Abstract
Sarcopenia, the age-related loss of muscle mass and function, imposes a dramatic burden on individuals and society. The development of preventive and therapeutic strategies against sarcopenia is therefore perceived as an urgent need by health professionals and has instigated intensive research on the pathophysiology of this syndrome. The pathogenesis of sarcopenia is multifaceted and encompasses lifestyle habits, systemic factors (e.g., chronic inflammation and hormonal alterations), local environment perturbations (e.g., vascular dysfunction), and intramuscular specific processes. In this scenario, derangements in skeletal myocyte mitochondrial function are recognized as major factors contributing to the age-dependent muscle degeneration. In this review, we summarize prominent findings and controversial issues on the contribution of specific mitochondrial processes – including oxidative stress, quality control mechanisms and apoptotic signaling – on the development of sarcopenia. Extramuscular alterations accompanying the aging process with a potential impact on myocyte mitochondrial function are also discussed. We conclude with presenting methodological and safety considerations for the design of clinical trials targeting mitochondrial dysfunction to treat sarcopenia. Special emphasis is placed on the importance of monitoring the effects of an intervention on muscle mitochondrial function and identifying the optimal target population for the trial.
Keywords: mitophagy, vascular dysfunction, fusion and fission, apoptosis, biomarkers
1. Introduction
Sarcopenia, the age-associated decline in skeletal muscle mass and function (Roseberg 1989), represents a well-established risk factor for major negative health-related conditions and events, including frailty, disability, institutionalization and mortality (Visser and Schaap 2011). The increasingly recognized clinical and public health relevance of this syndrome has been leading research activities at designing and developing novel preventive and therapeutic strategies (Cesari et al. 2008). The accomplishment of such task requires a thorough understanding of the cellular processes underlying the pathogenesis of sarcopenia, in order to identify selective targets for treatment.
Numerous pathways are proposed to be implicated in the age-dependent muscle degeneration (reviewed by Marzetti et al. 2009 and Buford et al. 2010). The vital functions carried out by mitochondria in the context of energy provision, redox homeostasis, and regulation of several catabolic pathways confer these organelles a central position in the maintenance of myocyte viability. The involvement of mitochondria in the regulation of skeletal myofiber plasticity further highlights the centrality of these organelles in muscle physiology. Adult skeletal myocytes are post-mitotic cells organized in syncitia of hundreds or thousands of nuclei, where each nucleus has jurisdiction over a surrounding volume of sarcoplasm (myonuclear domain or DNA unit) (Cheek 1985). The dynamic behavior of DNA units is believed to govern muscle fiber remodeling in response to load conditions, aging and diseases (Allen et al. 1999).
Although recently called into question (Bruusgaard and Gundersen 2008; Bruusgaard et al. 2012), this concept is supported by numerous studies reporting that modifications in fiber cross-sectional area (CSA) are associated with changes in the number of myonuclei (reviewed by Teixeira and Duarte 2011). Moreover, during muscle accretion, DNA units increase their size, while smaller myonuclear domains are observed during disuse- and aging-associated muscle atrophy (Van der Meer et al. 2011). A wealth of experimental evidence indicates that mitochondria are central in the regulation of myonuclear domain both in physiological and pathological conditions (reviewed by Calvani et al. 2013a). As such, mitochondrial decay is advocated as one of the major factors driving muscle aging (Johnson et al. 2013).
The interpretation of the consequences of mitochondrial dysfunction on muscle physiology is complicated by the existence of two populations of mitochondria in skeletal myocytes: subsarcolemmal mitochondria (SSM), located beneath the plasma membrane and accounting for approximately 20% of total mitochondrial mass, and interfibrillar mitochondria (IFM), arranged between the myofibrils and representing the remaining 80% of myofiber mitochondrial volume (Hoppeler 1986). These two subpopulations possess specific biochemical and functional properties and exhibit a distinct behavior during aging (Koves et al. 2005; Ferreira et al. 2010). For instance, SSM isolated from old muscles produce greater amounts of reactive oxygen species (ROS) and show higher rates of fragmentation and degradation relative to the IFM subfraction (Riley et al. 1990; Chabi et al. 2008; Seo et al. 2008; Wagatsuma et al. 2011). On the other hand, IFM are more prone than SSM to releasing apoptotic mediators under cell death stimuli (Adhihetty et al. 2005). These divergent properties raise the possibility that the two mitochondrial populations could be differentially involved in the pathogenesis of sarcopenia.
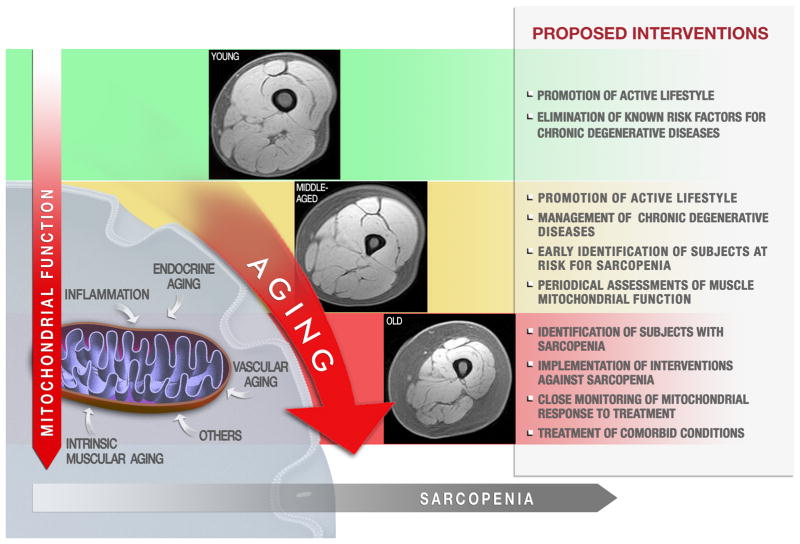
A further level of complexity is added by the fact that both mitochondrial function and muscle trophism are influenced by physical activity and anabolic hormones, which both decline with aging (Dela and Helge 2013). Other age-related processes, such as chronic, low-grade inflammation and vascular alterations, can also affect both muscle health and mitochondrial function (Terjung et al. 2002; Jensen 2008). Hence, segregating the impact of age per se from that of lifestyle habits and age-associated conditions on muscle pathophysiology poses a relevant challenge (Fig. 1).
Fig. 1.
Hypothetical progression of mitochondrial dysfunction during the development sarcopenia and possible windows for interventions. Multiple, interrelated factors can impact muscle mitochondria function over the life course, including intrinsic muscular aging, lifestyle habits, chronic inflammation, vascular dysfunction, hormonal changes, etc. Young individuals should be advised to adopt a healthy lifestyle, avoiding all of the known risk factors for chronic degenerative diseases. At middle age, subjects at risk for sarcopenia should be promptly identified, for instance through the computation of a “sarcopenia risk chart” (Calvani et al. 2013b). Eventual chronic degenerative diseases associated with accelerated development and/or progression of sarcopenia need to be managed appropriately. Periodical assessments of muscle mitochondrial function may allow detecting early signs of dysfunction. Once the presence of sarcopenia has been established, interventions must be implemented. A hypothetical treatment targeting mitochondria requires a close monitoring of mitochondrial response to the intervention. Artwork by Francesco Antognarelli.
In this review, we illustrate relevant mechanisms that are proposed to underlie the relationship among aging, muscle mitochondrial dysfunction and sarcopenia. First, we present a brief overview on the evidence linking mitochondrial dysfunction to human muscle aging. We then discuss prominent mitochondrial pathways that have been implicated in the pathogenesis of sarcopenia. Subsequently, we summarize extramuscular factors than can have an impact on myocyte mitochondrial function, thus potentially serving as targets for anti-sarcopenic interventions. Finally, methodological considerations for the design of clinical trials on mitochondrial dysfunction and sarcopenia are presented.
2. Involvement of mitochondrial dysfunction in muscle aging: evidence from human studies
One major consequence of the age-associated mitochondrial dysfunction is a decline in bioenergetics. Both resting and maximal oxygen (O2) consumption decreases with advancing age (Short et al. 2004). This decline is independent of fat-free mass, indicating that either muscle mitochondrial function or content (or both) is reduced as a function of age.
Studies on muscle specimens from healthy individuals have revealed age-related declines in mitochondrial mass (Welle et al. 2003), activities of tricarboxylic acid cycle enzymes (Crane et al. 2010), O2 consumption (Joseph et al. 2012; Coen et al. 2013), and ATP synthesis (Short et al. 2005). Moreover, a 40–50% decrease in oxidative phosphorylation (OXPHOS) activity has been detected in vivo via 31P nuclear magnetic resonance (31P-NMR) spectroscopy in older persons compared with younger controls (Conley et al. 2000; Petersen et al. 2003).
The impact of mitochondrial bioenergetic decline on muscle aging is witnessed by the existence of a correlation between ATP synthesis/O2 consumption and preferred walking speed in healthy elderly (Coen et al. 2013). In this regard, it is noteworthy that slow walking speed has been adopted as a defining criterion for sarcopenia (Cruz-Jentoft et al. 2010) and physical frailty (Fried et al. 2001). Another mechanism linking mitochondrial dysfunction to sarcopenia is the possible impact of ATP shortage on protein synthesis, which is reflected by the concomitant decrease in whole-body bioenergetics and muscle protein anabolism over the course of aging (Short et al. 2004). As discussed further on, the bioenergetic failure of the aged muscle is likely the result of a vicious cycle involving oxidant production, damage and depletion of mitochondrial DNA (mtDNA), and defective mitochondrial quality control (MQC) (Brunk and Terman 2002) (Fig. 2).
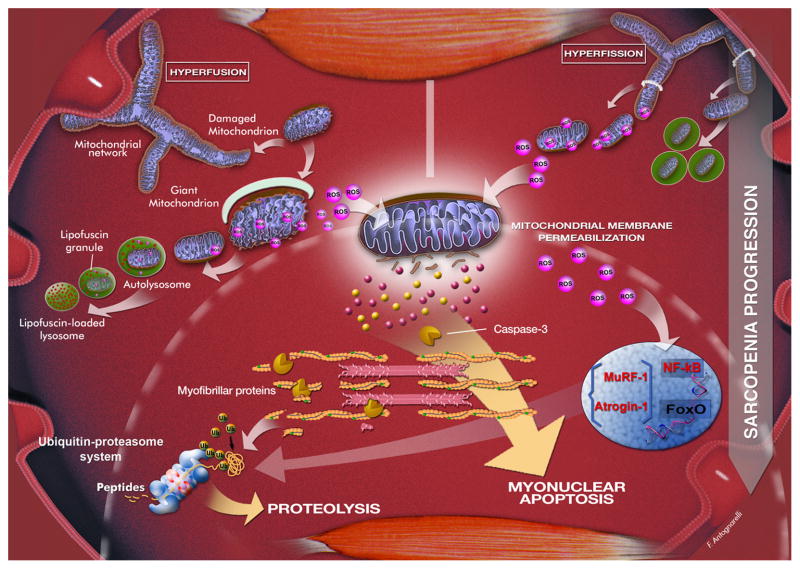
Fig. 2.
Possible scenarios resulting from mitochondrial quality control failure during the progression of sarcopenia. An imbalance in mitochondrial dynamics towards fusion is associated with the appearance of giant mitochondria, characterized by highly interconnected networks, aberrant morphology, reduced bioenergetic efficiency, and increased ROS production. Enlarged mitochondria cannot be efficiently removed due to their larger size. The accumulation of lipofuscin within lysosomes further contributes to impairing the autophagosomal-lysosomal axis. Oxidants generated by dysfunctional mitochondria compromise the surrounding tissue and amplify mitochondrial damage, eventually triggering apoptosis and proteolysis via ROS-mediated activation of nuclear factor κB (NF-κB) and Forkhead box O (FoxO) (Dodd et al. 2010). These transcription factors stimulate the expression of the muscle-specific ubiquitin ligases atrogin-1 and muscle-specific RING finger 1 (MuRF-1). Protein fragments derived from the action of caspase-3 on actomyosin complexes are eventually degraded by the ubiquitin-proteasome system. A shift of dynamics towards fission leads to mitochondrial network disintegration and overactivation of mitophagy. ROS generation by fragmented mitochondria is increased, which together with the upregulation of fission, stimulates muscle protein breakdown and myonuclear apoptosis through mechanisms similar to those described above. Artwork by Francesco Antognarelli.
A relevant consequence of mitochondrial dysfunction is the activation of apoptosis, a mechanism believed to represent a final common pathway through which sarcopenia and physical frailty ensue (Marzetti and Leeuwenburgh 2006) (Fig. 2). This idea is supported by the observation that mitochondrial apoptotic signaling correlates with slow walking speed and reduced muscle volume in older persons (Marzetti et al. 2012d).
3. Mechanisms and consequences of mitochondrial dysfunction in the sarcopenic muscle
3.1. The vicious cycle between oxidative stress and mitochondrial dysfunction in the aged muscle
Mitochondria are at the same time the major source of oxidants within cells and a primary target of oxidative stress. The mtDNA is intrinsically vulnerable to oxidative damage due to its proximity to the source of oxidants, the absence of histones and introns, and a less robust repair system compared with nuclear DNA (Yakes and Van Houten 1997). According to the widely accepted mitochondrial free radical theory of aging (MFRTA), mitochondrial dysfunction arising from oxidative damage to mtDNA would trigger a vicious cycle in which the synthesis of defective ETC subunits, results in OXPHOS impairment, decreased ATP production and further ROS generation (Harman 1972; Miquel et al. 1980).
The involvement of the mitochondrial free radical vicious cycle in muscle aging is supported by findings in preclinical models (Lee et al. 1998; Wanagat et al. 2001; Figueiredo et al. 2009; Lee et al. 2010) and humans (Bua et al. 2006). In an elegant study, Bua et al. (2006) analyzed the occurrence of ETC abnormalities and the abundance of mtDNA deletion-mutations along the length of laser-captured microdissected fibers isolated from human vastus lateralis (VL) muscle specimens. The number of fibers harboring ETC abnormalities, identified via histochemical staining for cytochrome c oxidase (COX) and succinate dehydrogenase (SDH) activities, increased from 6% at 49 years of age to 31% at 92 years. Fibers displaying a COX negative and SDH hyper-reactive (COX−/SDH++) phenotype co-localized with clonal expansions of somatically-derived mtDNA deletion-mutations and were more frequent in fiber segments with morphological aberrations. Conversely, mtDNA mutations and ETC abnormalities were absent in phenotypically normal regions within individual fibers.
The observation that sarcopenia develops prematurely in mice that express an error-prone mtDNA polymerase-γ has provided the proof of principle linking mtDNA damage, mitochondrial dysfunction and muscle atrophy (Kujoth et al. 2005; Dai et al. 2010; Hiona et al. 2010). Similar findings have recently been obtained in mice with double-strand mtDNA breaks caused by the transient expression of a mitochondrial-targeted endonuclease (PstI) (Wang et al. 2013).
The MFRTA suffers however a major pitfall: within cells, mitochondria fuse continuously with one another forming large syncytia. As a result, a constant mixing of the total cellular mtDNA pool occurs, disrupting the connection between genotype (damaged mtDNA) and phenotype (defective OXPHOS and increased ROS emission) (Sato et al. 2006). Nevertheless, the hypothesis linking mtDNA damage, ETC dysfunction and abnormal ROS generation may still retain validity inasmuch as MQC processes become inefficient in advanced age, as discussed in the next section.
3.2. Defective mitochondrial quality control in the sarcopenic muscle
Mitochondrial structural and functional integrity relies on the efficiency of quality control processes, including intramitochondrial oxidant-scavenging systems, protein repair and degradation pathways, and mitochondrial dynamics and turnover (reviewed by Twig et al. 2008b). MQC is especially relevant to the homeostasis of skeletal myocytes, given their high reliance on OXPHOS for energy provision and their post-mitotic nature, which impedes the dilution of mitochondrial dysfunction via cell division. Due to these vital responsibilities, the disruption of MQC processes is invoked as a mechanism contributing to muscle loss associated with aging and other atrophying conditions (Calvani et al. 2013a) (Fig. 2).
3.2.1 Mechanisms and consequences of altered mitochondrial dynamics in the aging muscle
Contrary to the traditional view of isolated, ovoidal organelles, mitochondria are indeed dynamic entities that form constantly changing networks within cells (Aon 2010). Mitochondrial dynamics are regulated by coordinated fusion and fission cycles performed by a complex molecular machinery (reviewed by Youle and van der Bliek 2012). Simplified, fusion allows joining mitochondria to mix their contents, thereby redistributing metabolites, proteins and mtDNA, and equilibrating the concentrations of nuclear-encoded proteins across organelles (Ono et al. 2001). Fission segregates components of the network that are irreversibly damaged or unnecessary, for subsequent removal (Twig et al. 2008a).
Derangements in fusion-fission have been proposed as a mechanism underlying the formation of aberrant mitochondria under stress conditions or during senescence (Yoon et al. 2006) (Fig. 2). Giant, dysfunctional mitochondria, characterized by highly interconnected networks and ultrastructural abnormalities, are frequently encountered in aging muscles (Beregi and Regius 1987). Remarkably, the exposure of cultured cells to subcytotoxic doses of hydrogen peroxide (H2O2) represses the expression of fission protein 1 (Fis1), thereby promoting the formation of elongated mitochondria with increased oxidant emission (Yoon et al. 2006). This finding, together with the observation that enlarged mitochondria accumulate in old skeletal myocytes, suggests that an imbalance in mitochondrial dynamics towards fusion might occur in the aged muscle (Fig. 2).
It is conceivable that mitochondrial hyperfusion could represent an attempt to cope with increased levels of mtDNA mutations through the dilution of mutant genomes along the network (Sato et al. 2006). In support to this hypothesis, disruption of mitochondrial fusion via mitofusin (Mfn) 1 deletion was found to increase mitochondrial dysfunction and lethality in mtDNA-mutator mice (Chen et al. 2010). Mitochondrial fragmentation and downregulation of fusion have also been detected in muscles from type-2-diabetic and obese subjects, in conjunction with impaired bioenergetics (Bach et al. 2005). It is worth mentioning that both diabetes mellitus and obesity are associated with accelerated development and/or progression of sarcopenia (Buford et al. 2010) (Fig. 1). Interestingly, depletion of the mitochondrial fusion factor optic atrophy protein 1 disintegrates the mitochondrial network and sensitizes cultured cells to apoptosis (Lee et al. 2004). On the contrary, blockade of Fis1 or dynamin-related protein 1 (Drp1) inhibits mitochondrial fragmentation and the execution of apoptosis in cell culture systems (Frank et al. 2001; Lee et al. 2004). Therefore, mitochondrial dynamics and apoptotic signaling appear to be intimately interconnected, which may be crucial for the modulation of cell death/survival pathways.
Mitochondrial hyperfusion has an important drawback. Severely damaged organelles within the network may be hard to single out by fission, thereby hindering their disposal. It can therefore be speculated that upregulation of fusion and/or downregulation of fission could allow maintaining myocyte viability until a critical threshold of pathogenic mtDNA is breached and the degree of mitochondrial interconnection becomes incompatible with the removal of damaged mitochondria (Calvani et al. 2013a) (Fig. 2). On a clinical level, this course of events could translate into a progressively reduced muscular performance and, eventually, the development of muscle atrophy.
In overt contrast to the mitochondrial hyperfusion hypothesis, recent findings show that fission signaling may indeed be upregulated in muscles from old rats, as evidenced by the higher expression of Fis1 and Drp1 relative to younger controls (Iqbal et al. 2013). A concomitant downregulation of Mfn2 is also reported, suggestive of decreased fusion. In addition, O’Leary et al. (2013) found that the expression of both fission and fusion proteins was upregulated in muscles from old rats. It should however be noted that in both of the aforementioned studies experiments were restricted to the SSM population, which does not allow extending those results to the more abundant IFM fraction.
An excessive activation of fission can also induce mitochondrial dysfunction and muscle atrophy (Fig. 2), at least under certain experimental paradigms. Using a transgenic mouse model, Romanello et al. (2010) showed that the expression of the fission machinery was sufficient to induce mitochondrial dysfunction, remodeling of the mitochondrial network, protein breakdown and fiber atrophy. Blockade of mitochondrial fission, in contrast, resulted in significant protection from atrophy during fasting as well as in mice with constitutively active Forkhead box O3 (FoxO3), a transcription factor that controls major catabolic pathways in skeletal myocytes (Sandri 2013).
In conclusion, the essential functions of mitochondrial morphogenesis in the context of MQC and the intersection with proteolytic and apoptotic pathways candidate mitochondrial shaping proteins as potential targets for interventions against sarcopenia. Further research is necessary to address several critical issues. First, it must be established whether distinct fusion and fission properties exist between IFM and SSM, which may help explain their divergent behavior during muscle aging. Second, the complex relationship among mitochondrial dynamics, muscle protein metabolism and apoptotic signaling needs to be fully deciphered. Lastly, studies are warranted to characterize the age-related changes in mitochondrial dynamics and their impact on muscle trophism in humans.
3.2.2. Contribution of impaired mitochondrial turnover to muscle aging
Mitochondrial renewal is performed by two opposing, yet coordinated processes: the degradation of dysfunctional or unnecessary mitochondria through a specialized form of autophagy (mitophagy) and the generation of new organelles via biogenesis. The description of the molecular pathways involved in mitophagy and mitochondrial biogenesis is beyond the scope of the present review, and the reader is referred elsewhere (for instance, Jornayvaz and Shulman 2010 and Bhatia-Kissova and Camougrand 2013).
Accumulating evidence indicates that mitochondrial turnover is altered during muscle aging, potentially affecting mitochondrial function and myocyte homeostasis (reviewed by Calvani et al. 2013a and Vina et al. 2009). A reduced expression of peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α), indicative of decreased mitochondriogenesis, has been detected in muscles of old experimental animals (Koltai et al. 2012) and elderly persons (Safdar et al. 2010). Disruption of PGC-1α signaling also influences protein metabolism. Indeed, PGC-1α attenuates muscle protein degradation by the ubiquitin-proteasome system (UPS) through blocking the activities of nuclear factor κB (NF-κB) and FoxO3 (Sandri et al. 2006; Brault et al. 2010). Furthermore, a splice variant of PGC-1α, PGC-1α4, stimulates protein synthesis and suppresses myostatin signaling and UPS activity in cultured myotubes and the murine tibialis anterior (TA) muscle (Ruas et al. 2012). The expression of both PGC-1α and PGC-1α4 is upregulated by physical exercise (Ruas et al. 2012), suggesting that, in older individuals, the beneficial effects of exercise training on muscle mitochondria biogenesis and protein metabolism could be mediated, at least in part, by the restoration of PGC-1α signaling. This hypothesis deserves further investigation.
A decline in autophagic function is a common trait of the aging process (Rajawat et al. 2009). This phenomenon, together with the progressive accumulation of macromolecular and organellar damage over the life course, has led to the proposition that impairments in the autophagosomal-lysosomal pathway could be centrally involved in the aging process (Brunk and Terman 2002). The autophagic failure experienced by old skeletal myocytes could be linked to the intralysosomal buildup of a non-degradable pigment called lipofuscin (Orlander et al. 1978; Terman and Brunk 2004) (Fig. 2). Lipofuscin-induced lysosomal clogging and mitochondrial decay concur to a self-perpetuating process in which mitochondrial ROS promote lipofuscinogenesis, while the accumulation of lipofuscin granules compromises mitochondrial autophagy (Terman et al. 2010). This vicious cycle forms the basis for the garbage catastrophe theory of aging, also known as the mitochondrial-lysosomal axis theory of aging (Brunk and Terman 2002).
Members of our group have shown that advanced age is associated with reduced transcript levels of the autophagic factor lysosomal-associated membrane protein 2 (LAMP-2) in the rat plantaris muscle, suggestive of decreased autophagy (Wohlgemuth et al. 2010). Life-long mild calorie restriction prevented the age-related decline in LAMP-2 expression, which was associated with an attenuation of oxidative stress and apoptotic DNA fragmentation. These data are in agreement with those by Russ et al. (2012) who showed that the ratio between microtubule-associated protein 1 light chain 3 (LC3)-II and LC3-I, an index of ongoing autophagy (Kadowaki and Karim 2009), was reduced in the gastrocnemius muscle of old rats, which was partially restored by voluntary wheel running. In another recent study, a 6-month weight loss program combined with moderate intensity exercise increased the mRNA abundance of LC3B, autophagy-related (Atg) protein 7 and LAMP-2 in the VL muscle of overweight older women (Wohlgemuth et al. 2011). The intervention also upregulated the gene expression of the mitochondrial biogenesis markers PGC-1α and mitochondrial transcription factor A (TFAM). Finally, Luo et al. (2013) found that 9 weeks of resistance training increased the expression of several autophagy regulatory proteins, including Beclin1, Atg5 and Atg7, and the lysosomal hydrolase cathepsin L in the gastrocnemius muscle of old rats. These adaptations were accompanied by downregulation of mitochondrial apoptotic signaling and improvements in muscle mass and strength. Hence, the stimulation of mitochondrial turnover appears to mediate, at least partly, the beneficial effect of exercise and weight loss on the skeletal muscle. In keeping with this idea, the abrogation of stress-induced autophagy (He et al. 2012) or the inhibition of mitochondrial biogenesis (Geng et al. 2010) has been shown to prevent several muscular metabolic adaptations to exercise training.
In contrast to the aforementioned studies, Wenz et al. (2009) found that the LC3-II/LC3-I ratio was increased in the biceps femoris muscle of old mice compared with younger controls. Mitochondrial damage and upregulation of autophagic markers were not detected in muscles of old mice with PGC-1α overexpression. It is possible that PGC-1α could intervene in the mitochondrial-lysosomal vicious cycle by promoting mitochondrial renewal and improving antioxidant defenses, eventually preventing the generation of lipofuscin. Consistent with these findings, the protein expression of Atg7 was shown to be elevated in the extensor digitorum longus muscle of old rats (O’Leary et al. 2013). The mitochondrial localization of Parkin, a mediator recruited to damaged mitochondria assisting in their mitophagic removal (Narendra et al. 2008), was also increased in aged animals (O’Leary et al. 2013). Finally, the protein content of the autophagic regulators Atg7 and Beclin1 was found to be higher in VL muscle samples of healthy older persons compared with younger controls, although the LC3-II/LC3-I ratio was unvarying (Fry et al. 2013).
These contrasting findings reflect the intricate relationships between mitochondrial (dys)function and autophagy during muscle aging. The interpretation of the effects of aging and behavioral interventions on mitochondrial autophagy in muscle is also hampered by the lack of use in most studies of standardized methods for monitoring this cellular pathway (Klionsky et al. 2012). The need for reliable biomarkers of the autophagic flux, combined with measures of mitochondrial network function, is especially stringent when considering that mitochondrial autophagy could follow a biphasic trajectory during muscle aging. A compensatory upregulation of autophagy may be put forward to cope with the progressive accumulation of dysfunctional mitochondria. Over the long term, this adaptation may impair the reserve capacity of the autophagic system that could therefore become unable to face an eventual additional perturbation (Kriete 2013). This view is supported by the observation that, in spite of an increased basal autophagic activation, old rat muscles cannot further upregulate autophagy during protracted immobilization (O’Leary et al. 2013).
The accumulation of lipofuscin granules into a growing number of lysosomes contributes to impairing the autophagosomal degradative capacity (Terman et al. 2010). Lipofuscin-loaded lysosomes consume a large part of newly produced hydrolases that, however, cannot digest lipofuscin (Fig. 2). At the same time, a smaller amount of lysosomal enzymes remains available for autophagic pathways, including mitophagy. The “futile cycle” of autophagic activation eventually leads to the collapse of the cell’s catabolic machinery (Brunk and Terman 2002). Bearing these considerations in mind, a “snapshot” assessment of protein or gene expression levels of autophagic mediators may not provide sufficient information on the efficiency of this pathway. Future studies adopting standardized methods to measure autophagy (Klionsky et al. 2012), in combination with assessments of mitochondrial network health, are required to conclusively establish whether targeting mitophagy to ameliorate MQC provides therapeutic gain against sarcopenia.
3.2.3. Mitochondrion-mediated apoptosis: a final common pathway in sarcopenia of aging?
According to the myonuclear domain paradigm, the elimination of myonuclei via an apoptosis-like process mediates the removal of DNA units during muscle atrophy (Allen et al. 1999) (Fig. 2). The hypothesis is supported by numerous studies showing that the extent of apoptotic DNA fragmentation increases in skeletal muscle over the course of aging, paralleling the development of sarcopenia and physical frailty (reviewed by Marzetti et al. 2010b). Given the central role of mitochondria in the integration of apoptotic signaling and the decline in muscle mitochondrial function with age, a great deal of research has been devoted to exploring the contribution of mitochondrion-mediated apoptosis to the pathogenesis of sarcopenia. Detailed descriptions of the mitochondrial apoptotic machinery are available in specialized reviews (for instance, Wang and Youle 2009).
Studies in rodent muscles have shown that advanced age is associated with changes in the expression pattern of B-cell leukemia-2 (Bcl-2) family proteins that favor outer mitochondrial membrane (OMM) permeabilization (Alway et al. 2002; Pistilli et al. 2006; Rice and Blough 2006; Song et al. 2006; Braga et al. 2008; Carter et al. 2011). An enhanced susceptibility towards opening of the permeability transition pore has also been demonstrated in muscles from old rats (Chabi et al. 2008; Seo et al. 2008; Picard et al. 2011). Noticeably, markers pertaining to both mitochondrial caspase-dependent (e.g., cytosolic cytochrome c and active caspase-9) and independent pathways [i.e., cytosolic and/or nuclear levels of apoptosis-inducing factor (AIF) and endonuclease G (EndoG)] are elevated in aged muscles (reviewed by Marzetti et al. 2010a).
Initial evidence on the possible involvement of mitochondrion-mediated apoptosis in human muscle aging has recently been reported. Park et al. (2010) found increased gene expression of AIF in the semitendinosus muscle of middle-aged men relative to younger controls, with no changes in caspase-dependent apoptotic signaling. However, since only mRNA levels of apoptotic regulators were assessed, these findings do not allow drawing conclusions about the actual activation of apoptosis. The involvement of caspase-independent apoptosis in human muscle aging is not supported by a recent study from our group, showing that only mediators pertaining to caspase-dependent apoptotic signaling are correlated with sarcopenia indices (i.e., muscle volume and gait speed) in VL muscle samples from community-dwelling elders (Marzetti et al. 2012d). The divergence between the two studies may be ascribed to methodological differences as well as to the different age of participants. The discrepancy also reflects the complexity of mitochondrion-mediated apoptosis regulation in the setting of human muscle aging.
Although substantial evidence supports the involvement of mitochondrial apoptotic signaling in sarcopenia, recent studies reported no apoptotic loss of myonuclei in murine muscles atrophied by denervation, nerve impulse block, or mechanical unloading (Bruusgaard and Gundersen 2008; Bruusgaard et al. 2012). Imaging of single fibers by in vivo time-lapse microscopy revealed no myonuclear depletion in either slow- or fast-twitch muscles, despite a 50% reduction in muscle CSA. The different timeframes during which acute atrophy and sarcopenia develop could explain these controversial findings. While apoptosis of myonuclei may be negligible in the short term, chronic atrophying stimuli have shown to induce a significant decrease in myonuclear count (Viguie et al. 1997). In addition, the young age of the mice used by Bruusgaard and colleagues could have protected skeletal myofibers against myonuclear apoptosis. In this regard, Salucci et al. (2013) found that well-functioning autophagy preserved the viability of C2C12 myotubes by conferring resistance towards pro-apoptotic stimuli. Accordingly, the induction of autophagy by resistance exercise training has been associated with downregulation of mitochondrial apoptotic signaling in the gastrocnemius muscle of old rats (Luo et al. 2013). It may therefore be hypothesized that the age-related decline in autophagic efficiency could render skeletal myocytes more prone to apoptosis, therefore providing an additional explanation for the increased severity of apoptosis experienced by old muscles.
The contribution of apoptosis to sarcopenia is strongly supported by the observation that both the activation of apoptosis and the severity of atrophy are generally greater in muscles with fast-twitch fiber dominance relative to those mainly populated by slow-twitch fibers (Marzetti and Leeuwenburgh 2006). Moreover, behavioral, pharmacological or genetic interventions proven to be effective at rescuing muscle mass and function in old age have also been shown to mitigate the severity of apoptosis (Marzetti et al. 2012b). Should the myonuclear domain paradigm be confuted, apoptotic signaling could still be involved in the pathogenesis of sarcopenia. Indeed, the activation of caspase-3, the final effector of intrinsic and extrinsic apoptotic pathways, is required for the initial breakdown of actomyosin complexes, yielding protein fragments of manageable size for degradation by the UPS (Du et al. 2004) (Fig. 2).
In conclusion, further research is required to clearly establish a mechanistic link between apoptosis and muscle atrophy, identify the most relevant apoptotic pathway(s) to target, and determine the optimal timing for intervention. Studies are also needed to investigate whether the network arrangement of mitochondria facilitates the propagation of the apoptotic signaling under severe muscle-atrophying conditions. The occurrence of such an amplification could provide a mechanistic explanation for the higher degree of atrophy experienced by older adults during periods of protracted disuse (e.g., prolonged bed rest) (Kortebein et al. 2007), therefore indicating new targets for treatment.
4. Impact of age-related conditions on muscle mitochondrial function: targeting extramuscular processes to treat sarcopenia
Muscle trophism and mitochondrial homeostasis are governed by complex interactions between intrinsic and extramuscular processes. Discerning the relative contribution of these factors to the pathogenesis of sarcopenia is a crucial issue researchers in the field need to address. The existence of an extramuscular component in the sarcopenia syndrome is supported by studies on muscle regeneration showing that the reduced regenerative potential of cross-transplanted muscle grafts is largely dictated by the age of the host rather than by the donor’s age (Carlson and Faulkner 1989 and 1996). The involvement of extrinsic factors in muscle aging is reinforced by findings in a model of heterochronic parabiosis where young and old mice shared the circulatory system (Conboy et al. 2005). The exposure of old muscles to blood supply from a young mouse restored the proliferative and regenerative capacity of aged satellite cells without recruitment of young cells from the shared circulation. These observations indicate that extramuscular factors have a great impact on muscle trophism and may therefore provide valuable targets for the prevention and treatment of sarcopenia. As discussed in the following subsections, several extrinsic processes may impact muscle trophism by acting directly or indirectly on mitochondria.
4.1. Chronic, low-grade inflammation
Systemic subacute inflammation is a salient characteristic of the aging process that has been implicated in the development of a number of chronic degenerative diseases, including atherosclerosis, osteoarthritis, cancer, diabetes, osteoporosis, cardiovascular diseases, and dementia (reviewed by Chung et al. 2009). Chronically elevated circulating levels of several pro-inflammatory mediators, most notably interleukin 6, C-reactive protein and tumor-necrosis factor α (TNF-α), have also been associated with the development of sarcopenia and physical frailty (Ferrucci et al. 2002; Visser et al. 2002; Cesari et al. 2005).
The induction of muscle protein breakdown has long been considered to be the major pathway underlying the relationship between inflammation and sarcopenia (Combaret et al. 2009). More recently, a link among inflammation, mitochondrial dysfunction and oxidative stress has been proposed (Salvioli et al. 2006; De la Fuente and Miquel 2009), possibly providing a further mechanistic explanation for the association between inflammation and sarcopenia.
One pathway through which inflammation may impact muscle mitochondrial function is the nitric oxide (NO•) signaling. NO• exerts important actions on mitochondria by modulating biogenesis, O2 consumption and redox homeostasis (Dai et al. 2013). Within the ETC, NO• competes with O2 for the substrate-binding site of complex IV (Carreras and Poderoso 2007). The outcome of such competition is dependent on intramitochondrial NO• concentrations, which in turn are determined by the NO• synthase (NOS) isoform expressed. Low levels of NO•-mediated ETC inhibition induced by endothelial and neuronal NOS (eNOS and nNOS) may be beneficial through dispensing O2 to cells at varying distances from blood vessels and by reducing oxidant generation (Gladwin and Shiva 2009). In skeletal myofibers, this mechanism could optimize O2 repartition between SSM and IFM (Marzetti et al. 2012c). On the other hand, excessive NO• production by inducible NOS (iNOS) leads to extensive ETC inhibition, amplification of oxidant production, and induction of apoptosis via OMM permeabilization (Boczkowski et al. 1999; Boyd and Cadenas 2002). Noteworthy, TNF-α is a potent inducer of iNOS (Nussler and Billiar 1993), which establishes a mechanistic link between inflammation and mitochondrial dysfunction.
To this end, we recently showed that downregulation of TNF-α expression was associated with upregulation of eNOS and repression of iNOS in the TA muscle of old rats treated with the angiotensin-converting enzyme inhibitor enalapril (Marzetti et al. 2012c). These adaptations were accompanied by decreased SSM emission of H2O2. In an earlier study from our group, enalapril administration downregulated mitochondrial caspase-dependent apoptotic signaling in the gastrocnemius of old rats, while attenuating the loss of muscle mass and strength (Carter et al. 2011).
Apart from its actions on NO• signaling, TNF-α can also trigger apoptosis through the death-receptor signaling pathway (Lavrik et al. 2005). Downstream of TNF-α, caspase-8 initiates the caspase cascade and also mediates crosstalk between the extrinsic and intrinsic apoptotic pathways via Bid truncation (Li et al. 1998). Once cleaved, Bid induces OMM permeabilization and release of apoptogenic factors from the intermembrane space (Scorrano et al. 2002). Interestingly, Bid localization to mitochondria was found to be increased in the gastrocnemius muscle of old rats, accompanied by elevation of markers pertaining to mitochondrial caspase-dependent and independent apoptotic signaling (Marzetti et al. 2008b).
Impairments in mitochondrial turnover have recently emerged as an additional mechanism linking inflammation to mitochondrial dysfunction. Elevated levels of TNF-α decrease the mRNA abundance of PGC-1α, TFAM and nuclear respiratory factor 1 in cultured C2C12 myoblasts, indicative of suppressed mitochondriogenesis (Remels et al. 2010). The effect, mediated by NF-κB, has been implicated in muscle wasting in patients with chronic obstructive pulmonary disease (COPD) (Remels et al. 2010). Inflammation may also inhibit autophagy, which in turn could amplify mitochondrial dysfunction by impairing the disposal of damaged organelles (Salminen et al. 2012).
In conclusion, the negative effect of chronically elevated levels of inflammatory cytokines on muscle mitochondrial function may underlie the well-known association between low-grade inflammation and sarcopenia. This implies that interventions targeting inflammation might help preserve skeletal muscle mass and function in advanced age, a concept starting to be supported by preclinical and epidemiological studies (Rieu et al. 2009; Landi et al. 2013). Further research is necessary to decipher the complex interaction between mitochondrial dysfunction and inflammation, select the most relevant inflammatory pathways to target, and identify circulating biomarkers that reflect the relationship among inflammation, muscle mitochondrial damage and sarcopenia.
4.2. Endocrine aging
The nature and magnitude of age-related alterations in circulating hormones and target tissue responsiveness have taken center stage in sarcopenia research (Sakuma and Yamaguchi 2012). Interestingly, recent studies have shown that the vast majority of endocrine factors associated with muscle aging may act on mitochondria. For instance, glucocorticoids, sex steroids and thyroid hormones regulate a wide range of muscle mitochondrial processes, including MQC pathways, OXPHOS activity, redox balance, and apoptotic signaling (Weber et al. 2002; Pitteloud et al. 2005; Casas et al. 2009; Musa et al. 2011; Weitzel and Iwen 2011; Guo et al. 2012; La Colla et al. 2013). The detection of steroid and thyroid hormone receptors in mitochondria and the presence of sequences similar to nuclear hormone responsive elements in the mitochondrial genome suggest these hormones may coordinate nuclear and mitochondrial gene transcription for adapting the cell to variable energy demands (Psarra and Sekeris 2008).
Studies have begun to unveil the role of sex steroid and thyroid hormones on mitochondrial function in the context of muscle aging. Casas et al. (2009) found that overexpression of p43, the mitochondrial triiodothyronine receptor, aggravated muscle loss in old mice. After an initial increase in mitochondrial mass, the mtDNA abundance declined significantly in the quadriceps muscle of aged transgenic mice. In addition, p43 overexpression was associated with enhanced oxidative stress in spite of increased antioxidant enzyme activities. As for sex steroids, Guo et al. (2012) showed that testosterone administration combined with low-intensity treadmill training ameliorated muscle mass and strength in old male mice to a greater extent than exercise alone. Such an effect was associated with upregulation of mitochondrial biogenesis, increased expression of markers of mitochondrial fission/fusion and mitophagy, and reduced oxidative stress.
Concurrent impairments in muscle mitochondrial function and insulin sensitivity are typically observed in advanced age (reviewed by Abbatecola et al. 2011). However, the association between insulin resistance and mitochondrial dysfunction and the actual direction of the relationship remain controversial (Dela and Helge 2013). Although mitochondrial decay has traditionally been attributed a causative role in insulin resistance, some intriguing findings point to impaired insulin sensitivity as a determinant of muscle mitochondrial dysfunction rather than the opposite (Stump et al. 2003; Asmann et al. 2006). Moreover, insulin sensitivity is similar in young and older men engaged in regular endurance exercise, in spite of a significant age-related reduction in mitochondrial oxidative capacity (Lanza et al. 2008). Declining insulin sensitivity is therefore likely related to changes in adiposity and to physical inactivity, rather than to alterations of mitochondrial function or mass.
Echoing these findings, a recently published cross-sectional study found a combination of muscle mitochondrial dysfunction, reduced muscle mass, ectopic lipid deposition and impaired glucose tolerance in sedentary non-obese elderly subjects (Johannsen et al. 2012). In this regard, crosstalk between muscle and adipose tissue, mediated by cytokines and peptides collectively named myokines and adipokines, may represent another aspect to take into account in the study of age-related mitochondria derangements. Indeed, leptin and adiponectin have shown to affect mitochondrial biogenesis and function in muscle (Iwabu et al. 2010; Li et al. 2011).
Although declining levels of growth hormone (GH) and insulin-like growth factor-1 (IGF-1) are often considered “the usual suspects” responsible for the deleterious changes in body composition and physical function with aging (Giovannini et al. 2008), controversies exist about the role of GH/IGF-1 axis modulation as a strategy to improve muscle strength and function (Taaffe et al. 1994; Yarasheski et al. 1995; Lange et al. 2002; Marzetti et al. 2008a). Notwithstanding, GH infusion was found to enhance mitochondrial bioenergetics in skeletal muscle of healthy young persons (Short et al. 2008). Whether GH supplementation produces a similar adaptation in older, sarcopenic individuals has not been explored. In any case, given the number of severe side effects associated with long-term GH supplementation, the Growth Hormone Research Society has warned against the use of GH or GH secretagogues as an anti-aging strategy (Thorner 2009).
Alterations in the renin-angiotensin system are indicated as potential contributors to sarcopenia through the promotion of muscular inflammation, mitochondrial dysfunction, and apoptosis (Carter et al. 2005). Angiotensin II (AngII) reduces mitochondrial mass in C2C12 cells in a dose-dependent manner by directly inhibiting the expression of genes involved in mitochondrial biogenesis (Mitsuishi et al. 2009). Similar effects were observed in skeletal muscle of mice after AngII infusion. Moreover, recent reports by our group show that the administration of enalapril to old rats protects against sarcopenia likely by attenuating mitochondrial oxidant generation and apoptosis (Carter et al. 2011; Marzetti et al. 2012c).
An extensive literature exists on the association between vitamin D deficiency and sarcopenia (reviewed by Cesari et al. 2011), but proof of causality and pathogenetic mechanisms are still controversial (Stockton et al. 2011). Nevertheless, a recent study reported that vitamin D supplementation to severely deficient persons produced marked improvements in fatigue that were accompanied, and perhaps mediated by augmented mitochondrial function, as measured in vivo by 31P-NMR spectroscopy (Sinha et al. 2013). Similar to steroid and thyroid hormones, the constitutive presence within mitochondria of vitamin D receptor (VDR) and its heterodimer partner retinoid X receptor has been detected in several cell types (Silvagno et al. 2010 and 2013). However, the function of mitochondrial VDR and its actual existence in skeletal myocytes have not yet been established.
In conclusion, although hormonal changes are likely involved in the pathogenesis of sarcopenia (Morley and Malmstrom 2013), several unresolved queries remain about the relationship between endocrine function and muscle mitochondrial physiology. Studies are also needed to identify possible differential effects of various hormones on SSM and IFM. Lastly, further research is warranted to determine if functional differences exist between systemic and local hormonal systems in advanced age, which could explain some of the controversial and disappointing results of hormonal supplementation in sarcopenic individuals.
4.3. Vascular aging
Vascular aging encompasses alterations in large conduit arteries as well as in smaller resistance arteries across multiple tissues. In skeletal muscle, aging is accompanied by reduced blood flow capacity (Lawrenson et al. 2003; Donato et al. 2006; DeVan et al. 2013) and impairment in endothelium-dependent vasodilation, due, in part, to altered ROS signaling and diminished NO• bioavailability (Muller-Delp et al. 2002).
Although the precise mechanisms underlying the alterations of ROS signaling within the aged microcirculation are not fully understood, emerging evidence suggests a fundamental role for mitochondrial dysfunction (Ungvari et al. 2008). Defective autophagy in endothelial cells (LaRocca et al. 2012) as well as increased ROS production (van der Loo et al. 2000; Ungvari et al. 2007) and impaired mitochondrial biogenesis (Ungvari et al. 2008) have been detected in vascular endothelium and smooth muscle cells. Enhanced mitochondrial ROS generation contributes to the adoption of a senescent phenotype in endothelial cells and to the activation of the redox-sensitive transcription factor NF-κB (Erusalimsky 2009). The latter leads to diminished endothelium-dependent vasodilation and upregulation of inflammatory gene expression in the aged vasculature (Ungvari et al. 2007; Pierce et al. 2009).
Given the role of mitochondrion-derived H2O2 from the myocyte as a potential feed-forward regulator of blood flow (Saitoh et al. 2006), an altered ROS signaling linked to age-related muscle mitochondrial dysfunction is more likely to affect the structural and dynamic behavior of the surrounding vasculature rather than the opposite. Regardless of the mechanisms responsible for vascular dysfunction in advanced age, the subsequent alteration in tissue perfusion may potentiate mitochondrial decay within skeletal muscle. In particular, age-dependent vascular alterations may disrupt the matching of substrate delivery (i.e., O2) with requirements in skeletal muscle both at rest and during exercise (DeLorey et al. 2004; Behnke et al. 2005; Herspring et al. 2008), resulting in a significantly lower microvascular oxygenation (and, by extension, intracellular oxygenation to maintain a given O2 uptake according to Fick’s law).
In turn, the decreased skeletal muscle oxygenation occurring with age could lead to the buildup of reducing equivalents (i.e., NADH and FADH2) within mitochondria (“reductive stress”), resulting in increased electron availability and enhanced generation of superoxide anions (Clanton 2007). Outside of skeletal muscle, it is possible that an altered vascular function in the adipose tissue (Davis et al. 2013; Padilla et al. 2013) may alter the synthesis and secretion of mitochondrial protective hormones such as IGF-1 (Kern et al. 1989), while enhancing the production of inflammatory cytokines (O’Rourke et al. 2011).
In conclusion, although there is no definite cause-effect evidence that the age-related vascular dysfunction drives muscle mitochondrial dysfunction or vice versa, it is likely that the reduced microvascular oxygenation (Behnke et al. 2005) and diminished lineal density of perfused capillaries (Russell et al. 2003) may contribute to impairing muscular mitochondrial function late in life. Furthermore, since H2O2 is involved in the regulation of vascular tone and muscle perfusion (Suvorava et al. 2005; Saitoh et al. 2007; Sindler et al. 2009), it is plausible that this oxidant may act as a paracrine signal regulating the local matching between O2 delivery and demand (Marvar et al. 2007; Golub and Pittman 2013). Hence, alterations in mitochondrial redox homeostasis may impair the local regulation of O2 delivery and exacerbate mitochondrial dysfunction with age.
More integrative research is needed to determine whether dysfunction in one compartment compromises the other and to identify targets for interventions that can protect both systems.
5. Towards clinical trials on mitochondrial dysfunction and sarcopenia: opportunities and challenges
The need of developing interventions to prevent or treat sarcopenia has been repeatedly evoked in literature (Brass and Sietsema 2011; Chumlea et al. 2011; Matthews et al. 2011). The design of a clinical trial targeting mitochondrial dysfunction to treat sarcopenia requires to preventively address several important issues. First of all, the effects of a hypothetical intervention on muscle mitochondrial function need to be carefully monitored in order to avoid potentially serious adverse events (Fig. 1). Just like most biological processes, the previously described mitochondrial pathways are not on/off phenomena, but proceed along a continuum with an optimal window of functioning, flanked by (detrimental) defective and excessive levels of operation.
Antioxidant supplementation is a paradigmatic example of how a simplistic way of tackling a complex issue produces unpredictable outcomes. Abnormal ROS emission likely contributes to muscle aging; hence, as predicated by the “Occam’s razor” principle, antioxidant supplementation would be the obvious remedy. Yet, oxidant generation within a hormetic range is needed for intracellular signaling (Handy and Loscalzo 2012) and optimal force production (Reid et al. 1993). Thus, antioxidants may paradoxically be harmful unless their effects on redox balance are closely monitored (Cerullo et al. 2012).
Likewise, while an excessive apoptotic activation may promote muscle atrophy, the indiscriminate abolition of apoptosis could result in the persistence of dysfunctional nuclei or damaged contractile elements, which may further aggravate muscle tissue dyshomeostasis. The consequences of varying levels of activation of mitochondrial fission and fusion on muscle trophism have already been discussed. As for autophagy, its unchecked stimulation could lead to autophagic cell death, while its abolition would abrogate the housekeeping function of this pathway, resulting in the accumulation of dysfunctional mitochondrial and other cellular waste (Gottlieb and Carreira 2010; Sandri 2010). Finally, excessive mitochondrial biogenesis is also maladaptive unless it is compensated by adequate upregulation of mitophagy (Gottlieb and Carreira 2010).
Another important aspect to take into account is the pleiotropic function of nearly all mediators of the mitochondrial pathways described (Gottlieb and Carreira 2010; Sack 2011; Galluzzi et al. 2012; Subramani and Malhotra 2013). Targeting a single molecule might therefore impact multiple processes. Moreover, manipulation of one pathway could elicit compensatory effects, impeding a clear distinction between primary and secondary outcomes and cause-effect relationships. These considerations highlight the need for reliable biomarkers of mitochondrial function to monitor the effects of the intervention under study.
The determination of muscle mitochondrial function in clinical research settings poses a relevant challenge (Marzetti et al. 2012a). Invasive approaches, such as muscle biopsy, may render more difficult the participation of older adults in a clinical trial, especially when considering that muscle specimens should be collected at least at two time-points to determine the effects of an intervention. In addition, sophisticated analyses of muscle samples are generally expensive, besides requiring specific laboratory equipment and solid personnel experience. Some mitochondrial measurements, such as O2 consumption or oxidant emission, need to be performed on fresh tissue and are highly laborious. This entails that, in the case of a multicenter trial, each of the participating sites must have its own laboratory, possibly introducing significant sources of variability, in addition to increasing the cost of research. Some of these limitations may be overcome by employing noninvasive approaches, like 31P-NMR spectroscopy (Wray et al. 2009). However, NMR is not widely available, besides being time-consuming and expensive.
Recent studies have shown that surrogate markers of mitochondrial function can be retrieved in biofluids, including “ATP profile” of peripheral white blood cells (Myhill et al. 2009), plasma creatine (Shaham et al. 2010), serum levels of fibroblast growth factor 21 (Suomalainen et al. 2011), and serum concentrations of acylcarnitines and non-esterified fatty acids (Sampey et al. 2012). If these analytes qualified as biomarkers for mitochondrial dysfunction in the setting of sarcopenia, their determination could serve for screening and follow-up purposes (Marzetti 2012). In this scenario, sophisticated analyses on muscle specimens would represent a second-level assessment for the identification of the pathway(s) compromised.
The design of an informative randomized controlled trial (RCT) necessarily goes through the correct selection of the study sample. The optimal target population of a RCT might be defined as a group of individuals who, for their sociodemographic, biological, and clinical characteristics, may best benefit from the intervention under study, with the lowest risk of experiencing treatment-related adverse events. In the case of sarcopenia, several criteria have been indicated to identify such a target population (Cesari and Pahor 2008). However, it should be well kept in mind that controversies still exist about the clinical identification of sarcopenia in older persons (Cruz-Jentoft et al. 2010; Muscaritoli et al. 2010; Fielding et al. 2011; Morley et al. 2011).
An optimal target individual for interventions against mitochondrial dysfunction and sarcopenia should present the condition of interest at the baseline. This implies that, in addition to mitochondrial parameters, muscle mass and strength must be objectively measured through imaging and physical performance tests (Marzetti 2012). Apart from quantitative parameters, the individual should also be assessed for the qualitative aspect of skeletal muscle. In this regard, the presence of sarcopenia can be judged by measuring the functional capacity expressed by unit of muscle (Goodpaster et al. 2001; Barbat-Artigas et al. 2012). Such approach might sufficiently confine the identification of sarcopenia to the skeletal muscle by reducing more systemic influences.
Since the primary reason for treating sarcopenia is to prevent disability, disabled subjects must be excluded from the trial. Therefore, selection criteria should not only include individuals with some physical impairment (e.g., reduced strength generated per unit of muscle), but also exclude those with overt disability (e.g., incapacity to walk 400 meters) or muscle-wasting disorders independent of aging (e.g., cachexia). It will also be necessary to exclude subjects in whom muscle mitochondrial function may be impaired by comorbid conditions (e.g., mitochondrial myopathies, peripheral vascular disease, type II diabetes, COPD, congestive heart failure, etc.). Such an approach, however, would significantly limit the representativeness of the sample for future generalization of the study results. Alternatively, individuals with comorbidities could still be considered eligible for participation, as long as investigators are aware of potential clinical confounders and take adequate countermeasures for balancing the randomization (Studenski 2009; Pahor and Cesari 2011). Same reasoning applies to medications. In other words, when designing their study, investigators should evaluate the ratio between “purity” of the future findings and the applicability of the results to a larger population (and real life).
Disquisitions about the optimal timing and duration of the intervention have been published elsewhere (Calvani et al. 2013b). It is however important to bear in mind that six months are typically indicated as the minimum timeframe to expect sizable changes in muscle mass (Cesari et al. 2012).
Taken together, these considerations highlight the number of safety and methodological issues researchers are called to address before embarking in the design of RCTs on mitochondrial dysfunction and sarcopenia. However, the possibility of relieving the individual and societal burden associated with muscle loss and disability in late life makes this challenging task worth pursuing.
6. Conclusion and future perspectives
Sarcopenia is one of the most burdensome geriatric syndromes. As such, the development of effective preventive and therapeutic measures is urgently needed. This task is hampered by the heterogeneity of clinical correlates of sarcopenia, reflecting the complexity of its pathogenesis. Derangements in mitochondrial function are currently invoked as major factors in the sarcopenia syndrome. Yet, exploiting mitochondrial pathways for therapeutic purposes is an ambitious task. First, most processes governing mitochondrial physiology exhibit a “yin-yang behavior”, but their optimal range of functioning is currently unclear. Moreover, no widely-accepted biomarkers of muscle mitochondrial dysfunction are presently available.
A deeper understanding of mitochondrial pathophysiology in sarcopenia also requires discerning the relative impact of intrinsic muscular aging from that of lifestyle habits and common age-associated conditions. At the patient level, it is possible that the phenotypic heterogeneity of sarcopenia could be the resultant of the diverse combination of factors acting at multiple levels, ranging from the extramuscular environment to specific mitochondrial processes in skeletal myocytes. Hence, future developments in the field will require the implementation of new analytical tools to achieve a global characterization of the sarcopenic patient. This multidimensional approach will assist in the design of personalized therapeutic programs and monitoring of the intervention effects.
The present review has not the pretension to being exhaustive. Our aim is to provide a coup d’oeil on the current evidence about the contribution of specific mitochondrial pathways to sarcopenia and emphasize the complexity of muscle aging. After all, understanding the inner foundations of sarcopenia means discovering why we age and how we can reduce the weathering and wearing effects of time.
Acknowledgments
This work was partly supported by funds from the Centro Studi Achille e Linda Lorenzon (E.M. and M.L.), the Italian Ministry of Economy and Finance to the CNR for the Project FaReBio di Qualità (R.C.), Chair of Excellence of the French Agence Nationale de le Recherche (M.C.), NIA RO1-AG21042, NIDDK RO1-DK090115-01A1, and the University of Florida’s Institute on Aging and Claude D. Pepper Older Americans Independence Center (NIA 1P30AG028740, C.L. and T.W.B.). The authors thank Dr. Simona Mastropaolo (Catholic University of Rome) for her critical reading of this manuscript and Mr. Francesco Antognarelli for his invaluable assistance with illustrations. We apologize to those authors whose excellent work could not be cited because of the vast literature on the subject and space limitations.
List of abbreviations
- 31P-NMR
31P nuclear magnetic resonance
- AIF
apoptosis-inducing factor
- AngII
angiotensin II
- Atg protein
autophagy-related protein
- Bcl-2
B-cell leukemia-2
- COPD
chronic obstructive pulmonary disease
- COX
cytochrome c oxidase
- CSA
cross-sectional area
- Drp1
dynamin-related protein 1
- EndoG
endonuclease G
- eNOS
endothelial nitric oxide synthase
- ETC
electron transport chain
- Fis1
fission protein 1
- FoxO3
Forkhead box O3
- GH
growth hormone
- IFM
interfibrillar mitochondria
- IGF-1
insulin-like growth factor-1
- iNOS
inducible nitric oxide synthase
- LAMP-2
lysosomal-associated membrane protein 2
- LC3
microtubule-associated protein 1 light chain 3
- MFRTA
mitochondrial free radical theory of aging
- Mfn
mitofusin
- MQC
mitochondrial quality control
- mtDNA
mitochondrial DNA
- NF-κB
nuclear factor κB
- nNOS
neuronal nitric oxide synthase
- NOS
nitric oxide synthase
- OMM
outer mitochondrial membrane
- OXPHOS
oxidative phosphorylation
- PGC-1α
peroxisome proliferator-activated receptor-γ coactivator-1α
- RCT
randomized controlled trial
- ROS
reactive oxygen species
- SDH
succinate dehydrogenase
- SSM
subsarcolemmal mitochondria
- TA
tibialis anterior
- TFAM
mitochondrial transcription factor A
- TNF-α
tumor-necrosis factor α
- UPS
ubiquitin-proteasome system
- VDR
vitamin D receptor
- VL
vastus lateralis
Footnotes
Conflict of interest statement: the authors declare that they have no conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbatecola AM, Paolisso G, Fattoretti P, Evans WJ, Fiore V, Dicioccio L, et al. Discovering pathways of sarcopenia in older adults: a role for insulin resistance on mitochondria dysfunction. J Nutr Health Aging. 2011;15:890–5. doi: 10.1007/s12603-011-0366-0. [DOI] [PubMed] [Google Scholar]
- Adhihetty PJ, Ljubicic V, Menzies KJ, Hood DA. Differential susceptibility of subsarcolemmal and intermyofibrillar mitochondria to apoptotic stimuli. Am J Physiol Cell Physiol. 2005;289:C994–C1001. doi: 10.1152/ajpcell.00031.2005. [DOI] [PubMed] [Google Scholar]
- Allen DL, Roy RR, Edgerton VR. Myonuclear domains in muscle adaptation and disease. Muscle Nerve. 1999;22:1350–60. doi: 10.1002/(sici)1097-4598(199910)22:10<1350::aid-mus3>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Alway SE, Degens H, Krishnamurthy G, Smith CA. Potential role for Id myogenic repressors in apoptosis and attenuation of hypertrophy in muscles of aged rats. Am J Physiol Cell Physiol. 2002;283:C66–C76. doi: 10.1152/ajpcell.00598.2001. [DOI] [PubMed] [Google Scholar]
- Aon MA. From isolated to networked: a paradigmatic shift in mitochondrial physiology. Front Physiol. 2010;1:20. doi: 10.3389/fphys.2010.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmann YW, Stump CS, Short KR, Coenen-Schimke JM, Guo Z, Bigelow ML, et al. Skeletal muscle mitochondrial functions, mitochondrial DNA copy numbers, and gene transcript profiles in type 2 diabetic and nondiabetic subjects at equal levels of low or high insulin and euglycemia. Diabetes. 2006;55:3309–19. doi: 10.2337/db05-1230. [DOI] [PubMed] [Google Scholar]
- Bach D, Naon D, Pich S, Soriano FX, Vega N, Rieusset J, et al. Expression of Mfn2, the Charcot-Marie-Tooth neuropathy type 2A gene, in human skeletal muscle: effects of type 2 diabetes, obesity, weight loss, and the regulatory role of tumor necrosis factor alpha and interleukin-6. Diabetes. 2005;54:2685–93. doi: 10.2337/diabetes.54.9.2685. [DOI] [PubMed] [Google Scholar]
- Barbat-Artigas S, Rolland Y, Zamboni M, Aubertin-Leheudre M. How to assess functional status: a new muscle quality index. J Nutr Health Aging. 2012;16:67–77. doi: 10.1007/s12603-012-0004-5. [DOI] [PubMed] [Google Scholar]
- Behnke BJ, Delp MD, Dougherty PJ, Musch TI, Poole DC. Effects of aging on microvascular oxygen pressures in rat skeletal muscle. Respir Physiol Neurobiol. 2005;146:259–68. doi: 10.1016/j.resp.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Beregi E, Regius O. Comparative morphological study of age related mitochondrial changes of the lymphocytes and skeletal muscle cells. Acta Morphol Hung. 1987;35:219–24. [PubMed] [Google Scholar]
- Bhatia-Kissova I, Camougrand N. Mitophagy: a process that adapts to the cell physiology. Int J Biochem Cell Biol. 2013;45:30–3. doi: 10.1016/j.biocel.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Boczkowski J, Lisdero CL, Lanone S, Samb A, Carreras MC, Boveris A, et al. Endogenous peroxynitrite mediates mitochondrial dysfunction in rat diaphragm during endotoxemia. FASEB J. 1999;13:1637–46. doi: 10.1096/fasebj.13.12.1637. [DOI] [PubMed] [Google Scholar]
- Boyd CS, Cadenas E. Nitric oxide and cell signaling pathways in mitochondrial-dependent apoptosis. Biol Chem. 2002;383:411–23. doi: 10.1515/BC.2002.045. [DOI] [PubMed] [Google Scholar]
- Braga M, Sinha Hikim AP, Datta S, Ferrini MG, Brown D, Kovacheva EL, et al. Involvement of oxidative stress and caspase 2-mediated intrinsic pathway signaling in age-related increase in muscle cell apoptosis in mice. Apoptosis. 2008;13:822–32. doi: 10.1007/s10495-008-0216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass EP, Sietsema KE. Considerations in the development of drugs to treat sarcopenia. J Am Geriatr Soc. 2011;59:530–5. doi: 10.1111/j.1532-5415.2010.03285.x. [DOI] [PubMed] [Google Scholar]
- Brault JJ, Jespersen JG, Goldberg AL. Peroxisome proliferator-activated receptor gamma coactivator 1alpha or 1beta overexpression inhibits muscle protein degradation, induction of ubiquitin ligases, and disuse atrophy. J Biol Chem. 2010;285:19460–71. doi: 10.1074/jbc.M110.113092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunk UT, Terman A. The mitochondrial-lysosomal axis theory of aging: accumulation of damaged mitochondria as a result of imperfect autophagocytosis. Eur J Biochem. 2002;269:1996–2002. doi: 10.1046/j.1432-1033.2002.02869.x. [DOI] [PubMed] [Google Scholar]
- Bruusgaard JC, Egner IM, Larsen TK, Dupre-Aucouturier S, Desplanches D, Gundersen K. No change in myonuclear number during muscle unloading and reloading. J Appl Physiol. 2012;113:290–6. doi: 10.1152/japplphysiol.00436.2012. [DOI] [PubMed] [Google Scholar]
- Bruusgaard JC, Gundersen K. In vivo time-lapse microscopy reveals no loss of murine myonuclei during weeks of muscle atrophy. J Clin Invest. 2008;118:1450–7. doi: 10.1172/JCI34022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bua E, Johnson J, Herbst A, Delong B, McKenzie D, Salamat S, et al. Mitochondrial DNA-deletion mutations accumulate intracellularly to detrimental levels in aged human skeletal muscle fibers. Am J Hum Genet. 2006;79:469–80. doi: 10.1086/507132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buford TW, Anton SD, Judge AR, Marzetti E, Wohlgemuth SE, Carter CS, et al. Models of accelerated sarcopenia: critical pieces for solving the puzzle of age-related muscle atrophy. Ageing Res Rev. 2010;9:369–83. doi: 10.1016/j.arr.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvani R, Joseph AM, Adhihetty PJ, Miccheli A, Bossola M, Leeuwenburgh C, et al. Mitochondrial pathways in sarcopenia of aging and disuse muscle atrophy. Biol Chem. 2013a;394:393–414. doi: 10.1515/hsz-2012-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvani R, Miccheli A, Landi F, Bossola M, Cesari M, Leeuwenburgh C, et al. Current nutritional recommendations and novel dietary strategies to manage sarcopenia. J Frailty Aging. 2013b;2:38–53. [PMC free article] [PubMed] [Google Scholar]
- Carlson BM, Faulkner JA. Muscle transplantation between young and old rats: age of host determines recovery. Am J Physiol. 1989;256:C1262–C1266. doi: 10.1152/ajpcell.1989.256.6.C1262. [DOI] [PubMed] [Google Scholar]
- Carlson BM, Faulkner JA. The regeneration of noninnervated muscle grafts and marcaine-treated muscles in young and old rats. J Gerontol A Biol Sci Med Sci. 1996;51:B43–B49. doi: 10.1093/gerona/51a.1.b43. [DOI] [PubMed] [Google Scholar]
- Carreras MC, Poderoso JJ. Mitochondrial nitric oxide in the signaling of cell integrated responses. Am J Physiol Cell Physiol. 2007;292:C1569–C1580. doi: 10.1152/ajpcell.00248.2006. [DOI] [PubMed] [Google Scholar]
- Carter CS, Giovannini S, Seo DO, Dupree J, Morgan D, Chung HY, et al. Differential effects of enalapril and losartan on body composition and indices of muscle quality in aged male Fischer 344 x Brown Norway rats. Age (Dordr ) 2011;33:167–83. doi: 10.1007/s11357-010-9196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Onder G, Kritchevsky SB, Pahor M. Angiotensin-converting enzyme inhibition intervention in elderly persons: effects on body composition and physical performance. J Gerontol A Biol Sci Med Sci. 2005;60:1437–46. doi: 10.1093/gerona/60.11.1437. [DOI] [PubMed] [Google Scholar]
- Casas F, Pessemesse L, Grandemange S, Seyer P, Baris O, Gueguen N, et al. Overexpression of the mitochondrial T3 receptor induces skeletal muscle atrophy during aging. PLoS ONE. 2009;4:e5631. doi: 10.1371/journal.pone.0005631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerullo F, Gambassi G, Cesari M. Rationale for antioxidant supplementation in sarcopenia. J Aging Res. 2012;2012:316943. doi: 10.1155/2012/316943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesari M, Ferrini A, Zamboni V, Pahor M. Sarcopenia: current clinical and research issues. Open Geriatr Med J. 2008;1:14–23. [Google Scholar]
- Cesari M, Fielding RA, Pahor M, Goodpaster B, Hellerstein M, Abellan van Kan G, et al. Biomarkers of sarcopenia in clinical trials. Recommendations from the International Working Group on sarcopenia. J Frailty Aging. 2012;1:102–10. doi: 10.1007/s13539-012-0078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesari M, Incalzi RA, Zamboni V, Pahor M. Vitamin D hormone: a multitude of actions potentially influencing the physical function decline in older persons. Geriatr Gerontol Int. 2011;11:133–42. doi: 10.1111/j.1447-0594.2010.00668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesari M, Kritchevsky SB, Baumgartner RN, Atkinson HH, Penninx BW, Lenchik L, et al. Sarcopenia, obesity, and inflammation--results from the Trial of Angiotensin Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors study. Am J Clin Nutr. 2005;82:428–34. doi: 10.1093/ajcn.82.2.428. [DOI] [PubMed] [Google Scholar]
- Cesari M, Pahor M. Target population for clinical trials on sarcopenia. J Nutr Health Aging. 2008;12:470–8. doi: 10.1007/BF02982708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabi B, Ljubicic V, Menzies KJ, Huang JH, Saleem A, Hood DA. Mitochondrial function and apoptotic susceptibility in aging skeletal muscle. Aging Cell. 2008;7:2–12. doi: 10.1111/j.1474-9726.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- Cheek DB. The control of cell mass and replication. The DNA unit--a personal 20-year study. Early Hum Dev. 1985;12:211–39. doi: 10.1016/0378-3782(85)90144-6. [DOI] [PubMed] [Google Scholar]
- Chen H, Vermulst M, Wang YE, Chomyn A, Prolla TA, McCaffery JM, et al. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell. 2010;141:280–9. doi: 10.1016/j.cell.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumlea WC, Cesari M, Evans WJ, Ferrucci L, Fielding RA, Pahor M, et al. Sarcopenia: designing phase IIB trials. J Nutr Health Aging. 2011;15:450–5. doi: 10.1007/s12603-011-0092-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, et al. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009;8:18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clanton TL. Hypoxia-induced reactive oxygen species formation in skeletal muscle. J Appl Physiol. 2007;102:2379–88. doi: 10.1152/japplphysiol.01298.2006. [DOI] [PubMed] [Google Scholar]
- Coen PM, Jubrias SA, Distefano G, Amati F, Mackey DC, Glynn NW, et al. Skeletal muscle mitochondrial energetics are associated with maximal aerobic capacity and walking speed in older adults. J Gerontol A Biol Sci Med Sci. 2013;68:447–55. doi: 10.1093/gerona/gls196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combaret L, Dardevet D, Bechet D, Taillandier D, Mosoni L, Attaix D. Skeletal muscle proteolysis in aging. Curr Opin Clin Nutr Metab Care. 2009;12:37–41. doi: 10.1097/MCO.0b013e32831b9c31. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–4. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- Conley KE, Jubrias SA, Esselman PC. Oxidative capacity and ageing in human muscle. J Physiol. 2000;526(Pt 1):203–10. doi: 10.1111/j.1469-7793.2000.t01-1-00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane JD, Devries MC, Safdar A, Hamadeh MJ, Tarnopolsky MA. The effect of aging on human skeletal muscle mitochondrial and intramyocellular lipid ultrastructure. J Gerontol A Biol Sci Med Sci. 2010;65:119–28. doi: 10.1093/gerona/glp179. [DOI] [PubMed] [Google Scholar]
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–23. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai DF, Chen T, Wanagat J, Laflamme M, Marcinek DJ, Emond MJ, et al. Age-dependent cardiomyopathy in mitochondrial mutator mice is attenuated by overexpression of catalase targeted to mitochondria. Aging Cell. 2010;9:536–44. doi: 10.1111/j.1474-9726.2010.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Z, Wu Z, Yang Y, Wang J, Satterfield MC, Meininger CJ, et al. Nitric oxide and energy metabolism in mammals. BioFactors. 2013 doi: 10.1002/biof.1099. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Davis RT, 3rd, Stabley JN, Dominguez JM, Ramsey MW, McCullough DJ, Lesniewski LA, et al. Differential effects of aging and exercise on intra-abdominal adipose arteriolar function and blood flow regulation. J Appl Physiol. 2013;114:808–15. doi: 10.1152/japplphysiol.01358.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Fuente M, Miquel J. An update of the oxidation-inflammation theory of aging: the involvement of the immune system in oxi-inflamm-aging. Curr Pharm Des. 2009;15:3003–26. doi: 10.2174/138161209789058110. [DOI] [PubMed] [Google Scholar]
- Dela F, Helge JW. Insulin resistance and mitochondrial function in skeletal muscle. Int J Biochem Cell Biol. 2013;45:11–5. doi: 10.1016/j.biocel.2012.09.019. [DOI] [PubMed] [Google Scholar]
- DeLorey DS, Kowalchuk JM, Paterson DH. Effect of age on O(2) uptake kinetics and the adaptation of muscle deoxygenation at the onset of moderate-intensity cycling exercise. J Appl Physiol. 2004;97:165–72. doi: 10.1152/japplphysiol.01179.2003. [DOI] [PubMed] [Google Scholar]
- DeVan AE, Eskurza I, Pierce GL, Walker AE, Jablonski KL, Kaplon RE, et al. Regular aerobic exercise protects against impaired fasting plasma glucose-associated vascular endothelial dysfunction with aging. Clin Sci (Lond) 2013;124:325–31. doi: 10.1042/CS20120291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd SL, Gagnon BJ, Senf SM, Hain BA, Judge AR. Ros-mediated activation of NF-kappaB and Foxo during muscle disuse. Muscle Nerve. 2010;41:110–3. doi: 10.1002/mus.21526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Uberoi A, Wray DW, Nishiyama S, Lawrenson L, Richardson RS. Differential effects of aging on limb blood flow in humans. Am J Physiol Heart Circ Physiol. 2006;290:H272–H278. doi: 10.1152/ajpheart.00405.2005. [DOI] [PubMed] [Google Scholar]
- Du J, Wang X, Miereles C, Bailey JL, Debigare R, Zheng B, et al. Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J Clin Invest. 2004;113:115–23. doi: 10.1172/JCI200418330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erusalimsky JD. Vascular endothelial senescence: from mechanisms to pathophysiology. J Appl Physiol. 2009;106:326–32. doi: 10.1152/japplphysiol.91353.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira R, Vitorino R, Alves RM, Appell HJ, Powers SK, Duarte JA, et al. Subsarcolemmal and intermyofibrillar mitochondria proteome differences disclose functional specializations in skeletal muscle. Proteomics. 2010;10:3142–54. doi: 10.1002/pmic.201000173. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Penninx BW, Volpato S, Harris TB, Bandeen-Roche K, Balfour J, et al. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc. 2002;50:1947–54. doi: 10.1046/j.1532-5415.2002.50605.x. [DOI] [PubMed] [Google Scholar]
- Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249–56. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo PA, Powers SK, Ferreira RM, Appell HJ, Duarte JA. Aging impairs skeletal muscle mitochondrial bioenergetic function. J Gerontol A Biol Sci Med Sci. 2009;64:21–33. doi: 10.1093/gerona/gln048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, et al. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell. 2001;1:515–25. doi: 10.1016/s1534-5807(01)00055-7. [DOI] [PubMed] [Google Scholar]
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- Fry CS, Drummond MJ, Glynn EL, Dickinson JM, Gundermann DM, Timmerman KL, et al. Skeletal muscle autophagy and protein breakdown following resistance exercise are similar in younger and older adults. J Gerontol A Biol Sci Med Sci. 2013;68:599–607. doi: 10.1093/gerona/gls209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Kepp O, Trojel-Hansen C, Kroemer G. Non-apoptotic functions of apoptosis-regulatory proteins. EMBO Rep. 2012;13:322–30. doi: 10.1038/embor.2012.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng T, Li P, Okutsu M, Yin X, Kwek J, Zhang M, et al. PGC-1alpha plays a functional role in exercise-induced mitochondrial biogenesis and angiogenesis but not fiber-type transformation in mouse skeletal muscle. Am J Physiol Cell Physiol. 2010;298:C572–C579. doi: 10.1152/ajpcell.00481.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannini S, Marzetti E, Borst SE, Leeuwenburgh C. Modulation of GH/IGF-1 axis: potential strategies to counteract sarcopenia in older adults. Mech Ageing Dev. 2008;129:593–601. doi: 10.1016/j.mad.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladwin MT, Shiva S. The ligand binding battle at cytochrome c oxidase: how NO regulates oxygen gradients in tissue. Circ Res. 2009;104:1136–8. doi: 10.1161/CIRCRESAHA.109.198911. [DOI] [PubMed] [Google Scholar]
- Golub AS, Pittman RN. Bang-bang model for regulation of local blood flow. Microcirculation. 2013 doi: 10.1111/micc.12051. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol. 2001;90:2157–65. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- Gottlieb RA, Carreira RS. Autophagy in health and disease. 5. Mitophagy as a way of life. Am J Physiol Cell Physiol. 2010;299:C203–C210. doi: 10.1152/ajpcell.00097.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Wong S, Li M, Liang W, Liesa M, Serra C, et al. Testosterone plus low-intensity physical training in late life improves functional performance, skeletal muscle mitochondrial biogenesis, and mitochondrial quality control in male mice. PLoS ONE. 2012;7:e51180. doi: 10.1371/journal.pone.0051180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handy DE, Loscalzo J. Redox regulation of mitochondrial function. Antioxid Redox Signal. 2012;16:1323–67. doi: 10.1089/ars.2011.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman D. The biologic clock: the mitochondria? J Am Geriatr Soc. 1972;20:145–7. doi: 10.1111/j.1532-5415.1972.tb00787.x. [DOI] [PubMed] [Google Scholar]
- He C, Bassik MC, Moresi V, Sun K, Wei Y, Zou Z, et al. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012;481:511–5. doi: 10.1038/nature10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herspring KF, Ferreira LF, Copp SW, Snyder BS, Poole DC, Musch TI. Effects of antioxidants on contracting spinotrapezius muscle microvascular oxygenation and blood flow in aged rats. J Appl Physiol. 2008;105:1889–96. doi: 10.1152/japplphysiol.90642.2008. [DOI] [PubMed] [Google Scholar]
- Hiona A, Sanz A, Kujoth GC, Pamplona R, Seo AY, Hofer T, et al. Mitochondrial DNA mutations induce mitochondrial dysfunction, apoptosis and sarcopenia in skeletal muscle of mitochondrial DNA mutator mice. PLoS ONE. 2010;5:e11468. doi: 10.1371/journal.pone.0011468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppeler H. Exercise-induced ultrastructural changes in skeletal muscle. Int J Sports Med. 1986;7:187–204. doi: 10.1055/s-2008-1025758. [DOI] [PubMed] [Google Scholar]
- Iqbal S, Ostojic O, Singh K, Joseph AM, Hood DA. Expression of mitochondrial fission and fusion regulatory proteins in skeletal muscle during chronic use and disuse. Muscle Nerve. 2013 doi: 10.1002/mus.23838. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Iwabu M, Yamauchi T, Okada-Iwabu M, Sato K, Nakagawa T, Funata M, et al. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature. 2010;464:1313–9. doi: 10.1038/nature08991. [DOI] [PubMed] [Google Scholar]
- Jensen GL. Inflammation: roles in aging and sarcopenia. JPEN J Parenter Enteral Nutr. 2008;32:656–9. doi: 10.1177/0148607108324585. [DOI] [PubMed] [Google Scholar]
- Johannsen DL, Conley KE, Bajpeyi S, Punyanitya M, Gallagher D, Zhang Z, et al. Ectopic lipid accumulation and reduced glucose tolerance in elderly adults are accompanied by altered skeletal muscle mitochondrial activity. J Clin Endocrinol Metab. 2012;97:242–50. doi: 10.1210/jc.2011-1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ML, Robinson MM, Nair KS. Skeletal muscle aging and the mitochondrion. Trends Endocrinol Metab. 2013;24:247–56. doi: 10.1016/j.tem.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jornayvaz FR, Shulman GI. Regulation of mitochondrial biogenesis. Essays Biochem. 2010;47:69–84. doi: 10.1042/bse0470069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph AM, Adhihetty PJ, Buford TW, Wohlgemuth SE, Lees HA, Nguyen LM, et al. The impact of aging on mitochondrial function and biogenesis pathways in skeletal muscle of sedentary high- and low-functioning elderly individuals. Aging Cell. 2012;11:801–9. doi: 10.1111/j.1474-9726.2012.00844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki M, Karim MR. Cytosolic LC3 ratio as a quantitative index of macroautophagy. Methods Enzymol. 2009;452:199–213. doi: 10.1016/S0076-6879(08)03613-6. [DOI] [PubMed] [Google Scholar]
- Kern PA, Svoboda ME, Eckel RH, Van Wyk JJ. Insulinlike growth factor action and production in adipocytes and endothelial cells from human adipose tissue. Diabetes. 1989;38:710–7. doi: 10.2337/diab.38.6.710. [DOI] [PubMed] [Google Scholar]
- Klionsky DJ, Abdalla FC, Abeliovich H, Abraham RT, Acevedo-Arozena A, Adeli K, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8:445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltai E, Hart N, Taylor AW, Goto S, Ngo JK, Davies KJ, et al. Age-associated declines in mitochondrial biogenesis and protein quality control factors are minimized by exercise training. Am J Physiol Regul Integr Comp Physiol. 2012;303:R127–R134. doi: 10.1152/ajpregu.00337.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortebein P, Ferrando A, Lombeida J, Wolfe R, Evans WJ. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA. 2007;297:1772–4. doi: 10.1001/jama.297.16.1772-b. [DOI] [PubMed] [Google Scholar]
- Koves TR, Noland RC, Bates AL, Henes ST, Muoio DM, Cortright RN. Subsarcolemmal and intermyofibrillar mitochondria play distinct roles in regulating skeletal muscle fatty acid metabolism. Am J Physiol Cell Physiol. 2005;288:C1074–C1082. doi: 10.1152/ajpcell.00391.2004. [DOI] [PubMed] [Google Scholar]
- Kriete A. Robustness and aging-A systems-level perspective. Biosystems. 2013;112:37–48. doi: 10.1016/j.biosystems.2013.03.014. [DOI] [PubMed] [Google Scholar]
- Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–4. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- La Colla A, Vasconsuelo A, Boland R. Estradiol exerts antiapoptotic effects in skeletal myoblasts via mitochondrial PTP and MnSOD. J Endocrinol. 2013;216:331–41. doi: 10.1530/JOE-12-0486. [DOI] [PubMed] [Google Scholar]
- Landi F, Marzetti E, Liperoti R, Pahor M, Russo A, Martone AM, et al. Nonsteroidal anti-Inflammatory drug (NSAID) use and sarcopenia in older people: results from the ilSIRENTE Study. J Am Med Dir Assoc. 2013 doi: 10.1016/j.jamda.2013.04.012. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Lange KH, Andersen JL, Beyer N, Isaksson F, Larsson B, Rasmussen MH, et al. GH administration changes myosin heavy chain isoforms in skeletal muscle but does not augment muscle strength or hypertrophy, either alone or combined with resistance exercise training in healthy elderly men. J Clin Endocrinol Metab. 2002;87:513–23. doi: 10.1210/jcem.87.2.8206. [DOI] [PubMed] [Google Scholar]
- Lanza IR, Short DK, Short KR, Raghavakaimal S, Basu R, Joyner MJ, et al. Endurance exercise as a countermeasure for aging. Diabetes. 2008;57:2933–42. doi: 10.2337/db08-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRocca TJ, Henson GD, Thorburn A, Sindler AL, Pierce GL, Seals DR. Translational evidence that impaired autophagy contributes to arterial ageing. J Physiol. 2012;590:3305–16. doi: 10.1113/jphysiol.2012.229690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavrik I, Golks A, Krammer PH. Death receptor signaling. J Cell Sci. 2005;118:265–7. doi: 10.1242/jcs.01610. [DOI] [PubMed] [Google Scholar]
- Lawrenson L, Poole JG, Kim J, Brown C, Patel P, Richardson RS. Vascular and metabolic response to isolated small muscle mass exercise: effect of age. Am J Physiol Heart Circ Physiol. 2003;285:H1023–H1031. doi: 10.1152/ajpheart.00135.2003. [DOI] [PubMed] [Google Scholar]
- Lee CM, Lopez ME, Weindruch R, Aiken JM. Association of age-related mitochondrial abnormalities with skeletal muscle fiber atrophy. Free Radic Biol Med. 1998;25:964–72. doi: 10.1016/s0891-5849(98)00185-3. [DOI] [PubMed] [Google Scholar]
- Lee HY, Choi CS, Birkenfeld AL, Alves TC, Jornayvaz FR, Jurczak MJ, et al. Targeted expression of catalase to mitochondria prevents age-associated reductions in mitochondrial function and insulin resistance. Cell Metab. 2010;12:668–74. doi: 10.1016/j.cmet.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Jeong SY, Karbowski M, Smith CL, Youle RJ. Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol Biol Cell. 2004;15:5001–11. doi: 10.1091/mbc.E04-04-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- Li L, Pan R, Li R, Niemann B, Aurich AC, Chen Y, et al. Mitochondrial biogenesis and peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1alpha) deacetylation by physical activity: intact adipocytokine signaling is required. Diabetes. 2011;60:157–67. doi: 10.2337/db10-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Lu AM, Wang Y, Hong A, Chen Y, Hu J, et al. Chronic resistance training activates autophagy and reduces apoptosis of muscle cells by modulating IGF-1 and its receptors, Akt/mTOR and Akt/FOXO3a signaling in aged rats. Exp Gerontol. 2013;48:427–36. doi: 10.1016/j.exger.2013.02.009. [DOI] [PubMed] [Google Scholar]
- Marvar PJ, Hammer LW, Boegehold MA. Hydrogen peroxide-dependent arteriolar dilation in contracting muscle of rats fed normal and high salt diets. Microcirculation. 2007;14:779–91. doi: 10.1080/10739680701444057. [DOI] [PubMed] [Google Scholar]
- Marzetti E. Imaging, functional and biological markers for sarcopenia: the pursuit of the golden ratio. J Frailty Aging. 2012;1:97–8. doi: 10.14283/jfa.2012.15. [DOI] [PubMed] [Google Scholar]
- Marzetti E, Buford TW, Bernabei R. Is misuse of the term sarcopenia due to a lack of biomarkers? J Appl Physiol. 2012a;113:680. [Google Scholar]
- Marzetti E, Calvani R, Bernabei R, Leeuwenburgh C. Apoptosis in skeletal myocytes: a potential target for interventions against sarcopenia and physical frailty - a mini-review. Gerontology. 2012b;58:99–106. doi: 10.1159/000330064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzetti E, Calvani R, Dupree J, Lees HA, Giovannini S, Seo DO, et al. Late-life enalapril administration induces nitric oxide-dependent and independent metabolic adaptations in the rat skeletal muscle. Age (Dordr) 2012c doi: 10.1007/s11357-012-9428-4. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzetti E, Groban L, Wohlgemuth SE, Lees HA, Lin M, Jobe H, et al. Effects of short-term GH supplementation and treadmill exercise training on physical performance and skeletal muscle apoptosis in old rats. Am J Physiol Regul Integr Comp Physiol. 2008a;294:R558–R567. doi: 10.1152/ajpregu.00620.2007. [DOI] [PubMed] [Google Scholar]
- Marzetti E, Hwang JC, Lees HA, Wohlgemuth SE, Dupont-Versteegden EE, Carter CS, et al. Mitochondrial death effectors: relevance to sarcopenia and disuse muscle atrophy. Biochim Biophys Acta. 2010a;1800:235–44. doi: 10.1016/j.bbagen.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzetti E, Lees HA, Manini TM, Buford TW, Aranda JM, Jr, Calvani R, et al. Skeletal muscle apoptotic signaling predicts thigh muscle volume and gait speed in community-dwelling older persons: an exploratory study. PLoS ONE. 2012d;7:e32829. doi: 10.1371/journal.pone.0032829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzetti E, Lees HA, Wohlgemuth SE, Leeuwenburgh C. Sarcopenia of aging: Underlying cellular mechanisms and protection by calorie restriction. BioFactors. 2009;35:28–35. doi: 10.1002/biof.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzetti E, Leeuwenburgh C. Skeletal muscle apoptosis, sarcopenia and frailty at old age. Exp Gerontol. 2006;41:1234–8. doi: 10.1016/j.exger.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Marzetti E, Privitera G, Simili V, Wohlgemuth SE, Aulisa L, Pahor M, et al. Multiple pathways to the same end: mechanisms of myonuclear apoptosis in sarcopenia of aging. ScientificWorldJournal. 2010b;10:340–9. doi: 10.1100/tsw.2010.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzetti E, Wohlgemuth SE, Lees HA, Chung HY, Giovannini S, Leeuwenburgh C. Age-related activation of mitochondrial caspase-independent apoptotic signaling in rat gastrocnemius muscle. Mech Ageing Dev. 2008b;129:542–9. doi: 10.1016/j.mad.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews GD, Huang CL, Sun L, Zaidi M. Translational musculoskeletal science: is sarcopenia the next clinical target after osteoporosis? Ann N Y Acad Sci. 2011;1237:95–105. doi: 10.1111/j.1749-6632.2011.06236.x. [DOI] [PubMed] [Google Scholar]
- Miquel J, Economos AC, Fleming J, Johnson JE., Jr Mitochondrial role in cell aging. Exp Gerontol. 1980;15:575–91. doi: 10.1016/0531-5565(80)90010-8. [DOI] [PubMed] [Google Scholar]
- Mitsuishi M, Miyashita K, Muraki A, Itoh H. Angiotensin II reduces mitochondrial content in skeletal muscle and affects glycemic control. Diabetes. 2009;58:710–7. doi: 10.2337/db08-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley JE, Abbatecola AM, Argiles JM, Baracos V, Bauer J, Bhasin S, et al. Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc. 2011;12:403–9. doi: 10.1016/j.jamda.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley JE, Malmstrom TK. Frailty, sarcopenia, and hormones. Endocrinol Metab Clin North Am. 2013;42:391–405. doi: 10.1016/j.ecl.2013.02.006. [DOI] [PubMed] [Google Scholar]
- Muller-Delp J, Spier SA, Ramsey MW, Lesniewski LA, Papadopoulos A, Humphrey JD, et al. Effects of aging on vasoconstrictor and mechanical properties of rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol. 2002;282:H1843–H1854. doi: 10.1152/ajpheart.00666.2001. [DOI] [PubMed] [Google Scholar]
- Musa M, Fernando SM, Chatterjee D, Monks DA. Subcellular effects of myocyte-specific androgen receptor overexpression in mice. J Endocrinol. 2011;210:93–104. doi: 10.1530/JOE-11-0071. [DOI] [PubMed] [Google Scholar]
- Muscaritoli M, Anker SD, Argiles J, Aversa Z, Bauer JM, Biolo G, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr. 2010;29:154–9. doi: 10.1016/j.clnu.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Myhill S, Booth NE, McLaren-Howard J. Chronic fatigue syndrome and mitochondrial dysfunction. Int J Clin Exp Med. 2009;2:1–16. [PMC free article] [PubMed] [Google Scholar]
- Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussler AK, Billiar TR. Inflammation, immunoregulation, and inducible nitric oxide synthase. J Leukoc Biol. 1993;54:171–8. [PubMed] [Google Scholar]
- O’Leary MF, Vainshtein A, Iqbal S, Ostojic O, Hood DA. Adaptive plasticity of autophagic proteins to denervation in aging skeletal muscle. Am J Physiol Cell Physiol. 2013;304:C422–C430. doi: 10.1152/ajpcell.00240.2012. [DOI] [PubMed] [Google Scholar]
- O’Rourke RW, White AE, Metcalf MD, Olivas AS, Mitra P, Larison WG, et al. Hypoxia-induced inflammatory cytokine secretion in human adipose tissue stromovascular cells. Diabetologia. 2011;54:1480–90. doi: 10.1007/s00125-011-2103-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono T, Isobe K, Nakada K, Hayashi JI. Human cells are protected from mitochondrial dysfunction by complementation of DNA products in fused mitochondria. Nat Genet. 2001;28:272–5. doi: 10.1038/90116. [DOI] [PubMed] [Google Scholar]
- Orlander J, Kiessling KH, Larsson L, Karlsson J, Aniansson A. Skeletal muscle metabolism and ultrastructure in relation to age in sedentary men. Acta Physiol Scand. 1978;104:249–61. doi: 10.1111/j.1748-1716.1978.tb06277.x. [DOI] [PubMed] [Google Scholar]
- Padilla J, Jenkins NT, Vieira-Potter VJ, Laughlin MH. Divergent phenotype of rat thoracic and abdominal perivascular adipose tissues. Am J Physiol Regul Integr Comp Physiol. 2013;304:R543–R552. doi: 10.1152/ajpregu.00567.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahor M, Cesari M. Designing Phase II B trials in sarcopenia: the best target population. J Nutr Health Aging. 2011;15:725–30. doi: 10.1007/s12603-011-0058-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Kim HY, Lee JH, Yoon KH, Chang MS, Park SK. The age-dependent induction of apoptosis-inducing factor (AIF) in the human semitendinosus skeletal muscle. Cell Mol Biol Lett. 2010;15:1–12. doi: 10.2478/s11658-009-0030-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140–2. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard M, Ritchie D, Thomas MM, Wright KJ, Hepple RT. Alterations in intrinsic mitochondrial function with aging are fiber type-specific and do not explain differential atrophy between muscles. Aging Cell. 2011;10:1047–55. doi: 10.1111/j.1474-9726.2011.00745.x. [DOI] [PubMed] [Google Scholar]
- Pierce GL, Lesniewski LA, Lawson BR, Beske SD, Seals DR. Nuclear factor-{kappa}B activation contributes to vascular endothelial dysfunction via oxidative stress in overweight/obese middle-aged and older humans. Circulation. 2009;119:1284–92. doi: 10.1161/CIRCULATIONAHA.108.804294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistilli EE, Siu PM, Alway SE. Molecular regulation of apoptosis in fast plantaris muscles of aged rats. J Gerontol A Biol Sci Med Sci. 2006;61:245–55. doi: 10.1093/gerona/61.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitteloud N, Mootha VK, Dwyer AA, Hardin M, Lee H, Eriksson KF, et al. Relationship between testosterone levels, insulin sensitivity, and mitochondrial function in men. Diabetes Care. 2005;28:1636–42. doi: 10.2337/diacare.28.7.1636. [DOI] [PubMed] [Google Scholar]
- Psarra AM, Sekeris CE. Steroid and thyroid hormone receptors in mitochondria. IUBMB Life. 2008;60:210–23. doi: 10.1002/iub.37. [DOI] [PubMed] [Google Scholar]
- Rajawat YS, Hilioti Z, Bossis I. Aging: central role for autophagy and the lysosomal degradative system. Ageing Res Rev. 2009;8:199–213. doi: 10.1016/j.arr.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Reid MB, Khawli FA, Moody MR. Reactive oxygen in skeletal muscle. III. Contractility of unfatigued muscle. J Appl Physiol. 1993;75:1081–7. doi: 10.1152/jappl.1993.75.3.1081. [DOI] [PubMed] [Google Scholar]
- Remels AH, Gosker HR, Schrauwen P, Hommelberg PP, Sliwinski P, Polkey M, et al. TNF-alpha impairs regulation of muscle oxidative phenotype: implications for cachexia? FASEB J. 2010;24:5052–62. doi: 10.1096/fj.09-150714. [DOI] [PubMed] [Google Scholar]
- Rice KM, Blough ER. Sarcopenia-related apoptosis is regulated differently in fast- and slow-twitch muscles of the aging F344/N x BN rat model. Mech Ageing Dev. 2006;127:670–9. doi: 10.1016/j.mad.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Rieu I, Magne H, Savary-Auzeloux I, Averous J, Bos C, Peyron MA, et al. Reduction of low grade inflammation restores blunting of postprandial muscle anabolism and limits sarcopenia in old rats. J Physiol. 2009;587:5483–92. doi: 10.1113/jphysiol.2009.178319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley DA, Slocum GR, Bain JL, Sedlak FR, Sowa TE, Mellender JW. Rat hindlimb unloading: soleus histochemistry, ultrastructure, and electromyography. J Appl Physiol. 1990;69:58–66. doi: 10.1152/jappl.1990.69.1.58. [DOI] [PubMed] [Google Scholar]
- Romanello V, Guadagnin E, Gomes L, Roder I, Sandri C, Petersen Y, et al. Mitochondrial fission and remodelling contributes to muscle atrophy. EMBO J. 2010;29:1774–85. doi: 10.1038/emboj.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseberg IH. Summary comments. Am J Clin Nutr. 1989;50:1231–3. [Google Scholar]
- Ruas JL, White JP, Rao RR, Kleiner S, Brannan KT, Harrison BC, et al. A PGC-1alpha isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell. 2012;151:1319–31. doi: 10.1016/j.cell.2012.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ DW, Krause J, Wills A, Arreguin R. “SR stress” in mixed hindlimb muscles of aging male rats. Biogerontology. 2012;13:547–55. doi: 10.1007/s10522-012-9399-y. [DOI] [PubMed] [Google Scholar]
- Russell JA, Kindig CA, Behnke BJ, Poole DC, Musch TI. Effects of aging on capillary geometry and hemodynamics in rat spinotrapezius muscle. Am J Physiol Heart Circ Physiol. 2003;285:H251–H258. doi: 10.1152/ajpheart.01086.2002. [DOI] [PubMed] [Google Scholar]
- Sack MN. Mitofusin function is dependent on the distinct tissue and organ specific roles of mitochondria. J Mol Cell Cardiol. 2011;51:881–2. doi: 10.1016/j.yjmcc.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safdar A, Hamadeh MJ, Kaczor JJ, Raha S, Debeer J, Tarnopolsky MA. Aberrant mitochondrial homeostasis in the skeletal muscle of sedentary older adults. PLoS ONE. 2010;5:e10778. doi: 10.1371/journal.pone.0010778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh S, Kiyooka T, Rocic P, Rogers PA, Zhang C, Swafford A, et al. Redox-dependent coronary metabolic dilation. Am J Physiol Heart Circ Physiol. 2007;293:H3720–H3725. doi: 10.1152/ajpheart.00436.2007. [DOI] [PubMed] [Google Scholar]
- Saitoh S, Zhang C, Tune JD, Potter B, Kiyooka T, Rogers PA, et al. Hydrogen peroxide: a feed-forward dilator that couples myocardial metabolism to coronary blood flow. Arterioscler Thromb Vasc Biol. 2006;26:2614–21. doi: 10.1161/01.ATV.0000249408.55796.da. [DOI] [PubMed] [Google Scholar]
- Sakuma K, Yamaguchi A. Sarcopenia and age-related endocrine function. Int J Endocrinol. 2012;2012:127362. doi: 10.1155/2012/127362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A, Kaarniranta K, Kauppinen A. Inflammaging: disturbed interplay between autophagy and inflammasomes. Aging (Albany NY) 2012;4:166–75. doi: 10.18632/aging.100444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salucci S, Burattini S, Baldassarri V, Battistelli M, Canonico B, Valmori A, et al. The peculiar apoptotic behavior of skeletal muscle cells. Histol Histopathol. 2013 doi: 10.14670/HH-28.1073. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Salvioli S, Capri M, Valensin S, Tieri P, Monti D, Ottaviani E, et al. Inflamm-aging, cytokines and aging: state of the art, new hypotheses on the role of mitochondria and new perspectives from systems biology. Curr Pharm Des. 2006;12:3161–71. doi: 10.2174/138161206777947470. [DOI] [PubMed] [Google Scholar]
- Sampey BP, Freemerman AJ, Zhang J, Kuan PF, Galanko JA, O’Connell TM, et al. Metabolomic profiling reveals mitochondrial-derived lipid biomarkers that drive obesity-associated inflammation. PLoS ONE. 2012;7:e38812. doi: 10.1371/journal.pone.0038812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandri M. Autophagy in health and disease. 3. Involvement of autophagy in muscle atrophy. Am J Physiol Cell Physiol. 2010;298:C1291–C1297. doi: 10.1152/ajpcell.00531.2009. [DOI] [PubMed] [Google Scholar]
- Sandri M. Protein breakdown in muscle wasting: Role of autophagy-lysosome and ubiquitin-proteasome. Int J Biochem Cell Biol. 2013 doi: 10.1016/j.biocel.2013.04.023. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandri M, Lin J, Handschin C, Yang W, Arany ZP, Lecker SH, et al. PGC-1alpha protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc Natl Acad Sci U S A. 2006;103:16260–5. doi: 10.1073/pnas.0607795103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A, Nakada K, Hayashi J. Mitochondrial dynamics and aging: Mitochondrial interaction preventing individuals from expression of respiratory deficiency caused by mutant mtDNA. Biochim Biophys Acta. 2006;1763:473–81. doi: 10.1016/j.bbamcr.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Scorrano L, Ashiya M, Buttle K, Weiler S, Oakes SA, Mannella CA, et al. A distinct pathway remodels mitochondrial cristae and mobilizes cytochrome c during apoptosis. Dev Cell. 2002;2:55–67. doi: 10.1016/s1534-5807(01)00116-2. [DOI] [PubMed] [Google Scholar]
- Seo AY, Xu J, Servais S, Hofer T, Marzetti E, Wohlgemuth SE, et al. Mitochondrial iron accumulation with age and functional consequences. Aging Cell. 2008;7:706–16. doi: 10.1111/j.1474-9726.2008.00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham O, Slate NG, Goldberger O, Xu Q, Ramanathan A, Souza AL, et al. A plasma signature of human mitochondrial disease revealed through metabolic profiling of spent media from cultured muscle cells. Proc Natl Acad Sci U S A. 2010;107:1571–5. doi: 10.1073/pnas.0906039107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, et al. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci U S A. 2005;102:5618–23. doi: 10.1073/pnas.0501559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short KR, Vittone JL, Bigelow ML, Proctor DN, Nair KS. Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am J Physiol Endocrinol Metab. 2004;286:E92–101. doi: 10.1152/ajpendo.00366.2003. [DOI] [PubMed] [Google Scholar]
- Silvagno F, Consiglio M, Foglizzo V, Destefanis M, Pescarmona G. Mitochondrial translocation of vitamin D receptor is mediated by the permeability transition pore in human keratinocyte cell line. PLoS ONE. 2013;8:e54716. doi: 10.1371/journal.pone.0054716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvagno F, De VE, Attanasio A, Gallo V, Mazzucco G, Pescarmona G. Mitochondrial localization of vitamin D receptor in human platelets and differentiated megakaryocytes. PLoS ONE. 2010;5:e8670. doi: 10.1371/journal.pone.0008670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindler AL, Delp MD, Reyes R, Wu G, Muller-Delp JM. Effects of ageing and exercise training on eNOS uncoupling in skeletal muscle resistance arterioles. J Physiol. 2009;587:3885–97. doi: 10.1113/jphysiol.2009.172221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha A, Hollingsworth KG, Ball S, Cheetham T. Improving the vitamin d status of vitamin d deficient adults is associated with improved mitochondrial oxidative function in skeletal muscle. J Clin Endocrinol Metab. 2013;98:E509–E513. doi: 10.1210/jc.2012-3592. [DOI] [PubMed] [Google Scholar]
- Song W, Kwak HB, Lawler JM. Exercise training attenuates age-induced changes in apoptotic signaling in rat skeletal muscle. Antioxid Redox Signal. 2006;8:517–28. doi: 10.1089/ars.2006.8.517. [DOI] [PubMed] [Google Scholar]
- Stockton KA, Mengersen K, Paratz JD, Kandiah D, Bennell KL. Effect of vitamin D supplementation on muscle strength: a systematic review and meta-analysis. Osteoporos Int. 2011;22:859–71. doi: 10.1007/s00198-010-1407-y. [DOI] [PubMed] [Google Scholar]
- Studenski S. Target population for clinical trials. J Nutr Health Aging. 2009;13:729–32. doi: 10.1007/s12603-009-0205-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stump CS, Short KR, Bigelow ML, Schimke JM, Nair KS. Effect of insulin on human skeletal muscle mitochondrial ATP production, protein synthesis, and mRNA transcripts. Proc Natl Acad Sci U S A. 2003;100:7996–8001. doi: 10.1073/pnas.1332551100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramani S, Malhotra V. Non-autophagic roles of autophagy-related proteins. EMBO Rep. 2013;14:143–51. doi: 10.1038/embor.2012.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomalainen A, Elo JM, Pietilainen KH, Hakonen AH, Sevastianova K, Korpela M, et al. FGF-21 as a biomarker for muscle-manifesting mitochondrial respiratory chain deficiencies: a diagnostic study. Lancet Neurol. 2011;10:806–18. doi: 10.1016/S1474-4422(11)70155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvorava T, Lauer N, Kumpf S, Jacob R, Meyer W, Kojda G. Endogenous vascular hydrogen peroxide regulates arteriolar tension in vivo. Circulation. 2005;112:2487–95. doi: 10.1161/CIRCULATIONAHA.105.543157. [DOI] [PubMed] [Google Scholar]
- Taaffe DR, Pruitt L, Reim J, Hintz RL, Butterfield G, Hoffman AR, et al. Effect of recombinant human growth hormone on the muscle strength response to resistance exercise in elderly men. J Clin Endocrinol Metab. 1994;79:1361–6. doi: 10.1210/jcem.79.5.7525633. [DOI] [PubMed] [Google Scholar]
- Teixeira CE, Duarte JA. Myonuclear domain in skeletal muscle fibers. A critical review. Archives of Exercise in Health and Disease. 2011;2:92–101. [Google Scholar]
- Terjung RL, Zarzeczny R, Yang HT. Muscle blood flow and mitochondrial function: influence of aging. Int J Sport Nutr Exerc Metab. 2002;12:368–78. doi: 10.1123/ijsnem.12.3.368. [DOI] [PubMed] [Google Scholar]
- Terman A, Brunk UT. Myocyte aging and mitochondrial turnover. Exp Gerontol. 2004;39:701–5. doi: 10.1016/j.exger.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Terman A, Kurz T, Navratil M, Arriaga EA, Brunk UT. Mitochondrial turnover and aging of long-lived postmitotic cells: the mitochondrial-lysosomal axis theory of aging. Antioxid Redox Signal. 2010;12:503–35. doi: 10.1089/ars.2009.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorner MO. Statement by the Growth Hormone Research Society on the GH/IGF-I axis in extending health span. J Gerontol A Biol Sci Med Sci. 2009;64:1039–44. doi: 10.1093/gerona/glp091. [DOI] [PubMed] [Google Scholar]
- Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008a;27:433–46. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twig G, Hyde B, Shirihai OS. Mitochondrial fusion, fission and autophagy as a quality control axis: the bioenergetic view. Biochim Biophys Acta. 2008b;1777:1092–7. doi: 10.1016/j.bbabio.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Labinskyy N, Gupte S, Chander PN, Edwards JG, Csiszar A. Dysregulation of mitochondrial biogenesis in vascular endothelial and smooth muscle cells of aged rats. Am J Physiol Heart Circ Physiol. 2008;294:H2121–H2128. doi: 10.1152/ajpheart.00012.2008. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith K, et al. Increased mitochondrial H2O2 production promotes endothelial NF-kappaB activation in aged rat arteries. Am J Physiol Heart Circ Physiol. 2007;293:H37–H47. doi: 10.1152/ajpheart.01346.2006. [DOI] [PubMed] [Google Scholar]
- van der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, et al. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192:1731–44. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Meer SF, Jaspers RT, Degens H. Is the myonuclear domain size fixed? J Musculoskelet Neuronal Interact. 2011;11:286–97. [PubMed] [Google Scholar]
- Viguie CA, Lu DX, Huang SK, Rengen H, Carlson BM. Quantitative study of the effects of long-term denervation on the extensor digitorum longus muscle of the rat. Anat Rec. 1997;248:346–54. doi: 10.1002/(SICI)1097-0185(199707)248:3<346::AID-AR7>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Vina J, Gomez-Cabrera MC, Borras C, Froio T, Sanchis-Gomar F, Martinez-Bello VE, et al. Mitochondrial biogenesis in exercise and in ageing. Adv Drug Deliv Rev. 2009;61:1369–74. doi: 10.1016/j.addr.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Visser M, Pahor M, Taaffe DR, Goodpaster BH, Simonsick EM, Newman AB, et al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002;57:M326–M332. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- Visser M, Schaap LA. Consequences of sarcopenia. Clin Geriatr Med. 2011;27:387–99. doi: 10.1016/j.cger.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Wagatsuma A, Kotake N, Mabuchi K, Yamada S. Expression of nuclear-encoded genes involved in mitochondrial biogenesis and dynamics in experimentally denervated muscle. J Physiol Biochem. 2011;67:359–70. doi: 10.1007/s13105-011-0083-5. [DOI] [PubMed] [Google Scholar]
- Wanagat J, Cao Z, Pathare P, Aiken JM. Mitochondrial DNA deletion mutations colocalize with segmental electron transport system abnormalities, muscle fiber atrophy, fiber splitting, and oxidative damage in sarcopenia. FASEB J. 2001;15:322–32. doi: 10.1096/fj.00-0320com. [DOI] [PubMed] [Google Scholar]
- Wang C, Youle RJ. The role of mitochondria in apoptosis. Annu Rev Genet. 2009;43:95–118. doi: 10.1146/annurev-genet-102108-134850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Pickrell AM, Rossi SG, Pinto M, Dillon LM, Hida A, et al. Transient systemic mtDNA damage leads to muscle wasting by reducing the satellite cell pool. Hum Mol Genet. 2013 doi: 10.1093/hmg/ddt251. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K, Bruck P, Mikes Z, Kupper JH, Klingenspor M, Wiesner RJ. Glucocorticoid hormone stimulates mitochondrial biogenesis specifically in skeletal muscle. Endocrinology. 2002;143:177–84. doi: 10.1210/endo.143.1.8600. [DOI] [PubMed] [Google Scholar]
- Weitzel JM, Iwen KA. Coordination of mitochondrial biogenesis by thyroid hormone. Mol Cell Endocrinol. 2011;342:1–7. doi: 10.1016/j.mce.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Welle S, Bhatt K, Shah B, Needler N, Delehanty JM, Thornton CA. Reduced amount of mitochondrial DNA in aged human muscle. J Appl Physiol. 2003;94:1479–84. doi: 10.1152/japplphysiol.01061.2002. [DOI] [PubMed] [Google Scholar]
- Wenz T, Rossi SG, Rotundo RL, Spiegelman BM, Moraes CT. Increased muscle PGC-1alpha expression protects from sarcopenia and metabolic disease during aging. Proc Natl Acad Sci U S A. 2009;106:20405–10. doi: 10.1073/pnas.0911570106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wohlgemuth SE, Lees HA, Marzetti E, Manini TM, Aranda JM, Daniels MJ, et al. An exploratory analysis of the effects of a weight loss plus exercise program on cellular quality control mechanisms in older overweight women. Rejuvenation Res. 2011;14:315–24. doi: 10.1089/rej.2010.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlgemuth SE, Seo AY, Marzetti E, Lees HA, Leeuwenburgh C. Skeletal muscle autophagy and apoptosis during aging: effects of calorie restriction and life-long exercise. Exp Gerontol. 2010;45:138–48. doi: 10.1016/j.exger.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray DW, Nishiyama SK, Monnet A, Wary C, Duteil SS, Carlier PG, et al. Antioxidants and aging: NMR-based evidence of improved skeletal muscle perfusion and energetics. Am J Physiol Heart Circ Physiol. 2009;297:H1870–H1875. doi: 10.1152/ajpheart.00709.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakes FM, Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc Natl Acad Sci U S A. 1997;94:514–9. doi: 10.1073/pnas.94.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarasheski KE, Zachwieja JJ, Campbell JA, Bier DM. Effect of growth hormone and resistance exercise on muscle growth and strength in older men. Am J Physiol. 1995;268:E268–E276. doi: 10.1152/ajpendo.1995.268.2.E268. [DOI] [PubMed] [Google Scholar]
- Yoon YS, Yoon DS, Lim IK, Yoon SH, Chung HY, Rojo M, et al. Formation of elongated giant mitochondria in DFO-induced cellular senescence: involvement of enhanced fusion process through modulation of Fis1. J Cell Physiol. 2006;209:468–80. doi: 10.1002/jcp.20753. [DOI] [PubMed] [Google Scholar]
- Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science. 2012;337:1062–5. doi: 10.1126/science.1219855. [DOI] [PMC free article] [PubMed] [Google Scholar]