Abstract
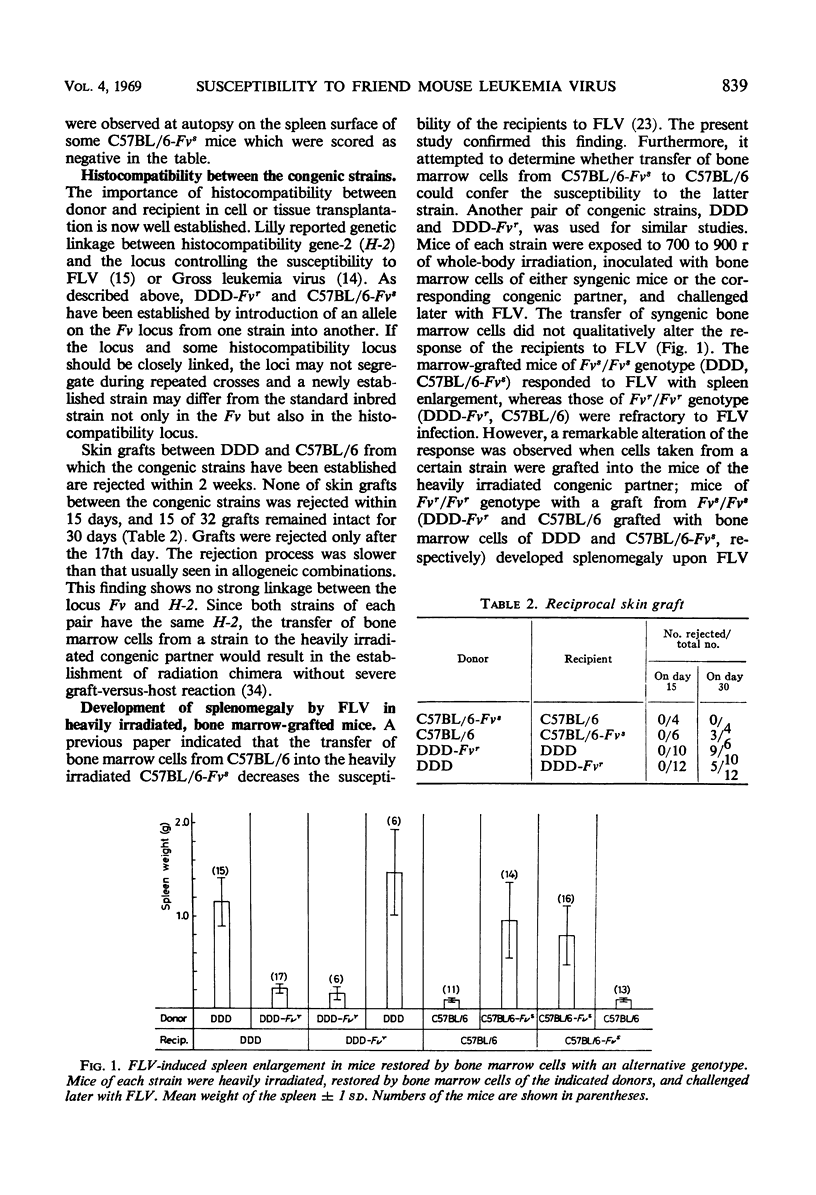
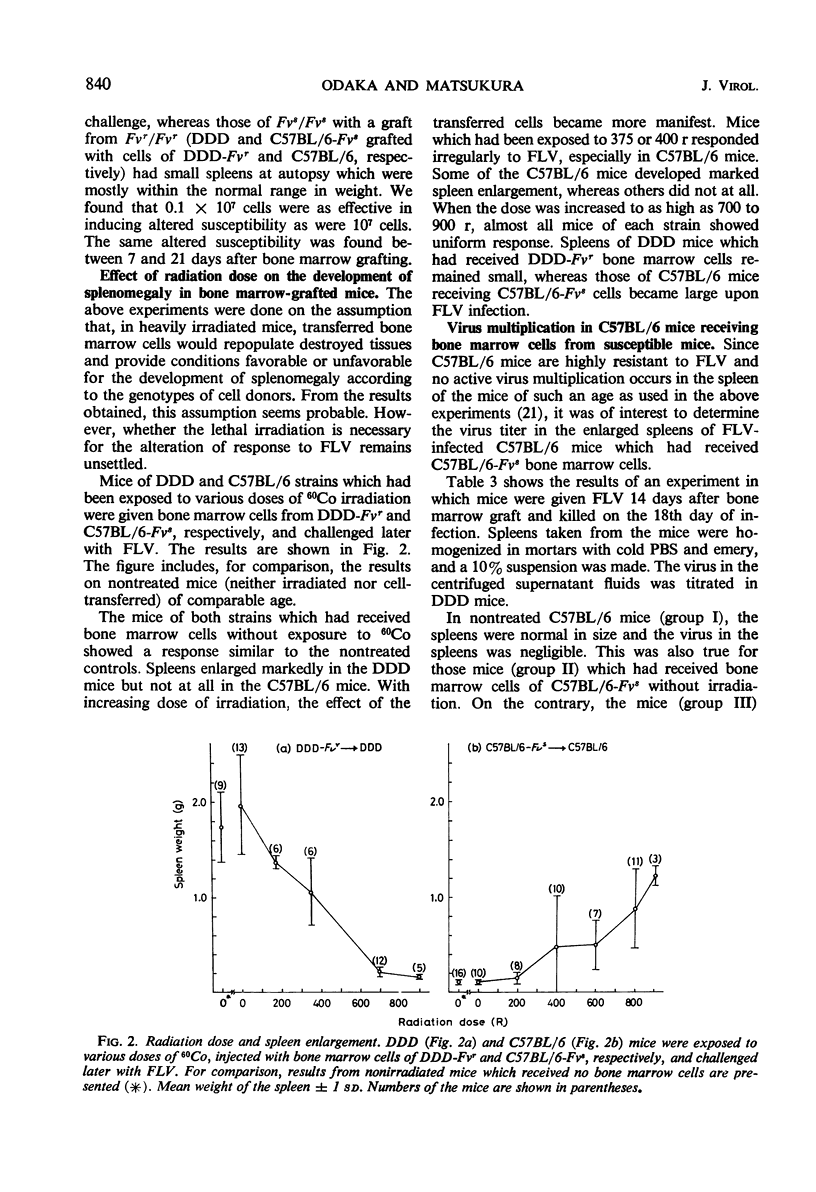
Two newly established mouse strains which are congenic with standard inbred strains were used for the study of the locus Fv which controls the susceptibility to Friend leukemia virus in mice. A strain in each congenic pair shares the major histocompatibility gene with the corresponding partner strain but differs from the latter in the Fv locus. Mice with Fvr/Fvr genotype (DDD-Fvr, C57BL/6) do not develop marked spleen enlargement upon virus challenge, whereas spleens of mice with Fvs/Fvs genotype (DDD, C57BL/6-Fvs) become large even with a virus inoculum 1/103 to 1/105 times that used for the resistant strains. Mice of each strain were heavily irradiated, inoculated with bone marrow cells taken from either syngenic or corresponding congenic mice, and challenged later with the leukemia virus. When Fvs/Fvs mice had been restored with bone marrow cells taken from Fvr/Fvr mice, the spleens remained small after the virus inoculation. In contrast, Fvr/Fvr mice receiving Fvs/Fvs cells responded to the virus with marked spleen enlargement. In the enlarged spleens of the C57BL/6 mice which do not otherwise allow the virus multiplication, a considerable amount of infectious virus was found. The altered response seems to be due to repopulation of destroyed tissues by the transplanted bone marrow cells. It is concluded that the locus Fv is expressed on hemopoietic cells, and cells derived from bone marrow play a predominant role in the development of splenomegaly by Friend leukemia virus.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdou N. I., Richter M. Cells involved in the immune response. VI. The immune response to red blood cells in irradiated rabbits after administration of normal, primed, or immune allogeneic rabbit bone marrow cells. J Exp Med. 1969 Apr 1;129(4):757–774. doi: 10.1084/jem.129.4.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrad A. Genetic and cellular basis of susceptibility or resistance to Friend leukemia virus infection in mice. Proc Can Cancer Conf. 1969;8:313–343. [PubMed] [Google Scholar]
- BERNSTEIN S. E., RUSSELL E. S. Implantation of normal bloodforming tissue in genetically anemic mice, without x-irradiation of host. Proc Soc Exp Biol Med. 1959 Aug-Sep;101:769–773. doi: 10.3181/00379727-101-25089. [DOI] [PubMed] [Google Scholar]
- Bang F. B., Warwick A. MOUSE MACROPHAGES AS HOST CELLS FOR THE MOUSE HEPATITIS VIRUS AND THE GENETIC BASIS OF THEIR SUSCEPTIBILITY. Proc Natl Acad Sci U S A. 1960 Aug;46(8):1065–1075. doi: 10.1073/pnas.46.8.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M., Steeves R. A., Cudkowicz G., Mirand E. A., Russell L. B. Mutant Sl alleles of mice affect susceptibility to Friend spleen focus-forming virus. Science. 1968 Nov 1;162(3853):564–565. doi: 10.1126/science.162.3853.564. [DOI] [PubMed] [Google Scholar]
- Crittenden L. B. Observations on the nature of a genetic cellular resistance to avian tumor viruses. J Natl Cancer Inst. 1968 Jul;41(1):145–153. [PubMed] [Google Scholar]
- De Maeyer E., De Maeyer-Guignard J. Influence of animal genotype and age on the amount of circulating interferoh induced by Newcastle disease virus. J Gen Virol. 1968 May;2(3):445–449. doi: 10.1099/0022-1317-2-3-445. [DOI] [PubMed] [Google Scholar]
- FRIEND C. Cell-free transmission in adult Swiss mice of a disease having the character of a leukemia. J Exp Med. 1957 Apr 1;105(4):307–318. doi: 10.1084/jem.105.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODMAN G. T., KOPROWSKI H. Macrophages as a cellular expression of inherited natural resistance. Proc Natl Acad Sci U S A. 1962 Feb;48:160–165. doi: 10.1073/pnas.48.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODMAN G. T., KOPROWSKI H. Study of the mechanism of innate resistance to virus infection. J Cell Comp Physiol. 1962 Jun;59:333–373. doi: 10.1002/jcp.1030590313. [DOI] [PubMed] [Google Scholar]
- Gatti R. A., Meuwissen H. J., Allen H. D., Hong R., Good R. A. Immunological reconstitution of sex-linked lymphopenic immunological deficiency. Lancet. 1968 Dec 28;2(7583):1366–1369. doi: 10.1016/s0140-6736(68)92673-1. [DOI] [PubMed] [Google Scholar]
- LINDENMANN J. INHERITANCE OF RESISTANCE TO INFLUENZA VIRUS IN MICE. Proc Soc Exp Biol Med. 1964 Jun;116:506–509. doi: 10.3181/00379727-116-29292. [DOI] [PubMed] [Google Scholar]
- Lilly F. The effect of histocompatibility-2 type on response to friend leukemia virus in mice. J Exp Med. 1968 Mar 1;127(3):465–473. doi: 10.1084/jem.127.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilly F. The inheritance of susceptibility to the Gross leukemia virus in mice. Genetics. 1966 Mar;53(3):529–539. doi: 10.1093/genetics/53.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubaroff D. M., Waksman B. H. Bone marrow as a source of cells in reactions of cellular hypersensitivity. I. Passive transfer of tuberculin sensitivity in syngeneic systems. J Exp Med. 1968 Dec 1;128(6):1425–1435. doi: 10.1084/jem.128.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathot C., Rothen A., Scher S. Strain influence on the immune response of mice to the Friend virus. Experientia. 1966 Dec 15;22(12):833–834. doi: 10.1007/BF01897448. [DOI] [PubMed] [Google Scholar]
- Micklem H. S., Ford C. E., Evans E. P., Gray J. Interrelationships of myeloid and lymphoid cells: studies with chromosome-marked cells transfused into lethally irradiated mice. Proc R Soc Lond B Biol Sci. 1966 Jul 19;165(998):78–102. doi: 10.1098/rspb.1966.0059. [DOI] [PubMed] [Google Scholar]
- Nossal G. J., Cunningham A., Mitchell G. F., Miller J. F. Cell to cell interaction in the immune response. 3. Chromosomal marker analysis of single antibody-forming cells in reconstituted, irradiated, or thymectomized mice. J Exp Med. 1968 Oct 1;128(4):839–853. doi: 10.1084/jem.128.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ODAKA T., EDAKA T., YAMAMOTTO T. Inheritance of susceptibility to Friend mouse leukemia virus. Jpn J Exp Med. 1962 Oct;32:405–413. [PubMed] [Google Scholar]
- Odaka T. Effect of bone marrow graft on the susceptibility of mice to Friend leukemia virus. Jpn J Exp Med. 1969 Feb;39(1):99–100. [PubMed] [Google Scholar]
- Odaka T. Inheritance of susceptibility to Friend mouse leukemia virus. V. Introduction of a gene responsible for susceptibility in the genetic complement of resistant mice. J Virol. 1969 Jun;3(6):543–548. doi: 10.1128/jvi.3.6.543-548.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odaka T. Inheritance of susceptibility to friend mouse leukemia virus. IV. Persistence of friend leukemia virus in C57BL-6 mice. Jpn J Exp Med. 1967 Feb;37(1):71–72. [PubMed] [Google Scholar]
- Odaka T., Yamamoto T. Inheritance of susceptibility to Friend mouse leukemia virus. 3. Susceptibility of F1 and genotypes of F2 and backcross progeny. Jpn J Exp Med. 1966 Feb;36(1):23–31. [PubMed] [Google Scholar]
- PRINCE A. M. Quantitative studies on Rous sarcoma virus. II. Mechanism of resistance of chick embryos to chorio-allantoic inoculation of Rous sarcoma virus. J Natl Cancer Inst. 1958 Apr;20(4):843–850. [PubMed] [Google Scholar]
- Rossi G. B., Friend C. Erythrocytic maturation of (Friend) virus-induced leukemic cells in spleen clones. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1373–1380. doi: 10.1073/pnas.58.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabin A. B. Nature of Inherited Resistance to Viruses Affecting the Nervous System. Proc Natl Acad Sci U S A. 1952 Jun;38(6):540–546. doi: 10.1073/pnas.38.6.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassa S., Takaku F., Nako K., Ikawa Y., Sugano H. Heme synthesis in Friend ascites tumor cells. Proc Soc Exp Biol Med. 1968 Feb;127(2):527–529. doi: 10.3181/00379727-127-32732. [DOI] [PubMed] [Google Scholar]
- Steeves R. A., Bennett M., Mirand E. A., Cudkowicz G. Genetic control by the W locus of susceptibility to (Friend) spleen focus-forming virus. Nature. 1968 Apr 27;218(5139):372–374. doi: 10.1038/218372a0. [DOI] [PubMed] [Google Scholar]
- Steeves R. A., Mirand E. A., Thomson S., Avila L. Enhancement of spleen focus formation and virus replication in Friend virus-infected mice. Cancer Res. 1969 May;29(5):1111–1116. [PubMed] [Google Scholar]
- Thomson S. A system for quantitative studies on interactions between Friend leukemia virus and hemopoietic cells. Proc Soc Exp Biol Med. 1969 Jan;130(1):227–232. doi: 10.3181/00379727-130-33527. [DOI] [PubMed] [Google Scholar]
- UPHOFF D. E., LAW L. W. An evaluation of some genetic factors influencing irradiation protection by bone marrow. J Natl Cancer Inst. 1959 Feb;22(2):229–241. [PubMed] [Google Scholar]
- Virolainen M. Hematopoietic origin of macrophages as studied by chromosome markers in mice. J Exp Med. 1968 May 1;127(5):943–952. doi: 10.1084/jem.127.5.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A. M., Till J. E., Siminovitch L., McCulloch E. A. A cytological study of the capacity for differentiation of normal hemopoietic colony-forming cells. J Cell Physiol. 1967 Apr;69(2):177–184. doi: 10.1002/jcp.1040690208. [DOI] [PubMed] [Google Scholar]
- van Furth R., Cohn Z. A. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968 Sep 1;128(3):415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]


