. THE UBIQUITIN-PROTEASOME PATHWAY AS A TARGET FOR METAL-BASED COMPOUNDS IN CANCER THERAPY
Protein homeostasis is critical to biological processes that are fundamental to cancer cell survival. Therefore, targeting the key features responsible for protein production and destruction, particularly factors responsible for the growth and proliferation of cancer cells, has been the focus of intense investigation [96]. Empirical findings indicate that many types of actively proliferating tumor cells are more sensitive to proteasome inhibitors than non cancerous cells, and that blockade of tumor proteasome activity results in cancer cell apoptosis [97]. Thus, targeting the ubiquitinproteasome pathway has emerged as a favorable strategy in the treatment of human cancers.
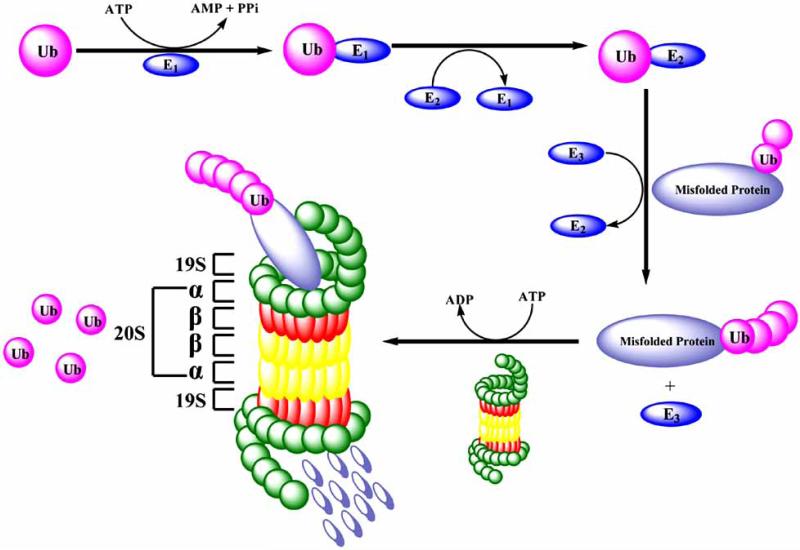
The ubiquitin-proteasome pathway (Fig. (2)) plays a crucial role in maintaining cellular homeostatic function by selectively degrading proteins involved in critical cellular functions. These include selective degradation of oxidatively damaged, mutated, or misfolded proteins, as well as those involved in cell proliferation, cell cycle progression, and apoptosis [98]. Proteins destined for degradation are first tagged with a chain of ubiquitin molecules by a multi-enzymatic system consisting of Ub-activating (E1), Ub-conjugating (E2), and Ub-ligating (E3) enzymes (Fig. (2)) [99]. The ubiquitin-tagged protein is then translocated to the 26S proteasome where it undergoes protein degradation, and the ubiquitin molecules are subsequently recycled [100, 101]. The 20S proteasome constitutes the proteolytic core of the 26S proteasome complex and mediates at least three distinct enzymatic activities, which function as a catalytic machine [102]. These activities include the chymotrypsin-like, trypsin-like, and peptidylglutamyl peptide hydrolyzing-like (PGPH) activities [103].
Fig. (2).
The ubiquitin-proteasome pathway plays a crucial role in maintaining cellular homeostatic function by selectively degrading various proteins including those involved in critical cellular functions. Proteins destined for degradation are first tagged with a chain of ubiquitin molecules by a multi-enzymatic system consisting of Ub-activating (E1), Ub-conjugating (E2), and Ub-ligating (E3) enzymes. The ubiquitin tagged protein is then translocated to the 26S proteasome where it undergoes degradation and the ubiquitin molecules are subsequently recycled. The 20S proteasome constitutes the proteolytic core and consists of a series of outer α-subunits and inner β-subunits mediated by atleast three distinct enzymatic activities. These include the chymotrypsin-like, trypsin-like, and peptidylglutamyl peptide hydrolyzing-like/PGPH.
The possibility of targeting the ubiquitin-proteasome pathway therapeutically was initially met with great skepticism in the scientific community owing to its importance in normal cellular development [104]. However, the demonstration that proteasome inhibitors were well tolerated and were found to be active in several cancer models in vivo, the proteasome inhibitor bortezomib (Velcade, PS-341) entered into clinical trials [105]. Based on clinical trial data that showed an acceptable therapeutic index with significant clinical benefit, bortezomib was ultimately approved by the FDA for the treatment of myeloma [106, 107]. Bortezomib has been studied extensively as a single agent and in combination with glucocorticoids, cytotoxic agents, immunomodulatory drugs and radiation as treatment for multiple myeloma and other hematological malignancies [108]. The results in some cases have been impressive. However, there is less evidence of botezomib's efficacy versus solid tumors. Overall, these studies provided proof-of-principle that targeting the proteasome is a valuable target in cancer therapy.
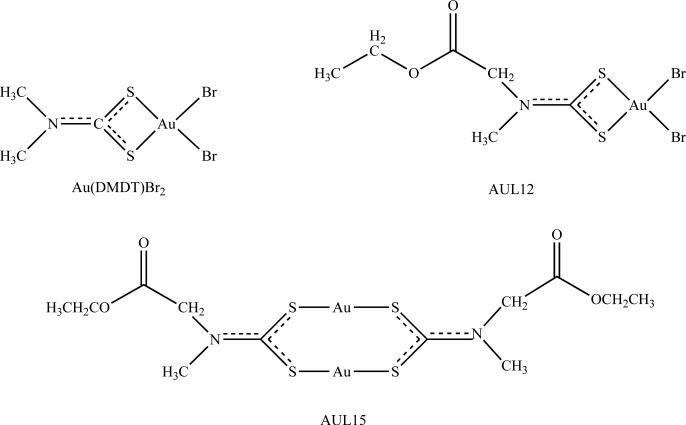
In order to achieve a higher cytotoxicity profile with a wider spectrum of activity compared to that of platinum-based compounds, the anticancer activity of gold coordination compounds has been investigated [109-111]. Studies have shown that interactions of gold(III) complexes with DNA, the favorable target of platinum, failed to pose a favorable binding mode, precipitating the investigation into gold-protein interactions [112, 113]. Because gold(III) is isoelectronic to platinum(II), and tetracoordinate gold(III) complexes are in the same square-planar geometries as cisplatin, this prompted the investigation of gold compounds as potential anti-cancer agents [111]. Initial studies focused on a series of synthetic gold(III) diothiocarbamate derivatives that were shown to be 1-to-4-fold more cytotoxic than cisplatin and were able to significantly overcome both intrinsic and acquired resistance [110]. However, these initial studies failed to establish a molecular link between these gold compounds and their anticancer activity. Studies from our laboratory highlight the proteasome as a molecular target for gold(III) dithiocarbamate derivatives, one such is referred as Au(DMDT)Br2 (Fig. (3)). We showed for the first time that this gold(III) dithiocarbamate analog inhibits the chymotrypsin-like activity of a purified rabbit 20S proteasome (IC50 = 7.4 μmol/L) and the 26S proteasome in highly metastatic MDA-MB-231 breast cancer cells [16]. Inhibition of proteasomal activity results in accumulation of proteasome target protein p27 and induction of apoptosis [16]. Interestingly, treatment of nude mice bearing breast cancer xenografts with this gold(III) compound caused inhibition of tumor growth, associated with proteasome inhibition and apoptosis induction [16].
Fig. (3).
Chemical structures of synthetic gold complexes as proteasome inhibitors: Au(DMDT)Br2, AUL12, and AUL15. Both Au(DMDT)Br2 and AUL12 have a trivalent oxidation state, whereas AUL15 is monovalent.
Recently our lab investigated the mechanism of two novel gold dithiocarbamate derivatives that differ in the oxidation state of the metal, namely (AUL12) with a trivalent oxidation state, and (AUL15) with a monovalent oxidation state (Fig. (3)). We found that both gold dithiocarbamate species were able to inhibit the chymotrypsin-like activity of purified 20S proteasome and 26S proteasome in human breast cancer cells, resulting in accumulation of ubiquitinated proteins, proteasome target proteins, and induction of cell death, but at significantly different levels [114]. Additionally, our studies found that inhibition of proteasome activity by gold(I) and gold(III) compounds were completely reversed by the addition of a reducing agent, N-acetyl-L-cysteine, suggesting the involvement of redox activity [114]. Interestingly, treatment of breast cancer cells with a gold(III) (AUL12), but not gold(I) (AUL15) derivative resulted in the significant production of reactive oxygen species [114]. Our study highlights the proteasome as an important molecular target of gold compounds, but shows distinct mechanisms of action responsible for their underlying biological activities, which depend on the oxidation state of the metal. A further in-depth analysis on the therapeutic potential of gold complexes as anticancer drugs has recently been published [104].
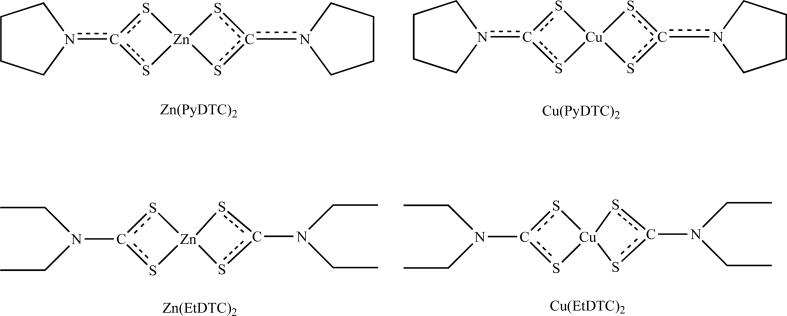
Our lab has also investigated the effects of well defined complexes of pyrrolidine diothiocarbamate complexes containing zinc, Zn(PyDTC)2, and copper, Cu(PyDTC)2, as potential proteasome inhibitors (Fig. (4)) [15]. Interestingly, these complexes were found to inhibit the chymotrypsin-like activity of cellular 26S, with much less inhibition of the 20S proteasome [15]. Based on these findings, we have proposed that inhibition of the JAMM domain in the 19S proteasome as a putative target for these metal compounds [15]. Since zinc is required for the catalytic activity of human deubiquitinating isopeptidase located on 19S, it is possible that this enzyme can be targeted by these complexes [115-117]. Further evidence to support this hypothesis comes from our findings comparing two synthetic diethyldithiocarbamate complexes containing copper and zinc [118]. We demonstrated that both Cu(EtDTC)2 and Zn(EtDTC)2 showed a much higher potency to inhibiting the 26S proteasome in intact breast cancer cells compared to their effects toward a purified 20S proteasome (Fig. (4)) [118]. While significant progress has been made in investigating the effects of metal complexes toward the ubiquitin-proteasome system, more detailed studies are necessary, especially to validate direct binding of the metal compounds to the JAMM domain of 19S proteasome.
Fig. (4).
Chemical structures of synthesized Zinc and Copper-containing complexes as proteasome inhibitors from the dithiocarbamate family, (PyDTC) and (EtDTC).
6. COPPER CHELATING LIGANDS ACTING AS A TUMOR PROTEASOME TARGETING STRATEGY
Copper is another essential trace metal that has been selected by nature to be a driving force in many biochemical processes including chemical redox reactions, cellular growth, development, and angiogenesis [7, 119]. Under biological conditions, copper exists in both (Cu+) and (Cu2+), which allows it to serve as a cofactor in redox reactions, such as cytochrome c oxidase (involved in the mitochondrial electron transport chain) and superoxide dismutase (involved in the detoxification of reactive oxygen species) [120, 121]. The acquisition and distribution of copper is a tightly regulated process to assure proper uptake, distribution, and to avoid unnecessary binding to biomolecules [122, 123].
Depending on the oxidation state, the coordination chemistry of copper is often distinct: Cu+ shows a preference for sulfur donor ligands, such as cysteine or methionine, whereas Cu2+ prefers nitrogen donors such as histidine or oxygen donors such as glutamate or aspartate [121]. Copper in its reduced form (Cu+) has a filled d10 configuration with no preference for geometry based on no LFSE (ligand field stabilization energy) and thus can exist in a wide range of geometries [124, 125]. The d9 configuration of Cu2+ favors a four to six coordination arrangement due to Jahn-Teller distortions [124, 125]. Geometries include four-coordinate square-planar, five-coordinate trigonal bipyramidal and six-coordinate axially distorted octahedral arrangements [125].
6.1. Copper, Angiogenesis, and Cancer
The role of copper in the growth and progression of malignancy has been the subject of intense investigation for the last two decades. This was born out of the realization that copper levels are altered in tumor bearing mice and humans [126, 127]. Additionally, high serum and tissue levels of copper were found in various human tumors including breast [128, 129], prostate [130, 131], colon [131], lung [132], and brain [133], compared to healthy subjects. The reasons for this elevation have not been fully elucidated and no firm conclusions could be established.
It was first noticed nearly 30 years ago that copper plays a critical role in angiogenesis [134, 135]. Angiogenesis is a complex process that is essential for the growth, invasion, and metastasis of tumor cells [136]. It has been shown that tumors cannot grow larger than 1-2 mm3 without the formation of new blood vessels [137]. In vitro studies have shown that copper acts as a critical angiogenic effector by stimulating the proliferation and migration of endothelial cells [138]. Based on the findings regarding the importance of angiogenesis and copper in tumor development, the concept of antiangiogenic therapy using copper chelators in cancer therapy has gained considerable interest [139, 140]. Indeed, a number of clinical trials have been initiated and have shown promising results [141, 142]. The following section will be devoted to a series of copper-chelating agents being investigated in our lab with special emphasis toward their cellular targets as a promising anticancer strategy.
6.2. Copper Chelating Ligands
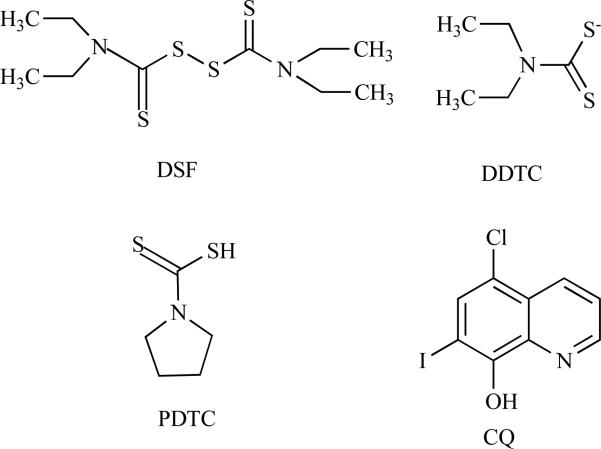
Disulfiram (tetraethylthiuram disulfide, DSF) (Fig. (5)) is a clinically employed drug used to treat patients afflicted with chronic alcoholism [143]. Upon consumption, alcohol is metabolized to acetaldehyde by alcohol dehydrogenases (ADH) and further converted to acetic acid by aldehyde dehydrogenase (ALDH) [144]. The chemical structure shows that DSF is able to react to copper with its thiol group [145]. To test this hypothesis, we mixed DSF with CuCl2 and found a dramatic color change that indicates that a chemical reaction between DSF and copper has occurred [146]. Based on findings that tumors contain higher levels of copper compared to normal tissue, our lab has undertaken an innovative strategy of targeting elevated copper levels with DSF, and hypothesized that the resultant DSF-copper complex could possess the potent tumor-specific killing activity by selectively inhibting the proteasome in tumor cells [122, 146].
Fig. (5).
Chemical structures of copper-chelating ligands DSF, DDTC PDTC, and CQ.
To verify our hypothesis that copper ions can be used as a novel, selective target for potential anticancer drugs, we examined the effect of DSF on human breast cancer cells [146]. Our studies found that in the presence of copper, DSF could inhibit proteasomal activity and induce apoptosis in cancer cells. However, in the absence of copper under the same experimental conditions, the effect of DSF was minimal [146]. We extended our studies further by testing whether DSF could react with endogenous levels of copper present in the tumor and convert the pro-angiogenic copper to an anticancer complex in vivo. We treated mouse-bearing breast cancer xenografts with DSF for 30 days and found that DSF significantly inhibited tumor growth by 74%, tightly associated with the inhibition of proteasome activity and induction of apoptosis in tumor tissues treated with DSF [146].
Along with DSF, diethyldithiocarbamate (DDTC) and pyrrolidine dithiocarbamate (PDTC) (Fig. (5)) are also members of the dithiocarbamate family that were found to act as potent metal chelators [7, 15, 122, 147, 148]. We found that DDTC was capable of binding copper and forming a new complex that potently inhibited the proteasomal chymotrypsin-like activity, induced apoptosis, decreased expression of androgen receptor (AR) and estrogen receptor α and (ERα and ERβ) proteins in both human prostate and breast cancer cells [148]. We also reported that after binding to copper, PDTC could inhibit the proteasomal chymotrypsin-like activity, suppress proliferation and induce apoptotic cell death in cultured human prostate cancer cells [147].
Along the same lines, we selected and tested clioquinol, another copper chelator, for its potential anticancer properties (Fig. (5)). Clioquinol (5-chloro-7-iodo-8-hydroxyquinoline), known as CQ, is an 8-hydroxyquinoline derivative that is clinically used for treatment of Alzheimer's disease [149] and Huntington's disease [150]. According to the chemical structure, CQ is similarly capable of forming stable complexes with copper(II) [151]. To test whether CQ can react with copper, we mixed a solution of CQ with a solution of CuCl2 at 1:1 molar ratio, and subsequently, a dramatic color change was observed [152]. To further verify whether the color change of the CQ-copper mixture indicated a formation of a stable CQ-copper complex, a series of samples was analyzed by X-ray absorption near-edge spectroscopy (XANES) and extended X-ray absorption fine structure spectroscopy (EXAFS) [152]. The results showed that the CQ-copper mixture had a different copper oxidation state than those of CuCl2 and the copper metal, confirming that a chemical reaction occurred between CQ and copper [152].
We further tested biological effects of the CQ-copper complex in human prostate cancer LNCaP (androgen-dependent) and C4-2B (androgen-independent) cells [152]. The results showed that inhibition of proteasome activity, reduction of androgen receptor (AR) expression, suppression of cell proliferation, and induction of apoptotic cell death occurred in both prostate cancer cell lines treated with CQ-copper complex, but not with CQ alone [152]. Furthermore, animal studies of mice bearing human C4-2B xenografts showed that treatment with CQ (10 mg/kg) for 30 days resulted in significant inhibition of tumor growth (66%) compared to the control [152]. The results also showed that proteasome inhibition, induction of apoptosis, suppression of AR expression and inhibition of angiogenesis occurred in the tumors tissues treated with CQ [152].
Elevated proteasome activity and high concentration of copper are unique features in human tumor tissue. Our findings have demonstrated that these unique features of tumor cells can be used as specific targets by already clinically approved drugs that, when complexed to copper, act as potent tumor cell killers.
7. TRIDENTATE [NN’O]-CONTAINING METAL COMPLEXES AS A SUITABLE PLATFORM IN THE DESIGN OF NOVEL ANTICANCER DRUG CANDIDATES
7.1. Tridentate [NN’O]-Containing Ligands with Ga3+ as Potential Tumor Proteasome Inhibitors and Anticancer Drug Candidates
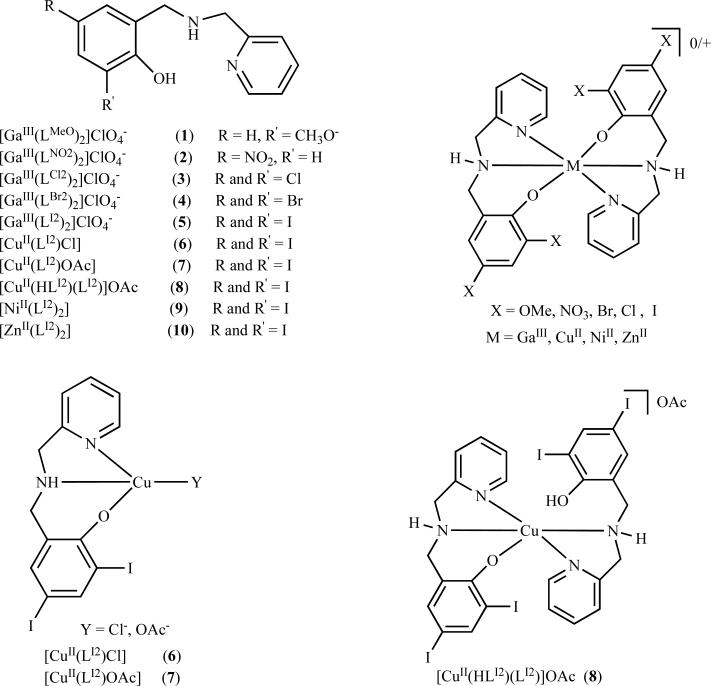
Our groups have been actively pursuing a strategy of developing novel complexes of well defined stoichiometry formed between asymmetric [NN’O] tridentate ligands-containing metals as potential anticancer drug candidates (Fig. (6)). Such ligands are an evolution from terbutylated analogues used as biomimetic models for galactose-oxidase [153, 154]. Moreover, a secondary amine in this ligand allows for the design of complexes with appended moieties to enhance water solubility [155, 156] or lipophilicity [157-159] to address concerns for drug design purposes. Our studies initially focused on the development of a series of gallium complexes described as [GaIII(Lx-NN’O)2]ClO4, with asymmetric NN’O-containing pyridine amino phenolate ligands (Fig. (6)) [160]. The phenolate moiety groups were appended with electron withdrawing and donating groups such as methoxy (1), nitro (2), chloro (3) bromo (4), and iodo (5) positioned at the 4th and 6th positions (Fig. (6)). The geometry of these complexes appears to be distorted octahedrally, but owing to the flexibility of the ligands, facial coordination takes place [160].
Fig. (6).
Asymmetric [NN’O]-containing metal complexes as proteasome inhibitors. R represents different groups at the 4th and 6th positions. M represents the metal ion, in this case, gallium(III), copper(II), nickel(II), and zinc(II) were used. Complexes were synthesized at a two ligand to one metal ratio.
The therapeutic efficacy of these complexes was first tested against cisplatin-resistant neuroblastoma cells [160]. Our studies reveal, in ranking order, methoxy (1) = nitro (2) < chloro (3) < bromo (4) < iodo (5), that the species containing halogen substituents showed preferential growth inhibition in human neuroblastoma cells with activity superior to that of cisplatin (Fig. (6)). Additionally, neuroblastoma cells treated with gallium chloride rendered cells predominantly viable, attaining similar activity as the methoxy substituted complexes [160]. Interestingly, these gallium (III) complexes were found to be relatively nontoxic at 25 μmol/L, only showing an increase in aberrant morphological changes above 50 μmol/L against normal human fibroblasts [160]. Thus, the formation of a coordination complex appended with halogen substituents appears to influence the cytotoxicity of these complexes [160].
We next set out to investigate a possible molecular mechanism for these gallium complexes that could provide insight into their growth inhibitory properties. Our studies reveal that complexes (3 Chloro) < (4 Bromo) < (5 Iodo) are able to target and inhibit proteasomal activity and induce apoptosis in various prostate cancer cell lines (Fig. (6)) [161]. The ability to inhibit proteasome activity was indicated by accumulation of ubiquitinated proteins and the proteasomal target protein p27. As observed with cisplatin-resistant neuroblastoma cells, complex (5 Iodo) achieved superior therapeutic efficacy in a human prostate cancer model. Accordingly, complex (5) exhibited potent proteasome inhibitory activity against both 26S proteasome (IC50 = 17 μmol/L) and purified 20S proteasome (IC50= 16 μmol/L) [161]. Consistently, this effect was associated with higher indices of apoptosis. It is notable to point out that treatment with the iodo-substitued ligand (HLI) alone showed only minimal levels of cytotoxicity in our cell culture system. Importantly, complex (5) was able to exert the same effect in vivo by inhibiting the growth of PC-3 bearing mice xenografts (66%), associated with proteasome inhibition and apoptosis induction [161]. The increased potency of complex (5) suggests that the iodo-substituted ligand attains certain physiochemical properties that after coordination with gallium, impart significant biological activity [161, 162]. It is possible that their biological activity is governed by the strong pi electron-donating group. Considering that all the ligands used in the coordination complexes of (3)-(5) contain electron-withdrawing appendages, it is reasonable to suggest that only their pi-donating ability could influence their anticancer properties (I > Br > Cl). Additionally, whether the coordination mode of the metal and geometries are instrumental in influencing its biological activity is rather speculative at this point and no firm conclusions could be established [161, 162].
7.2. Tridentate [NN’O]-Containing Ligands with Cu2+ as Potential Tumor Proteasome Inhibitors and Anticancer Drug Candidates
Since one of the important focuses in our labs involves investigating the use of copper chelating ligands and other transition metal-based complexes as proteasome inhibitors (discussed above) [122, 146, 152], we decided to extend our work by investigating the role of bivalent transition metals complexed to our same HLI platform to gain insight into their potential as anticancer agents.
Furthermore, we observed that in situ complexation of HLBr or HLI with Cu+2 salts leads to the generation of complexes that display superior anticancer activity when compared to equivalent gallium species (data unpublished). Based on the significantly higher potency conferred by the HLI ligand when formed in a coordination complex with gallium, subsequent studies relied on this model architecture with bivalent transition metals, such as copper and zinc, with emphasis on mechanistic properties. Spectrometric evaluation of the stoichiometric HLI:CuCl2:DMSO mixture led to the identification of both monomeric and dimeric fragments that may act as pharmacophores responsible for their biological activity [163]. These initial studies formed the basis of developing a series of copper(II) complexes with the ligand HLI, which were synthesized and characterized as (6), (7), and (8) (Fig. (6)). These complexes, with distinct stoichiometries, protonation states, and nature of the monodentate ligand, were designed considering that a metal ion coordinating to the ligand could bind to the proteasome, possibly via the N-terminal threonine or some other coordinating residue [163].
These copper complexes were first tested for their ability to impart cell death in human leukemia cells and compared to the activity of HLI and copper salt, independently [163]. Consequently, these complexes achieved a comparable IC50 (concentration at 50% cell death) value in the range of 3.8-4.0 μmol/L after 18 h treatment in human leukemia cells [163]. In comparison, treatment with either HLI or copper salt alone showed minimal proteasome inhibitory activity and cytotoxicty as high as 25 μmol/L [163]. Next, we tested whether the cellular proteasome was a molecular target for these copper complexes by using a human prostate cancer model [163]. When these complexes were tested in cultured prostate cancer cells, significant proteasome inhibition associated with higher levels of ubiquitinated proteins and massive apoptosis was apparent [163]. Since either HLI- or copper salt-treated cells failed to exhibit any considerable biological activity, we hypothesize that the formation of a coordination complex with copper is imperative for imparting biological activity [163]. However, when immortalized, nontransformed breast cells were treated with these copper complexes, they did not demonstrate loss of viability, suggesting resistance to the proteasome inhibitor effects [163]. Although a threshold level of selectivity was reached with these complexes that distinguish normal from tumor cells, further studies are warranted to provide more conclusive evidence [163]. Furthermore, our studies suggest the pharmacophore or active species as [CuLI] is needed for inhibition of proteasomal activity. Along this line it is possible that the 2:1 ligand to metal complex (8) (Fig. (6)) may act as a prodrug, and the loss of one of its ligands might occur to free up the metal for binding to coordinating residues on the proteasome. Although more detailed studies are needed, it is tempting to suggest that this pharmacophore would present an open coordination that facilitates interaction with available amino acids, potentially the N-terminal threonine, with high affinity for copper [163].
7.3. Comparative Activities of Nickel(II) and Zinc(II) Complexes of Tridentate [NN’O]-Containing Ligands as Proteasome Inhibitors
To further build upon our studies and further investigate our hypothesis that species [MLIA]+ is the necessary pharmacophore for proteasome inhibition, we compared the proteasome inhibition capabilities of two divalent transition metals as coordination complexes utilizing the same HLI ligand as a platform [164]. These complexes were synthesized and characterized as (9) and (10) (Fig. (6)) using various spectroscopic, spectrometric, and structural methods [164]. DFT calculations considering different isomers of (9) and (10) were carried out and show good agreement with the nickel complex, but fail to predict the appropriate geometry for the zinc-containing species because it lacks ligand field stabilization energy [164]. Furthermore, initial comparison studies considering coordination of a 1:1 [Zn(L)]+ fragment with threonine suggest a favorable coordination through the terminal hydroxyl group [164].
The pharmacological effects of these metal complexes along with those of the salts, NiCl2 and ZnCl2, were evaluated in vitro and in cultured prostate cancer cells in relation to their proteasome inhibiting and apoptosis-inducing capabilities [164]. We first tested the ability of these species to impart cell death in human leukemia cells. We found that cells treated with (10) exhibited superior cell death-inducing properties, with an IC50 of ~8 μmol/L. However, (9) and both of the metal salts failed to impart any level of cytotoxicity at as high as 20 μmol/L [164]. We next tested whether the proteasome is a target for these metal complexes in vitro and in cultured prostate cancer cells. Our results showed that that neither (9) nor NiCl2 have the ability to inhibit the proteasome activity at any significant levels. However, under cell-free conditions, ZnCl2 and (10) showed significant proteasome inhibiting versus the chymotrypsin-like activity of both the 26S proteasome (IC50 = 5.7 and 4.4 μmol/L, respectively) and the purified 20S proteasome (IC50 = 16.6 and 11.7 μmol/L, respectively) [164]. The observation that Zinc-containing dithiocarbamate derivatives as mentioned above showed only minimal inhibition toward 20S proteasome, suggests the nature of the dithiocarbamate ligand formed in a coordination complex with zinc may be influencing its targeting capabilities. However, no firm conclusion could be established and is purely speculative at this point. Additionally, decreased proteasome activity by (10) was associated with higher levels of ubiquitinated proteins and massive apoptosis, whereas treatment with (9) or either metal salt resulted in minimal effects. These interesting results coupled with their nontoxic effect toward nontransformed breast cells, present a compelling rationale for further investigating (10) as a potential anticancer drug candidate [164].
As observed with other metal complexes being investigated from our labs, considerable proteasome inhibition can be attained through 1:1 ligand-to-metal species, which we hypothesize to be the pharmacophore in all of these complexes under investigation. Therefore, it is tempting to suggest that an equilibrium [M(LIA)2]↔[M(LIA)2]+ + LIA- seems necessary to free up the pharmacophore with available coordination sites capable of interaction with the 20S proteasome. It is observed from the molecular structures and DFT calculations that covalent interactions prevail with (9), while the interactions in (10) (Fig. (6)) is governed by ionic bonds [164]. Taken together, these results strengthen the current working hypothesis that fast ligand exchange is necessary to generate the [Zn[LIA]+ fragment along with its gallium(III) and copper(II) congeners necessary to inhibit proteasome activity [164]. Further mechanistic understanding into the role of these metals and ligands and how their coordination and geometry influences their biological activity could lead to the development of next generation drug candidates using asymmetric [NN’O] tridentate metal complexes as selective tumor proteasome inhibitors.
8. CONCLUSION
The critical role that metals play in the functioning and maintenance of life highlights the extensive role that nature plays in regulating these vital components. The clinical success of cisplatin provided the “proof of concept” for investigating metals, essential or nonessential, and their coordination complexes as potential anticancer agents. Since the discovery of cisplatin, thousands of platinum analogs have been synthesized, with only carboplatin and oxaliplatin achieving widespread clinical use. Design strategies of novel platinum complexes have been under intense investigation to address shortcomings of previous generation platinum compounds. Targeting the ubiquitin-proteasome pathway with metal-based compounds is an emerging concept in developmental therapeutics. These include, but are not limited to, copper-, zinc- and gold-containing complexes which have made significant progress in the pursuit of developing novel anticancer drugs. Since higher concentrations of copper is a common trademark of many human tumors, targeting tumor cellular copper with copper chelating agents via inhibition of cancer cell proteasome has emerged as an exciting new avenue in cancer therapy. Along this line, metals including gallium(III), Zinc(II), and copper(II) when combined with asymmetric tridentate appended ligands represent an innovative new class of proteasome inhibitors. Since metals are endowed with unique properties that are absent in conventional carbon-based drugs, the positive trend in anticancer drug discovery can be continued by building on the underlying knowledge gained from medicinal inorganic chemistry.
ACKNOWLEDGEMENTS
We greatly thank Dr. Claudio Verani for the translation of Paracelsus’ quotation and for the critical reading of this manuscript. This work was partially supported by research funds from the Karmanos Cancer Institute of Wayne State University (to QPD) and the National Cancer Institute (1R01CA120009, 3R01CA120009-04S1, 1R21CA139386-01, to QPD) as well as a training grant from the National Cancer Institute (T32-CA009531 to MF).
Footnotes
Alle Ding’ sind Gift, und nichts ohn’ Gift; allein die Dosis macht, daß ein Ding kein Gift ist. Translation by Dr. Claudio Verani, Wayne State University
REFERENCES
- 1.DeVita VT, Jr., Chu E. A history of cancer chemotherapy. Cancer Res. 2008;68:8643–53. doi: 10.1158/0008-5472.CAN-07-6611. [DOI] [PubMed] [Google Scholar]
- 2.Hambley TW, Hait WN. Is anticancer drug development heading in the right direction? Cancer Res. 2009;69:1259–62. doi: 10.1158/0008-5472.CAN-08-3786. [DOI] [PubMed] [Google Scholar]
- 3.Neidle S, Thurston DE. Chemical approaches to the discovery and development of cancer therapies. Nat Rev Cancer. 2005;5:285–96. doi: 10.1038/nrc1587. [DOI] [PubMed] [Google Scholar]
- 4.Orvig C, Abrams MJ. Medicinal inorganic chemistry: introduction. Chem Rev. 1999;99:2201–4. doi: 10.1021/cr980419w. [DOI] [PubMed] [Google Scholar]
- 5.Yaman M, Kaya G, Yekeler H. Distribution of trace metal concentrations in paired cancerous and non-cancerous human stomach tissues. World J Gastroenterol. 2007;13:612–8. doi: 10.3748/wjg.v13.i4.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson KH, Orvig C. Boon and bane of metal ions in medicine. Science. 2003;300:936–9. doi: 10.1126/science.1083004. [DOI] [PubMed] [Google Scholar]
- 7.Chen D, Milacic V, Frezza M, Dou QP. Metal complexes, their cellular targets and potential for cancer therapy. Curr Pharm Des. 2009;15:777–91. doi: 10.2174/138161209787582183. [DOI] [PubMed] [Google Scholar]
- 8.Yan YK, Melchart M, Habtemariam A, Sadler PJ. Organometallic chemistry, biology and medicine: ruthenium arene anticancer complexes. Chem Commun (Camb) 2005:4764–76. doi: 10.1039/b508531b. [DOI] [PubMed] [Google Scholar]
- 9.Weiss RB, Christian MC. New cisplatin analogues in development. A review. Drugs. 1993;46:360–77. doi: 10.2165/00003495-199346030-00003. [DOI] [PubMed] [Google Scholar]
- 10.Criado JJ, Manzano JL, Rodriguez-Fernandez E. New organotropic compounds. Synthesis, characterization and reactivity of Pt(II) and Au(III) complexes with bile acids: DNA interactions and ‘in vitro’ anticancer activity. J Inorg Biochem. 2003;96:311–20. doi: 10.1016/s0162-0134(03)00240-x. [DOI] [PubMed] [Google Scholar]
- 11.Wong E, Giandomenico CM. Current status of platinum-based antitumor drugs. Chem Rev. 1999;99:2451–66. doi: 10.1021/cr980420v. [DOI] [PubMed] [Google Scholar]
- 12.Ho YP, Au-Yeung SC, To KK. Platinum-based anticancer agents: innovative design strategies and biological perspectives. Med Res Rev. 2003;23:633–55. doi: 10.1002/med.10038. [DOI] [PubMed] [Google Scholar]
- 13.Daniel KG, Chen D, Orlu S, Cui QC, Miller FR, Dou QP. Clioquinol and pyrrolidine dithiocarbamate complex with copper to form proteasome inhibitors and apoptosis inducers in human breast cancer cells. Breast Cancer Res. 2005;7:R897–908. doi: 10.1186/bcr1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daniel KG, Gupta P, Harbach RH, Guida WC, Dou QP. Organic copper complexes as a new class of proteasome inhibitors and apoptosis inducers in human cancer cells. Biochem Pharmacol. 2004;67:1139–51. doi: 10.1016/j.bcp.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 15.Milacic V, Chen D, Giovagnini L, Diez A, Fregona D, Dou QP. Pyrrolidine dithiocarbamate-zinc(II) and -copper(II) complexes induce apoptosis in tumor cells by inhibiting the proteasomal activity. Toxicol Appl Pharmacol. 2008;231:24–33. doi: 10.1016/j.taap.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milacic V, Chen D, Ronconi L, Landis-Piwowar KR, Fregona D, Dou QP. A novel anticancer gold(III) dithiocarbamate compound inhibits the activity of a purified 20S proteasome and 26S proteasome in human breast cancer cell cultures and xenografts. Cancer Res. 2006;66:10478–86. doi: 10.1158/0008-5472.CAN-06-3017. [DOI] [PubMed] [Google Scholar]
- 17.Hartinger CG, Jakupec MA, Zorbas-Seifried S, Groessl M, Egger A, Berger W, et al. KP1019, a new redox-active anticancer agent--preclinical development and results of a clinical phase I study in tumor patients. Chem Biodivers. 2008;5:2140–55. doi: 10.1002/cbdv.200890195. [DOI] [PubMed] [Google Scholar]
- 18.Hartinger CG, Zorbas-Seifried S, Jakupec MA, Kynast B, Zorbas H, Keppler BK. From bench to bedside--preclinical and early clinical development of the anticancer agent indazolium trans-[tetrachlorobis(1H-indazole)ruthenate(III)] (KP1019 or FFC14A). J Inorg Biochem. 2006;100:891–904. doi: 10.1016/j.jinorgbio.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Bowen ML, Orvig C. 99m-Technetium carbohydrate conjugates as potential agents in molecular imaging. Chem Commun (Camb) 2008:5077–91. doi: 10.1039/b809365b. [DOI] [PubMed] [Google Scholar]
- 20.Scott LE, Orvig C. Medicinal inorganic chemistry approaches to passivation and removal of aberrant metal ions in disease. Chem Rev. 2009;109:4885–910. doi: 10.1021/cr9000176. [DOI] [PubMed] [Google Scholar]
- 21.Haas KL, Franz KJ. Application of metal coordination chemistry to explore and manipulate cell biology. Chem Rev. 2009;109:4921–60. doi: 10.1021/cr900134a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao G, Lin H. Metal complexes with aromatic N-containing ligands as potential agents in cancer treatment. Curr Med Chem Anticancer Agents. 2005;5:137–47. doi: 10.2174/1568011053174873. [DOI] [PubMed] [Google Scholar]
- 23.Jakupec MA, Galanski M, Arion VB, Hartinger CG, Keppler BK. Antitumour metal compounds: more than theme and variations. Dalton Trans. 2008:183–94. doi: 10.1039/b712656p. [DOI] [PubMed] [Google Scholar]
- 24.Holm RH, Kennepohl P, Solomon EI. Structural and functional aspects of metal sites in biology. Chem Rev. 1996;96:2239–314. doi: 10.1021/cr9500390. [DOI] [PubMed] [Google Scholar]
- 25.Mertz W. Essential trace metals: new definitions based on new paradigms. Nutr Rev. 1993;51:287–95. doi: 10.1111/j.1753-4887.1993.tb03057.x. [DOI] [PubMed] [Google Scholar]
- 26.Fricker SP. Metal based drugs: from serendipity to design. Dalton Trans. 2007:4903–17. doi: 10.1039/b705551j. [DOI] [PubMed] [Google Scholar]
- 27.Meggers E. Targeting proteins with metal complexes. Chem Commun (Camb) 2009:1001–10. doi: 10.1039/b813568a. [DOI] [PubMed] [Google Scholar]
- 28.Cohen SM. New approaches for medicinal applications of bioinorganic chemistry. Curr Opin Chem Biol. 2007;11:115–20. doi: 10.1016/j.cbpa.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Ott I, Gust R. Non platinum metal complexes as anti-cancer drugs. Arch Pharm (Weinheim) 2007;340:117–26. doi: 10.1002/ardp.200600151. [DOI] [PubMed] [Google Scholar]
- 30.Hambley TW. Developing new metal-based therapeutics: challenges and opportunities. Dalton Trans. 2007:4929–37. doi: 10.1039/b706075k. [DOI] [PubMed] [Google Scholar]
- 31.Desoize B. Metals and metal compounds in cancer treatment. Anticancer Res. 2004;24:1529–44. [PubMed] [Google Scholar]
- 32.Jamieson ER, Lippard SJ. Structure, Recognition, and Processing of Cisplatin-DNA Adducts. Chem Rev. 1999;99:2467–98. doi: 10.1021/cr980421n. [DOI] [PubMed] [Google Scholar]
- 33.Kelland L. The resurgence of platinum-based cancer chemo-therapy. Nat Rev Cancer. 2007;7:573–84. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 34.Galanski M, Arion VB, Jakupec MA, Keppler BK. Recent developments in the field of tumor-inhibiting metal complexes. Curr Pharm Des. 2003;9:2078–89. doi: 10.2174/1381612033454180. [DOI] [PubMed] [Google Scholar]
- 35.Galanski M, Jakupec MA, Keppler BK. Update of the preclinical situation of anticancer platinum complexes: novel design strategies and innovative analytical approaches. Curr Med Chem. 2005;12:2075–94. doi: 10.2174/0929867054637626. [DOI] [PubMed] [Google Scholar]
- 36.Alama A, Tasso B, Novelli F, Sparatore F. Organometallic compounds in oncology: implications of novel organotins as antitumor agents. Drug Discov Today. 2009;14:500–8. doi: 10.1016/j.drudis.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 37.Harrap KR. Preclinical studies identifying carboplatin as a viable cisplatin alternative. Cancer Treat Rev. 1985;12(Suppl A):21–33. doi: 10.1016/0305-7372(85)90015-5. [DOI] [PubMed] [Google Scholar]
- 38.de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–47. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 39.Kostova I. Platinum complexes as anticancer agents. Recent Pat Anticancer Drug Discov. 2006;1:1–22. doi: 10.2174/157489206775246458. [DOI] [PubMed] [Google Scholar]
- 40.Reedijk J. New clues for platinum antitumor chemistry: kinetically controlled metal binding to DNA. Proc Natl Acad Sci USA. 2003;100:3611–6. doi: 10.1073/pnas.0737293100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montana AM, Batalla C. The rational design of anticancer platinum complexes: the importance of the structure-activity relationship. Curr Med Chem. 2009;16:2235–60. doi: 10.2174/092986709788453087. [DOI] [PubMed] [Google Scholar]
- 42.Coluccia M, Natile G. Trans-platinum complexes in cancer therapy. Anticancer Agents Med Chem. 2007;7:111–23. doi: 10.2174/187152007779314080. [DOI] [PubMed] [Google Scholar]
- 43.Abu-Surrah AS, Kettunen M. Platinum group antitumor chemistry: design and development of new anticancer drugs complementary to cisplatin. Curr Med Chem. 2006;13:1337–57. doi: 10.2174/092986706776872970. [DOI] [PubMed] [Google Scholar]
- 44.Lin X, Okuda T, Holzer A, Howell SB. The copper transporter CTR1 regulates cisplatin uptake in Saccharomyces cerevisiae. Mol Pharmacol. 2002;62:1154–9. doi: 10.1124/mol.62.5.1154. [DOI] [PubMed] [Google Scholar]
- 45.Fuertes MA, Alonso C, Perez JM. Biochemical modulation of Cisplatin mechanisms of action: enhancement of antitumor activity and circumvention of drug resistance. Chem Rev. 2003;103:645–62. doi: 10.1021/cr020010d. [DOI] [PubMed] [Google Scholar]
- 46.Jennerwein M, Andrews PA. Effect of intracellular chloride on the cellular pharmacodynamics of cis-diamminedichloroplatinum(II). Drug Metab Dispos. 1995;23:178–84. [PubMed] [Google Scholar]
- 47.Legendre F, Bas V, Kozelka J, Chottard JC. A complete kinetic study of GG versus AG plantination suggests that the doubly aquated derivatives of cisplatin are the actual DNA binding species. Chemistry. 2000;6:2002–10. doi: 10.1002/1521-3765(20000602)6:11<2002::aid-chem2002>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 48.Pizarro AM, Sadler PJ. Unusual DNA binding modes for metal anticancer complexes. Biochimie. 2009;91:1198–211. doi: 10.1016/j.biochi.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Knox RJ, Friedlos F, Lydall DA, Roberts JJ. Mechanism of cytotoxicity of anticancer platinum drugs: evidence that cisdiamminedichloroplatinum(II) and cis-diammine-(1,1-cyclobutanedicarboxylato)platinum(II) differ only in the kinetics of their interaction with DNA. Cancer Res. 1986;46:1972–9. [PubMed] [Google Scholar]
- 50.Jakupec MA, Galanski M, Keppler BK. Tumour-inhibiting platinum complexes--state of the art and future perspectives. Rev Physiol Biochem Pharmacol. 2003;146:1–54. doi: 10.1007/s10254-002-0001-x. [DOI] [PubMed] [Google Scholar]
- 51.Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4:307–20. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 52.Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–79. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- 53.Fuertes MA, Castilla J, Alonso C, Perez JM. Novel concepts in the development of platinum antitumor drugs. Curr Med Chem Anticancer Agents. 2002;2:539–51. doi: 10.2174/1568011023353958. [DOI] [PubMed] [Google Scholar]
- 54.Galanski M. Recent developments in the field of anticancer platinum complexes. Recent Pat Anticancer Drug Discov. 2006;1:285–95. doi: 10.2174/157489206777442287. [DOI] [PubMed] [Google Scholar]
- 55.Chaney SG, Vaisman A. Specificity of platinum-DNA adduct repair. J Inorg Biochem. 1999;77:71–81. doi: 10.1016/s0162-0134(99)00149-x. [DOI] [PubMed] [Google Scholar]
- 56.Raymond E, Faivre S, Woynarowski JM, Chaney SG. Oxaliplatin: mechanism of action and antineoplastic activity. Semin Oncol. 1998;25:4–12. [PubMed] [Google Scholar]
- 57.Kelland L. Broadening the clinical use of platinum drug-based chemotherapy with new analogues. Satraplatin and picoplatin. Expert Opin Investig Drugs. 2007;16:1009–21. doi: 10.1517/13543784.16.7.1009. [DOI] [PubMed] [Google Scholar]
- 58.Klein AV, Hambley TW. Platinum drug distribution in cancer cells and tumors. Chem Rev. 2009;109:4911–20. doi: 10.1021/cr9001066. [DOI] [PubMed] [Google Scholar]
- 59.Rademaker-Lakhai JM, Terret C, Howell SB, Baud CM, De Boer RF, Pluim D, et al. A Phase I and pharmacological study of the platinum polymer AP5280 given as an intravenous infusion once every 3 weeks in patients with solid tumors. Clin Cancer Res. 2004;10:3386–95. doi: 10.1158/1078-0432.CCR-03-0315. [DOI] [PubMed] [Google Scholar]
- 60.Choy H, Park C, Yao M. Current status and future prospects for satraplatin, an oral platinum analogue. Clin Cancer Res. 2008;14:1633–8. doi: 10.1158/1078-0432.CCR-07-2176. [DOI] [PubMed] [Google Scholar]
- 61.Hall MD, Mellor HR, Callaghan R, Hambley TW. Basis for design and development of platinum(IV) anticancer complexes. J Med Chem. 2007;50:3403–11. doi: 10.1021/jm070280u. [DOI] [PubMed] [Google Scholar]
- 62.Mellish KJ, Kelland LR. Mechanisms of acquired resistance to the orally active platinum-based anticancer drug bis-acetato-amminedichloro-cyclohexylamine platinum (i.v.) (JM216) in two human ovarian carcinoma cell lines. Cancer Res. 1994;54:6194–200. [PubMed] [Google Scholar]
- 63.Kozubik A, Vaculova A, Soucek K, Vondracek J, Turanek J, Hofmanova J. Novel anticancer platinum(IV) complexes with adamantylamine: their efficiency and innovative chemotherapy strategies modifying lipid metabolism. Met Based Drugs. 2008;2008:417897. doi: 10.1155/2008/417897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sternberg CN, Petrylak DP, Sartor O, Witjes JA, Demkow T, Ferrero JM, et al. Multinational, double-blind, phase III study of prednisone and either satraplatin or placebo in patients with castrate-refractory prostate cancer progressing after prior chemotherapy: the SPARC trial. J Clin Oncol. 2009;27:5431–8. doi: 10.1200/JCO.2008.20.1228. [DOI] [PubMed] [Google Scholar]
- 65.Kelland LR. An update on satraplatin: the first orally available platinum anticancer drug. Expert Opin Investig Drugs. 2000;9:1373–82. doi: 10.1517/13543784.9.6.1373. [DOI] [PubMed] [Google Scholar]
- 66.Mellish KJ, Barnard CF, Murrer BA, Kelland LR. DNA-binding properties of novel cis- and trans platinum-based anticancer agents in 2 human ovarian carcinoma cell lines. Int J Cancer. 1995;62:717–23. doi: 10.1002/ijc.2910620612. [DOI] [PubMed] [Google Scholar]
- 67.Bruijnincx CA, Sadler PJ. New trends for metal complexes with anticancer activity. Curr Opin Chem Bio. 2007;12:1–10. doi: 10.1016/j.cbpa.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.He Q, Liang CH, Lippard SJ. Steroid hormones induce HMG1 overexpression and sensitize breast cancer cells to cisplatin and carboplatin. Proc Natl Acad Sci USA. 2000;97:5768–72. doi: 10.1073/pnas.100108697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barnes KR, Kutikov A, Lippard SJ. Synthesis, characterization, and cytotoxicity of a series of estrogen-tethered platinum(IV) complexes. Chem Biol. 2004;11:557–64. doi: 10.1016/j.chembiol.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 70.Ang WH, Khalaila I, Allardyce CS, Juillerat-Jeanneret L, Dyson PJ. Rational design of platinum(IV) compounds to overcome glutathione-S-transferase mediated drug resistance. J Am Chem Soc. 2005;127:1382–3. doi: 10.1021/ja0432618. [DOI] [PubMed] [Google Scholar]
- 71.Bednarski PJ, Grunert R, Zielzki M, Wellner A, Mackay FS, Sadler PJ. Light-activated destruction of cancer cell nuclei by platinum diazide complexes. Chem Biol. 2006;13:61–7. doi: 10.1016/j.chembiol.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 72.Mackay FS, Woods JA, Moseley H, Ferguson J, Dawson A, Parsons S, et al. A photoactivated trans-diammine platinum complex as cytotoxic as cisplatin. Chemistry. 2006;12:3155–61. doi: 10.1002/chem.200501601. [DOI] [PubMed] [Google Scholar]
- 73.Ho E. Zinc deficiency, DNA damage and cancer risk. J Nutr Biochem. 2004;15:572–8. doi: 10.1016/j.jnutbio.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 74.Stefanidou M, Maravelias C, Dona A, Spiliopoulou C. Zinc: a multipurpose trace element. Arch Toxicol. 2006;80:1–9. doi: 10.1007/s00204-005-0009-5. [DOI] [PubMed] [Google Scholar]
- 75.Prasad AS. Zinc: an overview. Nutrition. 1995;11:93–9. [PubMed] [Google Scholar]
- 76.Prasad AS, Kucuk O. Zinc in cancer prevention. Cancer Metastasis Rev. 2002;21:291–5. doi: 10.1023/a:1021215111729. [DOI] [PubMed] [Google Scholar]
- 77.Fraker PJ, Lill-Elghanian DA. The many roles of apoptosis in immunity as modified by aging and nutritional status. J Nutr Health Aging. 2004;8:56–63. [PubMed] [Google Scholar]
- 78.Chang KL, Hung TC, Hsieh BS, Chen YH, Chen TF, Cheng HL. Zinc at pharmacologic concentrations affects cytokine expression and induces apoptosis of human peripheral blood mononuclear cells. Nutrition. 2006;22:465–74. doi: 10.1016/j.nut.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 79.Franklin RB, Costello LC. The important role of the apoptotic effects of zinc in the development of cancers. J Cell Biochem. 2009;106:750–7. doi: 10.1002/jcb.22049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Provinciali M, Di Stefano G, Fabris N. Dose-dependent opposite effect of zinc on apoptosis in mouse thymocytes. Int J Immunopharmacol. 1995;17:735–44. doi: 10.1016/0192-0561(95)00063-8. [DOI] [PubMed] [Google Scholar]
- 81.Murakami M, Hirano T. Intracellular zinc homeostasis and zinc signaling. Cancer Sci. 2008;99:1515–22. doi: 10.1111/j.1349-7006.2008.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Federico A, Iodice P, Federico P, Del Rio A, Mellone MC, Catalano G. Effects of selenium and zinc supplementation on nutritional status in patients with cancer of digestive tract. Eur J Clin Nutr. 2001;55:293–7. doi: 10.1038/sj.ejcn.1601157. [DOI] [PubMed] [Google Scholar]
- 83.Prasad AS, Beck FW, Doerr TD, Shamsa FH, Penny HS, Marks SC, et al. Nutritional and zinc status of head and neck cancer patients: an interpretive review. J Am Coll Nutr. 1998;17:409–18. doi: 10.1080/07315724.1998.10718787. [DOI] [PubMed] [Google Scholar]
- 84.Chakravarty PK, Ghosh A, Chowdhury JR. Zinc in human malignancies. Neoplasma. 1986;33:85–90. [PubMed] [Google Scholar]
- 85.Franklin RB, Costello LC. Zinc as an anti-tumor agent in prostate cancer and in other cancers. Arch Biochem Biophys. 2007;463:211–7. doi: 10.1016/j.abb.2007.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gupta SK, Singh SP, Shukla VK. Copper, zinc, and Cu/Zn ratio in carcinoma of the gallbladder. J Surg Oncol. 2005;91:204–8. doi: 10.1002/jso.20306. [DOI] [PubMed] [Google Scholar]
- 87.Margalioth EJ, Schenker JG, Chevion M. Copper and zinc levels in normal and malignant tissues. Cancer. 1983;52:868–72. doi: 10.1002/1097-0142(19830901)52:5<868::aid-cncr2820520521>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 88.Schwartz AE, Leddicotte GW, Fink RW, Friedman EW. Trace elements in noraml and malignant human breast tissue. Surgery. 1974;76:325–9. [PubMed] [Google Scholar]
- 89.Zhao H, Eide D. The yeast ZRT1 gene encodes the zinc transporter protein of a high-affinity uptake system induced by zinc limitation. Proc Natl Acad Sci USA. 1996;93:2454–8. doi: 10.1073/pnas.93.6.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li M, Zhang Y, Liu Z, Bharadwaj U, Wang H, Wang X, et al. Aberrant expression of zinc transporter ZIP4 (SLC39A4) significantly contributes to human pancreatic cancer pathogenesis and progression. Proc Natl Acad Sci USA. 2007;104:18636–41. doi: 10.1073/pnas.0709307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Costello LC, Franklin RB. The clinical relevance of the metabolism of prostate cancer; zinc and tumor suppression: connecting the dots. Mol Cancer. 2006;5:17. doi: 10.1186/1476-4598-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Manning DL, Robertson JF, Ellis IO, Elston CW, McClelland RA, Gee JM, et al. Oestrogen-regulated genes in breast cancer: association of pLIV1 with lymph node involvement. Eur J Cancer. 1994;30A:675–8. doi: 10.1016/0959-8049(94)90543-6. [DOI] [PubMed] [Google Scholar]
- 93.Taylor KM, Morgan HE, Smart K, Zahari NM, Pumford S, Ellis IO, et al. The emerging role of the LIV-1 subfamily of zinc transporters in breast cancer. Mol Med. 2007;13:396–406. doi: 10.2119/2007-00040.Taylor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006;66:8319–26. doi: 10.1158/0008-5472.CAN-06-0410. [DOI] [PubMed] [Google Scholar]
- 95.Kagara N, Tanaka N, Noguchi S, Hirano T. Zinc and its transporter ZIP10 are involved in invasive behavior of breast cancer cells. Cancer Sci. 2007;98:692–7. doi: 10.1111/j.1349-7006.2007.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Adams J. The development of proteasome inhibitors as anticancer drugs. Cancer Cell. 2004;5:417–21. doi: 10.1016/s1535-6108(04)00120-5. [DOI] [PubMed] [Google Scholar]
- 97.Adams J. The proteasome: a suitable antineoplastic target. Nat Rev Cancer. 2004;4:349–60. doi: 10.1038/nrc1361. [DOI] [PubMed] [Google Scholar]
- 98.Nalepa G, Rolfe M, Harper JW. Drug discovery in the ubiquitinproteasome system. Nat Rev Drug Discov. 2006;5:596–613. doi: 10.1038/nrd2056. [DOI] [PubMed] [Google Scholar]
- 99.Newton K, Vucic D. Ubiquitin ligases in cancer: ushers for degradation. Cancer Invest. 2007;25:502–13. doi: 10.1080/07357900701508041. [DOI] [PubMed] [Google Scholar]
- 100.Dou QP, Li B. Proteasome inhibitors as potential novel anticancer agents. Drug Resist Updat. 1999;2:215–23. doi: 10.1054/drup.1999.0095. [DOI] [PubMed] [Google Scholar]
- 101.Hochstrasser M. Ubiquitin, proteasomes, and the regulation of intracellular protein degradation. Curr Opin Cell Biol. 1995;7:215–23. doi: 10.1016/0955-0674(95)80031-x. [DOI] [PubMed] [Google Scholar]
- 102.Adams J, Palombella VJ, Elliott PJ. Proteasome inhibition: a new strategy in cancer treatment. Invest New Drugs. 2000;18:109–21. doi: 10.1023/a:1006321828515. [DOI] [PubMed] [Google Scholar]
- 103.Rajkumar SV, Richardson PG, Hideshima T, Anderson KC. Proteasome inhibition as a novel therapeutic target in human cancer. J Clin Oncol. 2005;23:630–9. doi: 10.1200/JCO.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 104.Milacic V, Fregona D, Dou QP. Gold complexes as prospective metal-based anticancer drugs. Histol Histopathol. 2008;23:101–8. doi: 10.14670/HH-23.101. [DOI] [PubMed] [Google Scholar]
- 105.Orlowski RZ, Eswara JR, Lafond-Walker A, Grever MR, Orlowski M, Dang CV. Tumor growth inhibition induced in a murine model of human Burkitt's lymphoma by a proteasome inhibitor. Cancer Res. 1998;58:4342–8. [PubMed] [Google Scholar]
- 106.Dou QP, Goldfarb RH. Bortezomib (millennium pharmaceuticals). IDrugs. 2002;5:828–34. [PubMed] [Google Scholar]
- 107.Orlowski RZ, Stinchcombe TE, Mitchell BS, Shea TC, Baldwin AS, Stahl S, et al. Phase I trial of the proteasome inhibitor PS-341 in patients with refractory hematologic malignancies. J Clin Oncol. 2002;20:4420–7. doi: 10.1200/JCO.2002.01.133. [DOI] [PubMed] [Google Scholar]
- 108.Yang H, Zonder JA, Dou QP. Clinical development of novel proteasome inhibitors for cancer treatment. Expert Opin Investig Drugs. 2009;18:957–71. doi: 10.1517/13543780903002074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Messori L, Abbate F, Marcon G, Orioli P, Fontani M, Mini E, et al. Gold(III) complexes as potential antitumor agents: solution chemistry and cytotoxic properties of some selected gold(III) compounds. J Med Chem. 2000;43:3541–8. doi: 10.1021/jm990492u. [DOI] [PubMed] [Google Scholar]
- 110.Ronconi L, Giovagnini L, Marzano C, Bettio F, Graziani R, Pilloni G, et al. Gold dithiocarbamate derivatives as potential antineoplastic agents: design, spectroscopic properties, and in vitro antitumor activity. Inorg Chem. 2005;44:1867–81. doi: 10.1021/ic048260v. [DOI] [PubMed] [Google Scholar]
- 111.Ronconi L, Marzano C, Zanello P, Corsini M, Miolo G, Macca C, et al. Gold(III) dithiocarbamate derivatives for the treatment of cancer: solution chemistry, DNA binding, and hemolytic properties. J Med Chem. 2006;49:1648–57. doi: 10.1021/jm0509288. [DOI] [PubMed] [Google Scholar]
- 112.Marcon G, Carotti S, Coronnello M, Messori L, Mini E, Orioli P, et al. Gold(III) complexes with bipyridyl ligands: solution chemistry, cytotoxicity, and DNA binding properties. J Med Chem. 2002;45:1672–7. doi: 10.1021/jm010997w. [DOI] [PubMed] [Google Scholar]
- 113.Messori L, Orioli P, Tempi C, Marcon G. Interactions of selected gold(III) complexes with calf thymus DNA. Biochem Biophys Res Commun. 2001;281:352–60. doi: 10.1006/bbrc.2001.4358. [DOI] [PubMed] [Google Scholar]
- 114.Zhang X, Frezza M, Milacic V, Ronconi L, Fan Y, Bi C, et al. Inhibition of tumor proteasome activity by gold-dithiocarbamato complexes via both redox-dependent and -independent processes. J Cell Biochem. 2010;109:162–72. doi: 10.1002/jcb.22394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ambroggio XI, Rees DC, Deshaies RJ. JAMM: a metalloprotease-like zinc site in the proteasome and signalosome. PLoS Biol. 2004;2:E2. doi: 10.1371/journal.pbio.0020002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gallery M, Blank JL, Lin Y, Gutierrez JA, Pulido JC, Rappoli D, et al. The JAMM motif of human deubiquitinase Poh1 is essential for cell viability. Mol Cancer Ther. 2007;6:262–8. doi: 10.1158/1535-7163.MCT-06-0542. [DOI] [PubMed] [Google Scholar]
- 117.Verma R, Aravind L, Oania R, McDonald WH, Yates JR, 3rd, Koonin EV, et al. Role of Rpn11 metalloprotease in deubiquitination and degradation by the 26S proteasome. Science. 2002;298:611–5. doi: 10.1126/science.1075898. [DOI] [PubMed] [Google Scholar]
- 118.Cvek B, Milacic V, Taraba J, Dou QP. Ni(II), Cu(II), and Zn(II) diethyldithiocarbamate complexes show various activities against the proteasome in breast cancer cells. J Med Chem. 2008;51:6256–8. doi: 10.1021/jm8007807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tapiero H, Townsend DM, Tew KD. Trace elements in human physiology and pathology. Copper. Biomed Pharmacother. 2003;57:386–98. doi: 10.1016/s0753-3322(03)00012-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gupte A, Mumper RJ. Elevated copper and oxidative stress in cancer cells as a target for cancer treatment. Cancer Treat Rev. 2009;35:32–46. doi: 10.1016/j.ctrv.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 121.Kim BE, Nevitt T, Thiele DJ. Mechanisms for copper acquisition, distribution and regulation. Nat Chem Biol. 2008;4:176–85. doi: 10.1038/nchembio.72. [DOI] [PubMed] [Google Scholar]
- 122.Chen D, Dou QP. New uses for old copper-binding drugs: converting the pro-angiogenic copper to a specific cancer cell death inducer. Expert Opin Ther Targets. 2008;12:739–48. doi: 10.1517/14728222.12.6.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Radisky D, Kaplan J. Regulation of transition metal transport across the yeast plasma membrane. J Biol Chem. 1999;274:4481–4. doi: 10.1074/jbc.274.8.4481. [DOI] [PubMed] [Google Scholar]
- 124.Rorabacher DB. Electron transfer by copper centers. Chem Rev. 2004;104:651–97. doi: 10.1021/cr020630e. [DOI] [PubMed] [Google Scholar]
- 125.Tisato F, Marzano C, Porchia M, Pellei M, Santini C. Copper in diseases and treatments, and copper-based anticancer strategies. Med Res Rev. 2009 doi: 10.1002/med.20174. [Eupb ahead of print] [DOI] [PubMed] [Google Scholar]
- 126.Apelgot S, Coppey J, Fromentin A, Guille E, Poupon MF, Roussel A. Altered distribution of copper (64Cu) in tumor-bearing mice and rats. Anticancer Res. 1986;6:159–64. [PubMed] [Google Scholar]
- 127.Zowczak M, Iskra M, Torlinski L, Cofta S. Analysis of serum copper and zinc concentrations in cancer patients. Biol Trace Elem Res. 2001;82:1–8. doi: 10.1385/BTER:82:1-3:001. [DOI] [PubMed] [Google Scholar]
- 128.Kuo HW, Chen SF, Wu CC, Chen DR, Lee JH. Serum and tissue trace elements in patients with breast cancer in Taiwan. Biol Trace Elem Res. 2002;89:1–11. doi: 10.1385/BTER:89:1:1. [DOI] [PubMed] [Google Scholar]
- 129.Rizk SL, Sky-Peck HH. Comparison between concentrations of trace elements in normal and neoplastic human breast tissue. Cancer Res. 1984;44:5390–4. [PubMed] [Google Scholar]
- 130.Habib FK, Dembinski TC, Stitch SR. The zinc and copper content of blood leucocytes and plasma from patients with benign and malignant prostates. Clin Chim Acta. 1980;104:329–35. doi: 10.1016/0009-8981(80)90390-3. [DOI] [PubMed] [Google Scholar]
- 131.Nayak SB, Bhat VR, Upadhyay D, Udupa SL. Copper and ceruloplasmin status in serum of prostate and colon cancer patients. Indian J Physiol Pharmacol. 2003;47:108–10. [PubMed] [Google Scholar]
- 132.Diez M, Arroyo M, Cerdan FJ, Munoz M, Martin MA, Balibrea JL. Serum and tissue trace metal levels in lung cancer. Oncology. 1989;46:230–4. doi: 10.1159/000226722. [DOI] [PubMed] [Google Scholar]
- 133.Turecky L, Kalina P, Uhlikova E, Namerova S, Krizko J. Serum ceruloplasmin and copper levels in patients with primary brain tumors. Klin Wochenschr. 1984;62:187–9. doi: 10.1007/BF01731643. [DOI] [PubMed] [Google Scholar]
- 134.McAuslan BR, Reilly W. Endothelial cell phagokinesis in response to specific metal ions. Exp Cell Res. 1980;130:147–57. doi: 10.1016/0014-4827(80)90051-8. [DOI] [PubMed] [Google Scholar]
- 135.Lowndes SA, Harris AL. Copper chelation as an antiangiogenic therapy. Oncol Res. 2004;14:529–39. doi: 10.3727/0965040042707952. [DOI] [PubMed] [Google Scholar]
- 136.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 137.Ryan CJ, Wilding G. Angiogenesis inhibitors. New agents in cancer therapy. Drugs Aging. 2000;17:249–55. doi: 10.2165/00002512-200017040-00001. [DOI] [PubMed] [Google Scholar]
- 138.Hu GF. Copper stimulates proliferation of human endothelial cells under culture. J Cell Biochem. 1998;69:326–35. doi: 10.1002/(sici)1097-4644(19980601)69:3<326::aid-jcb10>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 139.Pan Q, Kleer CG, van Golen KL, Irani J, Bottema KM, Bias C, et al. Copper deficiency induced by tetrathiomolybdate suppresses tumor growth and angiogenesis. Cancer Res. 2002;62:4854–9. [PubMed] [Google Scholar]
- 140.Yoshii J, Yoshiji H, Kuriyama S, Ikenaka Y, Noguchi R, Okuda H, et al. The copper-chelating agent, trientine, suppresses tumor development and angiogenesis in the murine hepatocellular carcinoma cells. Int J Cancer. 2001;94:768–73. doi: 10.1002/ijc.1537. [DOI] [PubMed] [Google Scholar]
- 141.Brewer GJ, Dick RD, Grover DK, LeClaire V, Tseng M, Wicha M, et al. Treatment of metastatic cancer with tetrathiomolybdate, an anticopper, antiangiogenic agent: Phase I study. Clin Cancer Res. 2000;6:1–10. [PubMed] [Google Scholar]
- 142.Redman BG, Esper P, Pan Q, Dunn RL, Hussain HK, Chenevert T, et al. Phase II trial of tetrathiomolybdate in patients with advanced kidney cancer. Clin Cancer Res. 2003;9:1666–72. [PubMed] [Google Scholar]
- 143.Krampe H, Stawicki S, Wagner T, Bartels C, Aust C, Ruther E, et al. Follow-up of 180 alcoholic patients for up to 7 years after outpatient treatment: impact of alcohol deterrents on outcome. Alcohol Clin Exp Res. 2006;30:86–95. doi: 10.1111/j.1530-0277.2006.00013.x. [DOI] [PubMed] [Google Scholar]
- 144.Crabb DW, Matsumoto M, Chang D, You M. Overview of the role of alcohol dehydrogenase and aldehyde dehydrogenase and their variants in the genesis of alcohol-related pathology. Proc Nutr Soc. 2004;63:49–63. doi: 10.1079/pns2003327. [DOI] [PubMed] [Google Scholar]
- 145.Cen D, Brayton D, Shahandeh B, Meyskens FL, Jr, Farmer PJ. Disulfiram facilitates intracellular Cu uptake and induces apoptosis in human melanoma cells. J Med Chem. 2004;47:6914–20. doi: 10.1021/jm049568z. [DOI] [PubMed] [Google Scholar]
- 146.Chen D, Cui QC, Yang H, Dou QP. Disulfiram, a clinically used anti-alcoholism drug and copper-binding agent, induces apoptotic cell death in breast cancer cultures and xenografts via inhibition of the proteasome activity. Cancer Res. 2006;66:10425–33. doi: 10.1158/0008-5472.CAN-06-2126. [DOI] [PubMed] [Google Scholar]
- 147.Chen D, Peng F, Cui QC, Daniel KG, Orlu S, Liu J, et al. Inhibition of prostate cancer cellular proteasome activity by a pyrrolidine dithiocarbamate-copper complex is associated with suppression of proliferation and induction of apoptosis. Front Biosci. 2005;10:2932–9. doi: 10.2741/1749. [DOI] [PubMed] [Google Scholar]
- 148.Pang H, Chen D, Cui QC, Dou QP. Sodium diethyldithiocarbamate, an AIDS progression inhibitor and a copper-binding compound, has proteasome-inhibitory and apoptosis-inducing activities in cancer cells. Int J Mol Med. 2007;19:809–16. [PubMed] [Google Scholar]
- 149.Ritchie CW, Bush AI, Masters CL. Metal-protein attenuating compounds and Alzheimer's disease. Expert Opin Investig Drugs. 2004;13:1585–92. doi: 10.1517/13543784.13.12.1585. [DOI] [PubMed] [Google Scholar]
- 150.Nguyen T, Hamby A, Massa SM. Clioquinol down-regulates mutant huntingtin expression in vitro and mitigates pathology in a Huntington's disease mouse model. Proc Natl Acad Sci USA. 2005;102:11840–5. doi: 10.1073/pnas.0502177102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Di Vaira M, Bazzicalupi C, Orioli P, Messori L, Bruni B, Zatta P. Clioquinol, a drug for Alzheimer's disease specifically interfering with brain metal metabolism: structural characterization of its zinc(II) and copper(II) complexes. Inorg Chem. 2004;43:3795–7. doi: 10.1021/ic0494051. [DOI] [PubMed] [Google Scholar]
- 152.Chen D, Cui QC, Yang H, Barrea RA, Sarkar FH, Sheng S, et al. Clioquinol, a therapeutic agent for Alzheimer's disease, has proteasome-inhibitory, androgen receptor-suppressing, apoptosis-inducing, and antitumor activities in human prostate cancer cells and xenografts. Cancer Res. 2007;67:1636–44. doi: 10.1158/0008-5472.CAN-06-3546. [DOI] [PubMed] [Google Scholar]
- 153.Itoh S, Taki M, Takayama S, Nagatomo S, Kitagawa T, Sakurada N, et al. Oxidation of Benzyl Alcohol with Cu(II) and Zn(II) Complexes of the Phenoxyl Radical as a Model of the Reaction of Galactose Oxidase. Angew Chem Int Ed Engl. 1999;38:2774–6. doi: 10.1002/(sici)1521-3773(19990917)38:18<2774::aid-anie2774>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 154.Vaidyanathan M, Viswanathan R, Palaniandavar M, Balasubramanian T, Prabhaharan P, Muthiah TP. Copper(II) complexes with unusual axial phenolate coordination as structural models for the active site in galactose oxidase: X-ray crystal structures and spectral and redox properties of [Cu(bpnp)X] complexes. Inorg Chem. 1998;37:6418–27. doi: 10.1021/ic971567s. [DOI] [PubMed] [Google Scholar]
- 155.Melchior M, Rettig SJ, Liboiron BD, Thompson KH, Yuen VG, McNeill JH, et al. Insulin-enhancing vanadium(III) complexes. Inorg Chem. 2001;40:4686–90. doi: 10.1021/ic000984t. [DOI] [PubMed] [Google Scholar]
- 156.Storr T, Sugai Y, Barta CA, Mikata Y, Adam MJ, Yano S, et al. Carbohydrate-appended 2,2'-dipicolylamine metal complexes as potential imaging agents. Inorg Chem. 2005;44:2698–705. doi: 10.1021/ic048528i. [DOI] [PubMed] [Google Scholar]
- 157.Kirin SI, Dubon P, Weyhermuller T, Bill E, Metzler-Nolte N. Amino acid and peptide bioconjugates of copper(II) and zinc(II) complexes with a modified N,N-bis(2-picolyl)amine ligand. Inorg Chem. 2005;44:5405–15. doi: 10.1021/ic048343b. [DOI] [PubMed] [Google Scholar]
- 158.Shakya R, Hindo SS, Wu L, Allard MM, Heeg MJ, Hratchian HP, et al. Archetypical modeling and amphiphilic behavior of cobalt(II)-containing soft-materials with asymmetric tridentate ligands. Inorg Chem. 2007;46:9808–18. doi: 10.1021/ic7011815. [DOI] [PubMed] [Google Scholar]
- 159.Shakya R, Imbert C, Hratchian HP, Lanznaster M, Heeg MJ, McGarvey BR, et al. Structural, spectroscopic, and electrochemical behavior of trans-phenolato cobalt(III) complexes of asymmetric NN'O ligands as archetypes for metallomesogens. Dalton Trans. 2006:2517–25. doi: 10.1039/b514190g. [DOI] [PubMed] [Google Scholar]
- 160.Shakya R, Peng F, Liu J, Heeg MJ, Verani CN. Synthesis, structure, and anticancer activity of gallium(III) complexes with asymmetric tridentate ligands: growth inhibition and apoptosis induction of cisplatin-resistant neuroblastoma cells. Inorg Chem. 2006;45:6263–8. doi: 10.1021/ic060106g. [DOI] [PubMed] [Google Scholar]
- 161.Chen D, Frezza M, Shakya R, Cui QC, Milacic V, Verani CN, et al. Inhibition of the proteasome activity by gallium(III) complexes contributes to their anti prostate tumor effects. Cancer Res. 2007;67:9258–65. doi: 10.1158/0008-5472.CAN-07-1813. [DOI] [PubMed] [Google Scholar]
- 162.Frezza M, Verani CN, Chen D, Dou QP. The therapeutic potential of gallium-based complexes in anti-tumor drug design. Lett Drug Design Discov. 2007;4:311–7. [Google Scholar]
- 163.Hindo SS, Frezza M, Tomco D, Heeg MJ, Hryhorczuk L, McGarvey BR, et al. Metals in anticancer therapy: copper(II) complexes as inhibitors of the 20S proteasome. Eur J Med Chem. 2009;44:4353–61. doi: 10.1016/j.ejmech.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Frezza M, Hindo SS, Tomco D, Allard MM, Cui QC, Heeg MJ, et al. Comparative activities of nickel(II) and zinc(II) complexes of asymmetric [NN'O] ligands as 26S proteasome inhibitors. Inorg Chem. 2009 doi: 10.1021/ic900276g. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]