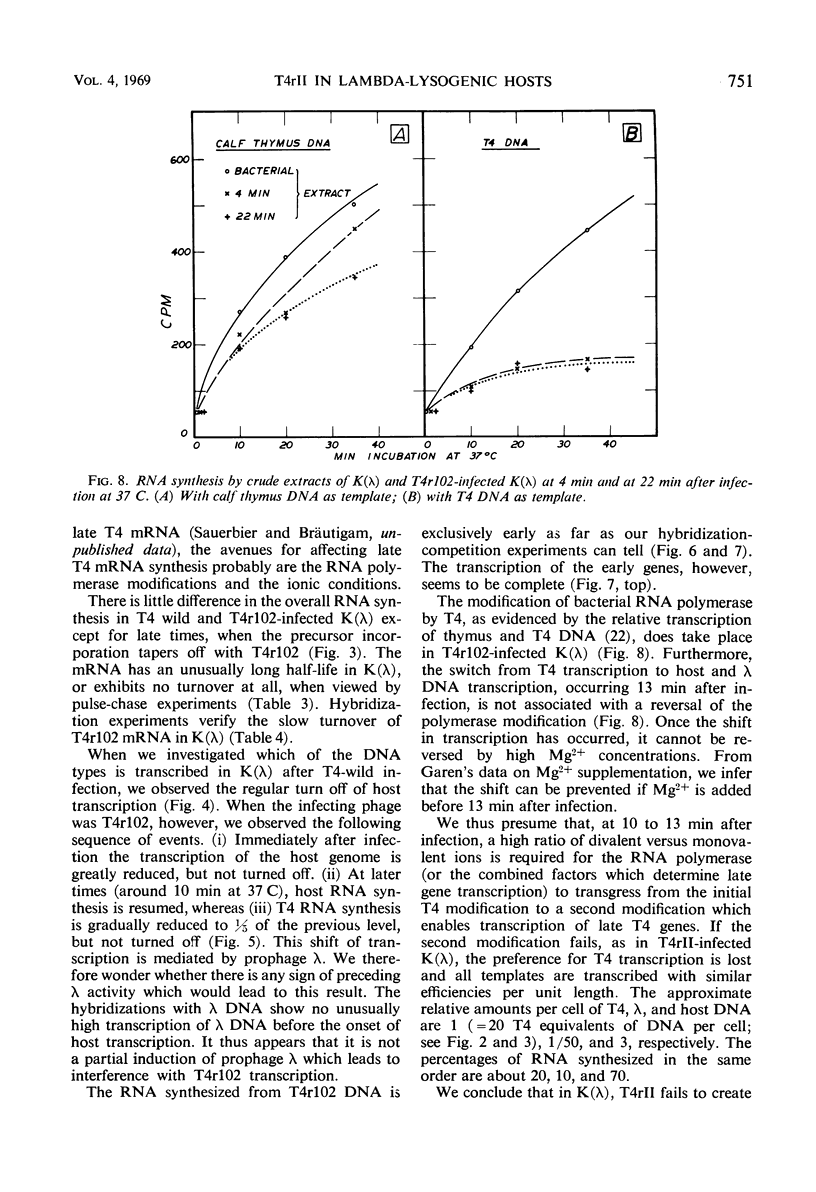
Abstract
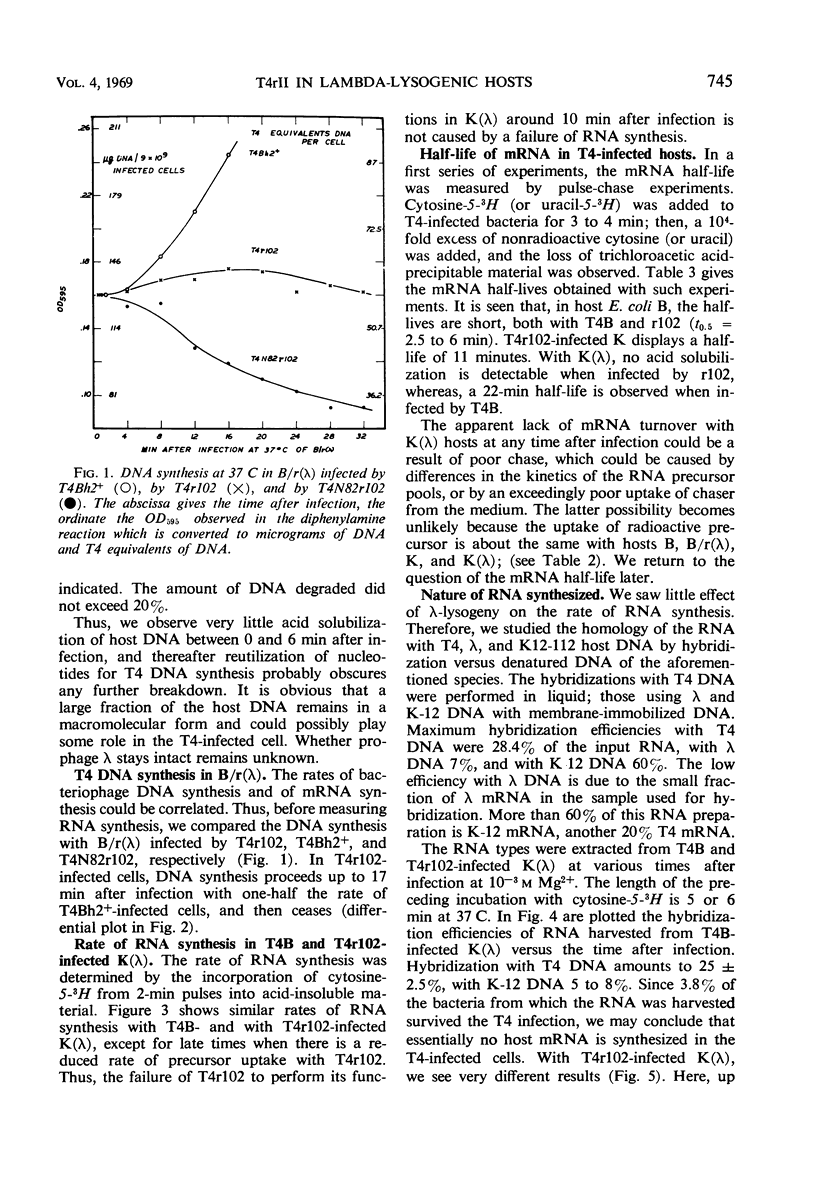
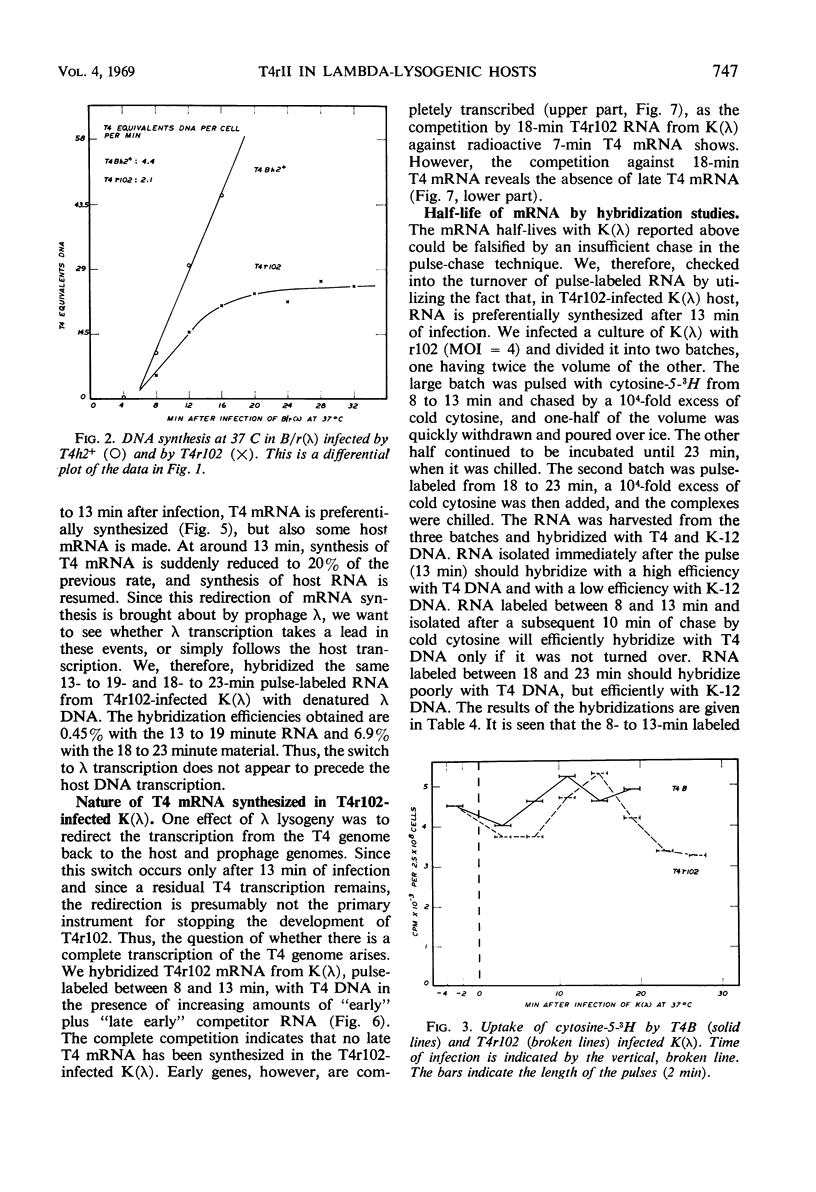
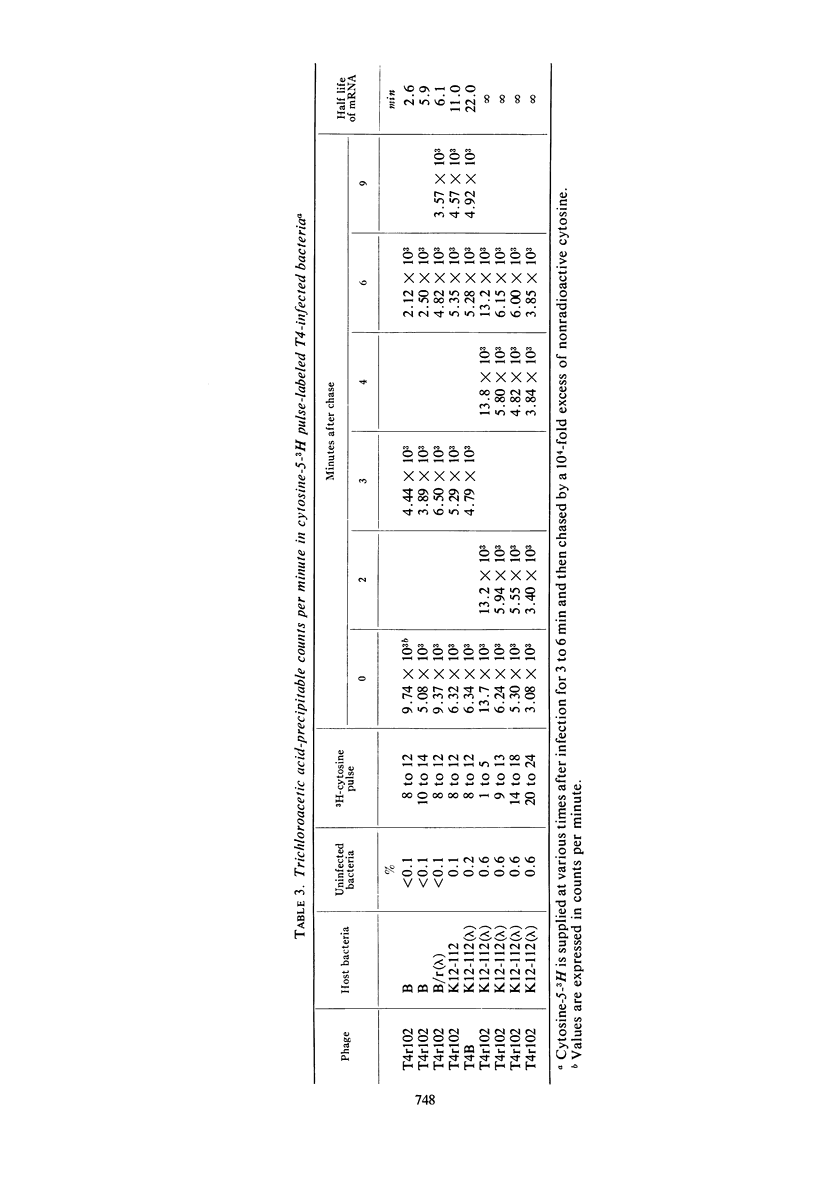
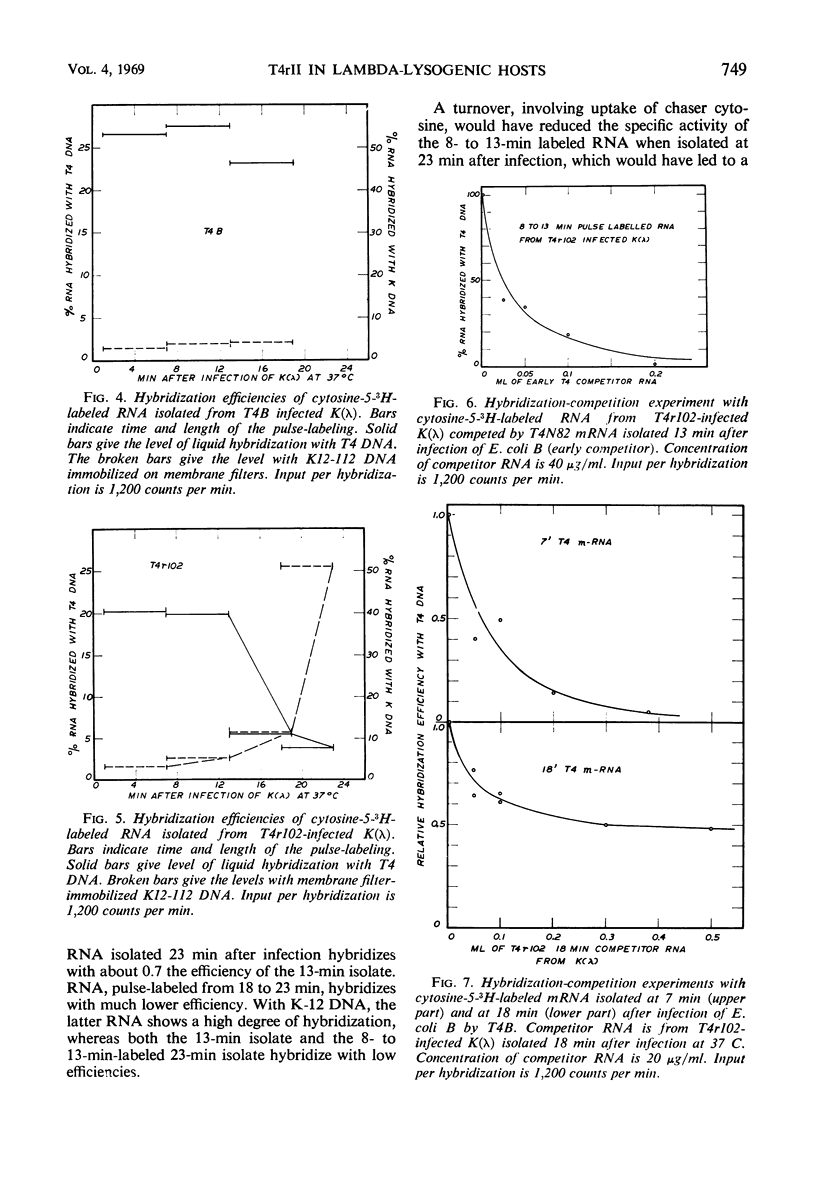
Deoxyribonucleic acid (DNA) synthesis in T4rII-infected, lambda-lysogenic strains of Escherichia coli proceeds with one-half the rate of T4 wild-infected bacteria and stops 16 min after infection at 37 C. The rates of ribonucleic acid (RNA) synthesis, however, are the same with T4rII and T4 wild. The turnover of pulse-labeled RNA is slow in K strains (half-lives 10 to 20 min) as compared with B strains (half-lives 2.5 to 6 min). Lambda-lysogeny increases the apparent messenger (m) RNA half-lives in pulse-chase experiments. The shutoff of host RNA synthesis in T4rII infected K(λ) is incomplete. Moreover, the preferential transcription of T4 DNA ceases 13 min after infection, and transcription of host and prophage λ DNA is resumed. The T4 RNA synthesized in rII-infected K(λ) contains no late T4 mRNA. The early portion of the T4 genome, however, is transcribed completely. The T4-induced early modification of bacterial RNA polymerase does occur. Resumption of host DNA transcription at 13 min after infection is not associated with a reversal of the above polymerase modification. It is concluded that in lambdalysogenic bacteria T4rII infections are abortive because RNA polymerase is prevented from transcribing late T4 genes.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROCK M. L. THE EFFECTS OF POLYAMINES ON THE REPLICATION OF T4RII MUTANTS IN ESCHERICHIA COLI K-12 (LAMBDA). Virology. 1965 Jun;26:221–227. doi: 10.1016/0042-6822(65)90049-8. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautz E. K., Bautz F. A., Dunn J. J. E. coli sigma factor: a positive control element in phage T4 development. Nature. 1969 Sep 6;223(5210):1022–1024. doi: 10.1038/2231022a0. [DOI] [PubMed] [Google Scholar]
- Benzer S. FINE STRUCTURE OF A GENETIC REGION IN BACTERIOPHAGE. Proc Natl Acad Sci U S A. 1955 Jun 15;41(6):344–354. doi: 10.1073/pnas.41.6.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDGAR R. S., LIELAUSIS I. TEMPERATURE-SENSITIVE MUTANTS OF BACTERIOPHAGE T4D: THEIR ISOLATION AND GENETIC CHARACTERIZATION. Genetics. 1964 Apr;49:649–662. doi: 10.1093/genetics/49.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchse, Millette R. L., Zillig W., Walter G. Influence of salts on RNA synthesis by DNA-dependent RNA-polymerase from Escherichia coli. Eur J Biochem. 1967 Dec;3(2):183–193. doi: 10.1111/j.1432-1033.1967.tb19514.x. [DOI] [PubMed] [Google Scholar]
- GAREN A. Physiological effects of rII mutations in bacteriophage T4. Virology. 1961 Jun;14:151–163. doi: 10.1016/0042-6822(61)90190-8. [DOI] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- HALL B. D., NYGAARD A. P., GREEN M. H. CONTROL OF T2-SPECIFIC RNA SYNTHESIS. J Mol Biol. 1964 Jul;9:143–153. doi: 10.1016/s0022-2836(64)80096-6. [DOI] [PubMed] [Google Scholar]
- Howard B. D. Phage lambda mutants deficient in r-II exclusion. Science. 1967 Dec 22;158(3808):1588–1589. doi: 10.1126/science.158.3808.1588. [DOI] [PubMed] [Google Scholar]
- KAISER A. D., JACOB F. Recombination between related temperate bacteriophages and the genetic control of immunity and prophage localization. Virology. 1957 Dec;4(3):509–521. doi: 10.1016/0042-6822(57)90083-1. [DOI] [PubMed] [Google Scholar]
- KASATIYA S. S., LIEB M. EFFECT OF HEAT SHOCK ON T4RII MULTIPLICATION IN ESCHERICHIA COLI. J Bacteriol. 1964 Dec;88:1585–1589. doi: 10.1128/jb.88.6.1585-1589.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai F., Streisinger G., Miller B. The mechanism of lysis in phage T4-infected cells. Virology. 1967 Nov;33(3):398–404. doi: 10.1016/0042-6822(67)90115-8. [DOI] [PubMed] [Google Scholar]
- NOMURA M. DNA synthesized in Escherichia coli K12 (lambda) after infection with an rII mutant of bacteriophage T4. Virology. 1961 Jun;14:164–166. doi: 10.1016/0042-6822(61)90191-x. [DOI] [PubMed] [Google Scholar]
- Rutberg B., Rutberg L. Bacteriophage-induced functions in Escherichia coli K (lambda) infected with rII mutants of bacteriophage T4. J Bacteriol. 1966 Jan;91(1):76–80. doi: 10.1128/jb.91.1.76-80.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi M. Studies on the physiological defect in rII mutants of bacteriophage T4. J Mol Biol. 1966 Apr;16(2):503–522. doi: 10.1016/s0022-2836(66)80188-2. [DOI] [PubMed] [Google Scholar]
- Tomizawa J., Ogawa T. Inhibition of growth of rII mutants of bacteriophage T4 by immunity substance of bacteriophage lambda. J Mol Biol. 1967 Jan 28;23(2):277–280. doi: 10.1016/s0022-2836(67)80033-0. [DOI] [PubMed] [Google Scholar]
- Walter G., Seifert W., Zillig W. Modified DNA-dependent RNA polymerase from E. coli infected with bacteriophage T4. Biochem Biophys Res Commun. 1968 Feb 15;30(3):240–247. doi: 10.1016/0006-291x(68)90441-5. [DOI] [PubMed] [Google Scholar]


