Abstract
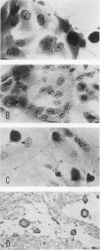
Four equine herpesviruses (equine abortion virus, equine herpesvirus types 2 and 3, and equine cytomegalovirus) were compared. The equine abortion virus did not cross-neutralize with any of the other viruses, but the other three did show varying degrees of cross-neutralization among themselves. Equine abortion virus grew more quickly in tissue cultures than did the others, and attained higher titers of infectivity in the culture fluid; it also formed plaques in a wider range of tissue culture species, although the other three were not specific for one tissue culture system only, in that they would multiply in rabbit and cat kidney cultures. The densities of the deoxyribonucleic acids of all four viruses were in the range 1.716 to 1.717 g/ml (a guanine plus cytosine content of 57 to 58%). Taxonomic separation, as a distinct serotype, of equine abortion virus from the other herpesviruses seems to be justified. The other three are closely related to one another. They should perhaps be regarded as separate viruses and termed horse herpesviruses types 2, 3, and 4, although an alternative view would be to regard them as variants of a single virus type. The question of whether types 2, 3, and 4, or any other herpesviruses, should be placed in a phylogenetically distinct subgroup, known as cytomegaloviruses, is a moot point.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DOLL E. R., RICHARDS M. G., WALLACE M. E. Adaptation of the equine abortion virus to suckling Syrian hamsters. Cornell Vet. 1953 Oct;43(4):551–558. [PubMed] [Google Scholar]
- Hsiung G. D., Fischman H. R., Fong C. K., Green R. H. Characterization of a cytomegalo-like virus isolated from spontaneously degenerated equine kidney cell culture. Proc Soc Exp Biol Med. 1969 Jan;130(1):80–84. doi: 10.3181/00379727-130-33492. [DOI] [PubMed] [Google Scholar]
- Karpas A. Characterization of a new herpes-like virus isolated from foal kidney. Ann Inst Pasteur (Paris) 1966 May;110(5):688–696. [PubMed] [Google Scholar]
- PLUMMER G., WATERSON A. P. Equine herpes viruses. Virology. 1963 Mar;19:412–416. doi: 10.1016/0042-6822(63)90083-7. [DOI] [PubMed] [Google Scholar]
- RUSSELL W. C., CRAWFORD L. V. PROPERTIES OF THE NUCLEIC ACIDS FROM SOME HERPES GROUP VIRUSES. Virology. 1964 Feb;22:288–292. doi: 10.1016/0042-6822(64)90017-0. [DOI] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]