To the Editor
The precise mechanisms by which rhinovirus (RV) causes exacerbation of asthma remain unclear. The current explanation is that RV infection of airway epithelial cells induces release of chemokines, thereby attracting inflammatory cells and augmenting pre-existing allergic inflammation. Genes encoding chemokines are upregulated following experimental RV infection of the nasal epithelium,1 and experimentally infected asthmatic subjects show increased airway neutrophils, lymphocytes, and eosinophils.2 However, the notion that viral infection simply augments asthmatic airway inflammation may not explain how asthmatic subjects suffer manifestations of lower airways disease after colds while subjects without asthma do not. Could asthmatic subjects have a qualitatively different immune response to rhinovirus infection than do subjects without asthma?
To address this question, we developed a mouse model of RV-induced asthma exacerbation.3 Mice were treated with intraperitoneal and intranasal ovalbumin and then infected with RV. Following ovalbumin and RV, airways inflammation and hyper-responsiveness increased significantly over that generated by either ovalbumin or RV alone. In addition, we found that CD68-positive mononuclear cells, rather than or in addition to epithelial cells, were a major source of CCL11, IL-4,3 and CCL24 expression. While RV nonproductively infects bronchoalveolar macrophages in vitro,5 the former data demonstrate that monocytes may contribute to RV-induced airway responses in vivo.
To examine the role of monocytes/macrophages in RV infection in humans, we performed a preliminary study examining lower airway biopsy specimens from control and allergic asthmatic subjects experimentally infected with RV16. Subjects were originally enrolled between 2002 and 2004.6 All the subjects experienced cold symptoms. We studied samples taken during the acute cold phase (4 days after infection). To unmask antigens, tissue slides were heated under pressure for 90 secondsin 150 mM NaCl and 0.01 M citrate buffer (pH 6.0). To block unreacted formaldehyde groups, samples were incubated in 100 mM ammonium chloride in 70% ethanol or 0.02% sodium borohydride in PBS for 10 minutes. Tissues were blocked for 2 hours with 5% goat serum in Tris-buffered saline with 0.05% Tween-20 (pH 7.6). Sections were incubated with AlexaFluor 594 (Molecular Probes, Portland, Ore)-conjugated R16-7,6 an antibody that binds to the RV16 VP2 capsid protein and, depending on the experiment, mouse antihuman CD68 (clone KP1; EBioscience, San Diego, Calif), CD11b, CD11c, and/or isotype controls IgG1 κ, IgG2b, or IgG2a (all from Biolegend, San Diego, Calif). Antibodies were conjugated with AlexaFluor 488 or 633. Nuclei were stained with Hoescht 33258. Images were visualized by using an Axioplan microscope with Apotome attachment or LSM 510 confocal microscope (Carl Zeiss, Thornwood, NY).
Samples from 4 controls and 11 asthmatic subjects were obtained (for tabulation of staining results, see Table E1 in this article's Online Repository at www.jacionline.org). Because of prior use of the blocks and high autofluorescence, only a limited number of slides were available for study. Three of 4 controls and 8 of 11 asthmatic subjects showed a positive RV16 signal. Of the 3 control samples with RV, 2 demonstrated positive staining for RV but no CD68 staining, indicating successful epithelial infection with RV and an absence of macrophages (Fig 1, A-E). The remaining control sample demonstrated a few CD68-positive cells present in the epithelial layer, but no colocalization with RV. Of the 8 asthma samples with a positive RV signal, 7 were stained for CD68 (the eighth was not stained). Six showed colocalization of RV and CD68. Three samples contained an ample epithelial layer studded with RV-positive and RV-, CD68-double positive cells (Fig 1, F-J). Some CD68-positive cells displayed a different morphology than did surrounding cells. The remaining samples from asthma patients demonstrated epithelial damage (all 6 samples from asthmatic subjects with colocalization are shown in Fig E1 in the Online Repository at www.jacionline.org). In these cases, RV-, CD68-double positive cells were present in the airway lumen.
Fig 1.
Images of bronchial epithelium from control (A-E) and asthmatic (F-J) subjects experimentally infected with RV16. A, F, Hematoxylin and eosin. Arrows in F mark corresponding RV16-, CD68-positive cells. B, G, Merged immunofluorescence image showing staining for RV16 capsid protein (red), CD68 (green), and DAPI, a nuclear stain (blue). Colocalization is yellow. C, H, CD68 staining. D, I, RV16 staining. E, J, Isotype control IgGs. (Original magnification, ×640). DAPI, 4′-6-Diamidino-2-phenylindole, dihydrochloride.
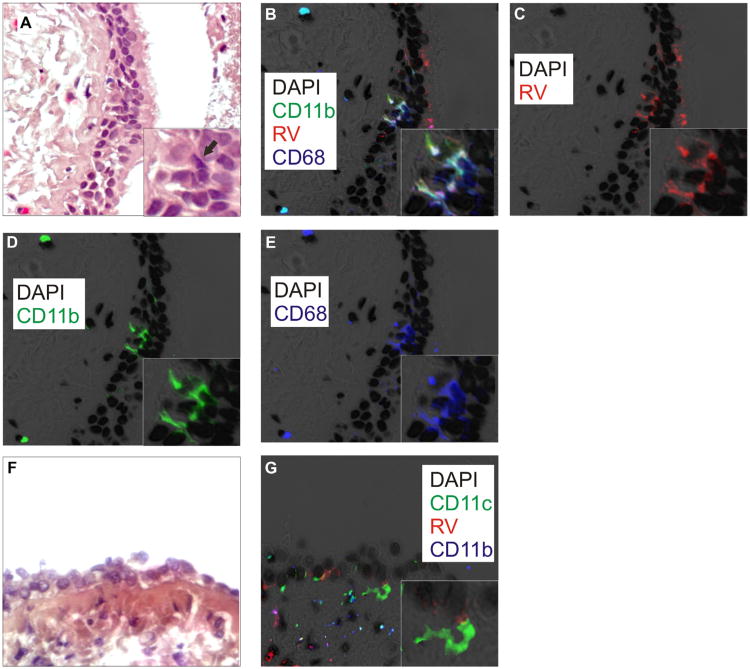
While CD68 is an accepted marker for monocytes/macrophages, other markers exist, including CD14, CD11c (resident macrophages), and CD11b (recruited macrophages). Because of intense nonspecific staining, we were unable to reliably test for CD14. However, we were able to stain 4 samples from asthmatic subjects for CD11b and CD11c. We observed colocalization of CD11b and RV16 in all 4 samples studied (samples with colocalization are shown in Fig E2 in the Online Repository at www.jacionline.org). In 2 cases, we found colocalization of RV, CD68, and CD11b (Fig 2, A-E). Finally, all 4 slides tested demonstrated CD11c-positive cells, but in only 1 instance was there colocalization with RV16. In 1 subject, CD11c-positive cells took on the appearance of dendritic cells (Fig 2, F and G). Together, these data suggest that following experimental infection of patients with allergic asthma, RV colocalizes with CD68 and CD11b in airway monocyte/macrophages.
Fig 2.
Asthmatic tissue stained with DAPI (black), R16-7 (red), anti-CD11b (green), and anti-CD68 (blue). A, F, Hematoxylin and eosin. The arrow in A marks a corresponding RV16-, CD11b-, CD68-positive cell. B, Colocalization of CD11b and CD68 is cyan; colocalization of 3 markers is white. C, RV16. D, CD11b. E, CD68. (Original magnification, ×640; insets show group of costained cells.) G, Image showing RV (red), CD11c (green), and CD11b (blue). CD11c-positive stellate cell underlying infected epithelial cell (inset). DAPI, 4′-6-Diamidino-2-phenylindole, dihydrochloride.
Macrophages play a critical role in innate immunity, including defense against pulmonary pathogens and the inflammatory response. Mice with allergic airways disease show increased alternatively activated, M2-polarized lung macrophages that produce type 2 cytokines in response to RV stimulation.3,4 Recruited CD11b-positive macrophages promote eosinophilic airway inflammation in mouse asthma models.4,7 Enhanced numbers of alternatively activated macrophages are found in the airways of subjects with asthma.8 It is therefore conceivable that the enhanced inflammatory response to RV in patients with allergic asthma is due to infection of recruited, activated macrophages that are normally not present in control individuals.
This study has limitations that should be considered in interpreting these findings, including the age of tissues, limited number of slides, and harsh unmasking protocols. We do not know whether the colocalization we observed represents replicative infection of airway macrophages, or simple endocytosis of the virus. Finally, under specific conditions, airway epithelial cells may take on features of phagocytic cells, including CD68 expression.9 Nevertheless, based on costaining with CD11b and morphologic differences, RV-, CD68-double positive cells in the airway epithelium are likely to be monocytes.
We conclude that RV is ingested by recruited CD68-positive and CD11b-positive macrophages in asthmatic humans, providing direct evidence that tissue macrophages in the lower airways contribute to anti-RV responses in vivo. A prospective study is needed to definitively characterize the role of monocytic infection in RV-induced asthma exacerbations.
Supplementary Material
Fig E1. Images of bronchial epithelium from 6 adult subjects with asthma experimentally infected with RV16. A, C, E, G, I, K, Hematoxylin and eosin staining. B, D, F, H, J, L, Merged immunofluorescence images show staining for RV16 (red), CD68 (green), and DAPI (black). Sections show colocalization of RV16 and CD68 (yellow). (Original magnification, ×640). DAPI, 4′-6-Diamidino-2-phenylindole, dihydrochloride.
Fig E2. Images of bronchial epithelium from 4 subjects with asthma infected with RV16. A, C, E, G, Hematoxylin and eosin staining. B, D, F, H, Merged immunofluorescence images show staining for RV16 (red), CD11b (green), and DAPI (black). Sections show colocalization of RV16 and CD11b (yellow). (Original magnification, ×640). DAPI, 4′-6-Diamidino-2-phenylindole, dihydrochloride.
Table E1. RV16, CD68, CD11b, and CD11c in tissue samples from controls and asthmatic subjects experimentally infected with RV16*
*Empty cells indicate that the staining was not completed.
Acknowledgments
This work was supported by National Institutes of Health grants HL081420 (to M.B.H.) and U19 AI070503 (to J.E.G.).
Footnotes
Disclosure of potential conflict of interest: J. K. Bentley and M. B. Dzaman have been supported by one or more grants from the National Institutes of Health (NIH) (HL081420). J. E. Gern has received consultancy fees from GlaxoSmithKline, Biota, Centocor, Boehringer Ingelheim, MedImmune, Theraclone, and Merck and has received one or more grants from Merck, AstraZeneca, and GlaxoSmithKline. M. B. Hershenson has been supported by one or more grants from the NIH and has consultancy arrangements with Boehringer Ingelheim, Gilead, and Almirall. U. S. Sajjan has received one or more grants from or has one or more grants pending with the NIH. The rest of the authors declare that they have no relevant conflicts of interest.
References
- 1.Proud D, Turner RB, Winther B, Wiehler S, Tiesman JP, Reichling TD, et al. Gene expression profiles during in vivo human rhinovirus infection: insights into the host response. Am J Respir Crit Care Med. 2008;178:962–8. doi: 10.1164/rccm.200805-670OC. [DOI] [PubMed] [Google Scholar]
- 2.Message SD, Laza-Stanca V, Mallia P, Parker HL, Zhu J, Kebadze T, et al. Rhino-virus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proc Natl Acad Sci U S A. 2008;105:13562–7. doi: 10.1073/pnas.0804181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagarkar DR, Bowman ER, Schneider D, Wang Q, Shim J, Zhao Y, et al. Rhinovirus infection of allergen-sensitized and -challenged mice induces eo-taxin release from functionally polarized macrophages. J Immunol. 2010;185:2525–35. doi: 10.4049/jimmunol.1000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schneider D, Hong JY, Bowman ER, Chung Y, Nagarkar DR, McHenry CL, et al. Macrophage/epithelial cell CCL2 contributes to rhinovirus-induced hyperrespon-siveness and inflammation in a mouse model of allergic airways disease. Am J Physiol Lung Cell Mol Physiol. 2013;304:L162–9. doi: 10.1152/ajplung.00182.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gern JE, Dick EC, Lee WM, Murray S, Meyer K, Handzel ZT, et al. Rhinovirus enters but does not replicate inside monocytes and airway macrophages. J Immunol. 1996;156:621–7. [PubMed] [Google Scholar]
- 6.Mosser AG, Vrtis R, Burchell L, Lee WM, Dick CR, Weisshaar E, et al. Quantitative and qualitative analysis of rhinovirus infection in bronchial tissues. Am J Respir Crit Care Med. 2005;171:645–51. doi: 10.1164/rccm.200407-970OC. [DOI] [PubMed] [Google Scholar]
- 7.Moon KA, Kim SY, Kim TB, Yun ES, Park CS, Cho YS, et al. Allergen-induced CD11b+ CD11cint CCR3+ macrophages in the lung promote eosinophilic airway inflammation in a mouse asthma model. Int Immunol. 2007;19:1371–81. doi: 10.1093/intimm/dxm108. [DOI] [PubMed] [Google Scholar]
- 8.Melgert BN, ten Hacken NH, Rutgers B, Timens W, Postma DS, Hylkema MN. More alternative activation of macrophages in lungs of asthmatic patients. J Allergy Clin Immunol. 2011;127:831–3. doi: 10.1016/j.jaci.2010.10.045. [DOI] [PubMed] [Google Scholar]
- 9.Travaglione S, Falzano L, Fabbri A, Stringaro A, Fais S, Fiorentini C. Epithelial cells and expression of the phagocytic marker CD68: scavenging of apoptotic bodies following Rho activation. Toxicol In Vitro. 2002;16:405–11. doi: 10.1016/s0887-2333(02)00028-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig E1. Images of bronchial epithelium from 6 adult subjects with asthma experimentally infected with RV16. A, C, E, G, I, K, Hematoxylin and eosin staining. B, D, F, H, J, L, Merged immunofluorescence images show staining for RV16 (red), CD68 (green), and DAPI (black). Sections show colocalization of RV16 and CD68 (yellow). (Original magnification, ×640). DAPI, 4′-6-Diamidino-2-phenylindole, dihydrochloride.
Fig E2. Images of bronchial epithelium from 4 subjects with asthma infected with RV16. A, C, E, G, Hematoxylin and eosin staining. B, D, F, H, Merged immunofluorescence images show staining for RV16 (red), CD11b (green), and DAPI (black). Sections show colocalization of RV16 and CD11b (yellow). (Original magnification, ×640). DAPI, 4′-6-Diamidino-2-phenylindole, dihydrochloride.
Table E1. RV16, CD68, CD11b, and CD11c in tissue samples from controls and asthmatic subjects experimentally infected with RV16*
*Empty cells indicate that the staining was not completed.