Abstract
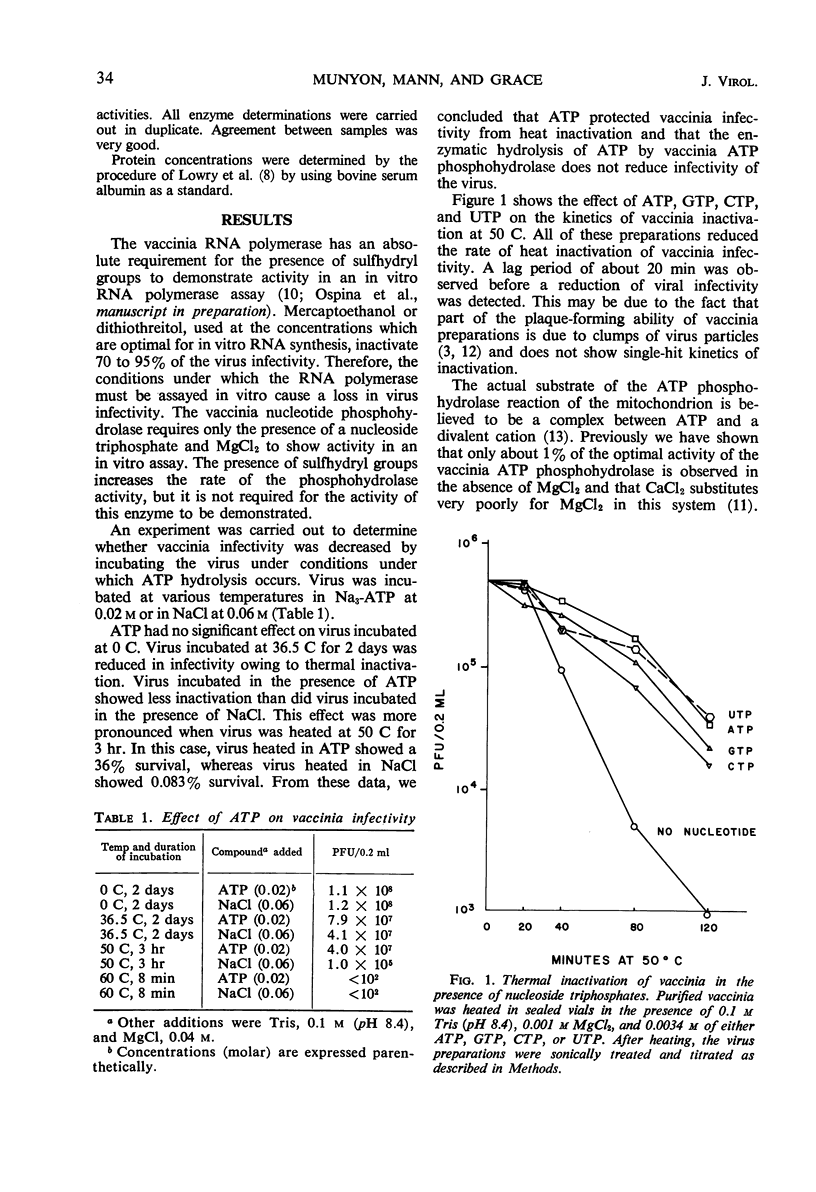
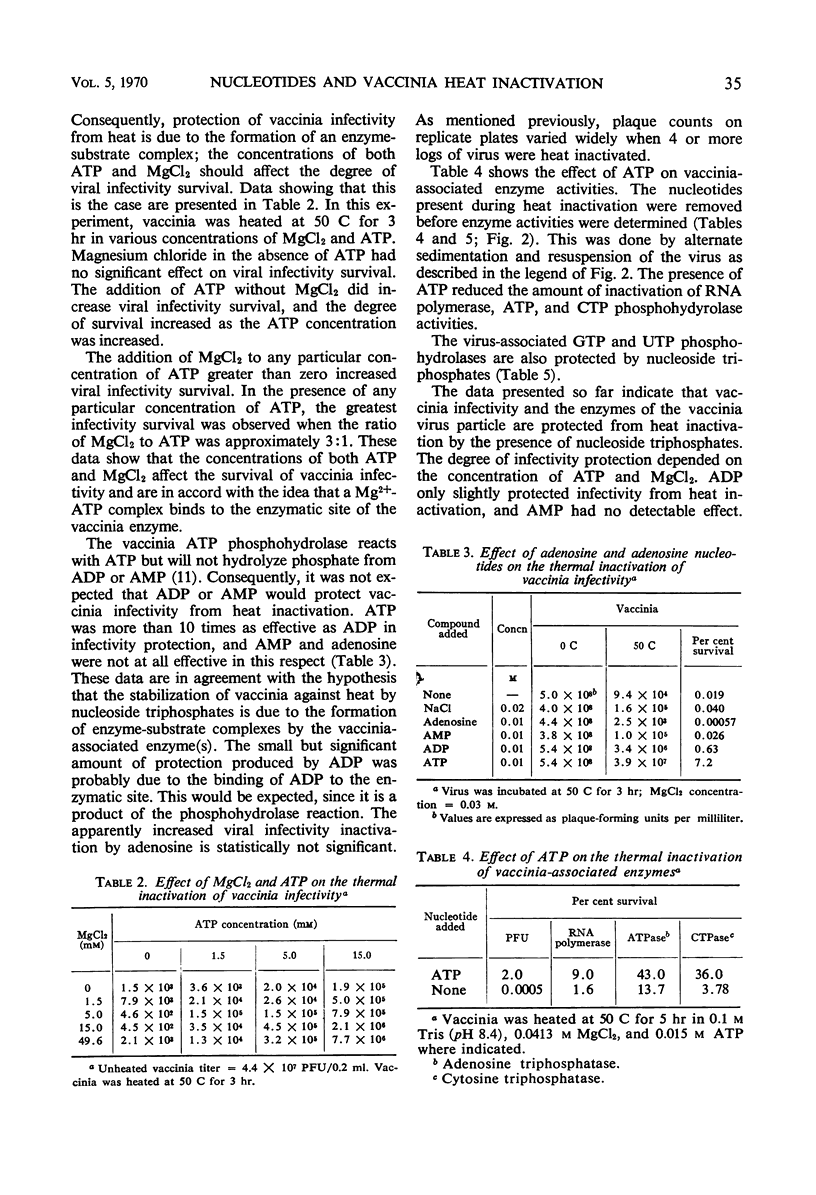
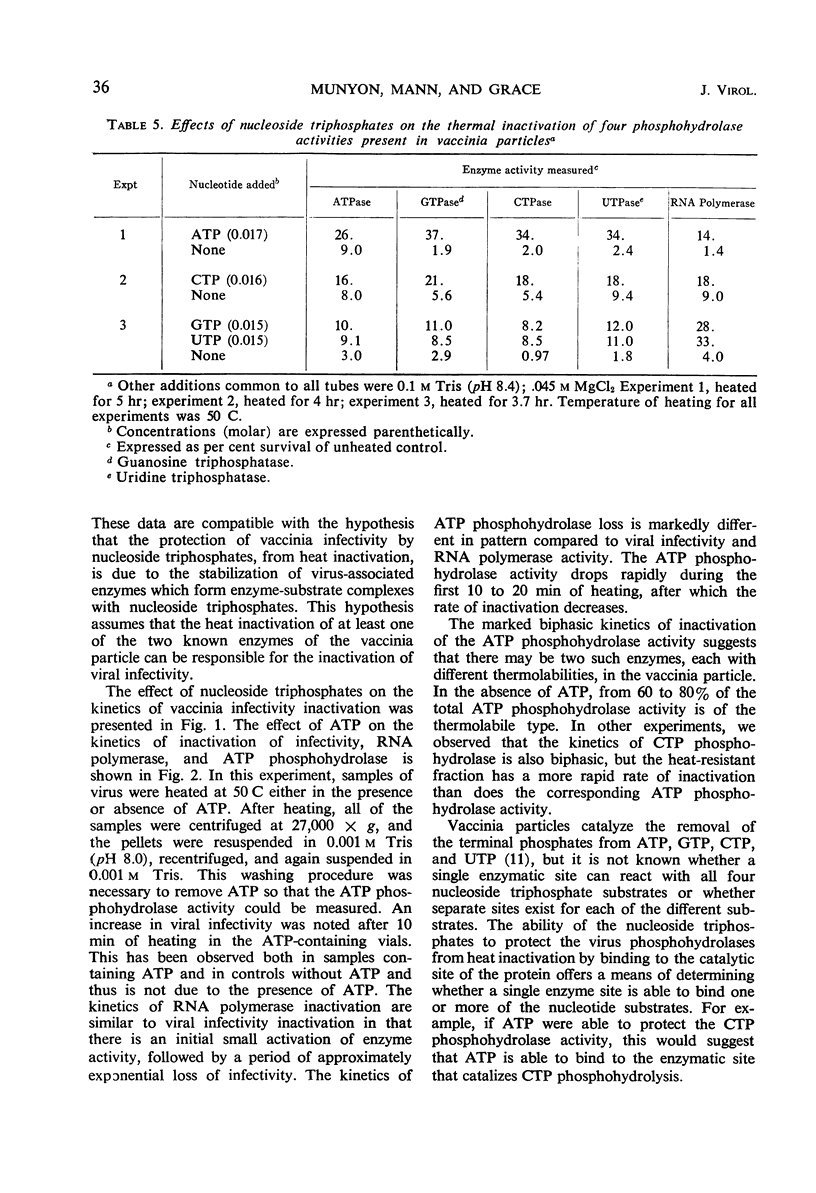
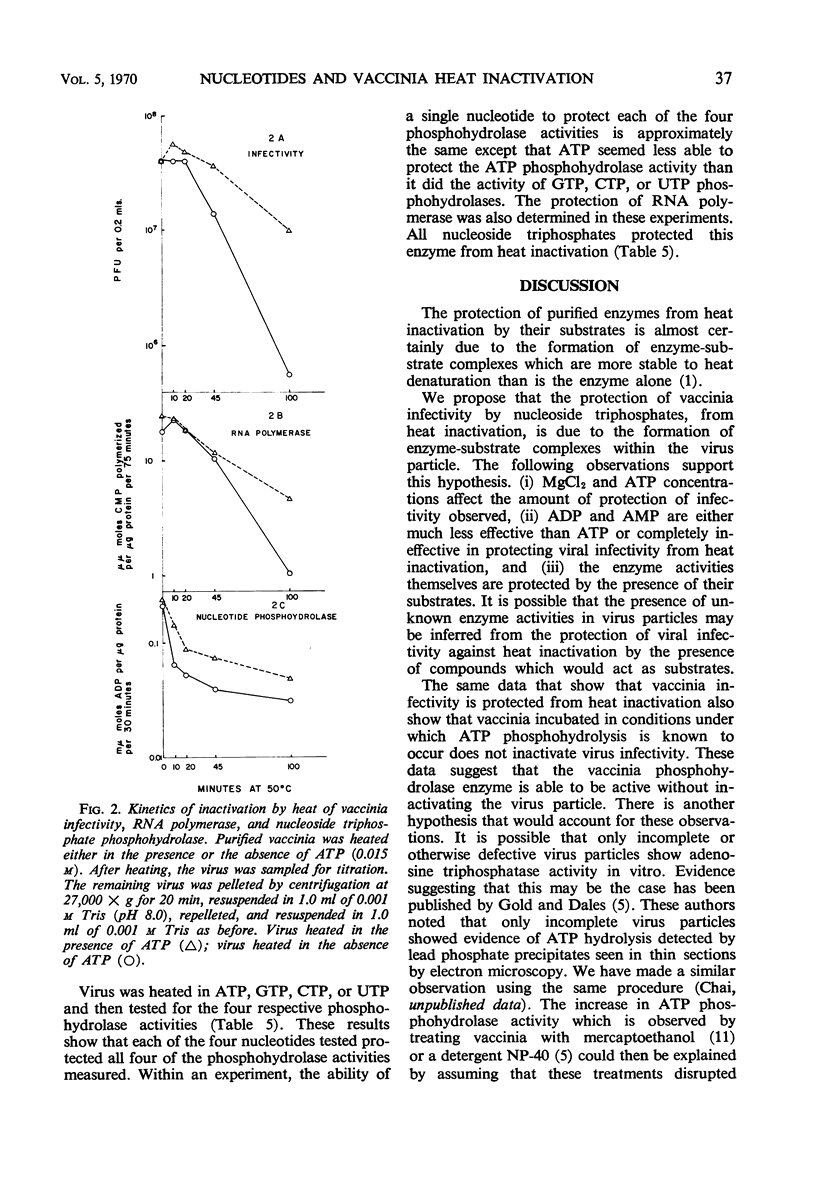
The presence of adenosine triphosphate, guanosine triphosphate, cytosine triphosphate, or uridine triphosphate reduced the rate of inactivation of vaccinia when heated at 50 C. The virus-associated nucleoside triphosphate phosphohydrolases (adenosine triphosphatase, guanosine triphosphatase, cytosine triphosphatase, and uridine triphosphatase) and ribonucleic acid polymerase were also protected from heat inactivation by these compounds. These obervations are best explained by postulating that ribonucleoside triphosphates bind to enzymes in the virus particle, and that these enzyme-substrate complexes are more resistant to thermal denaturation than are the enzymes without their substrates. The kinetics of heat inactivation of the vaccinia ATP phosphohydrolase activity is biphasic, suggesting that there are two proteins in the vaccinia particle that have this enzyme activity but they have different kinetics of heat inactivation. Any of the vaccinia-associated nucleotide phosphohydrolase activities are protected from heat inactivation by the presence of any one of the respective nucleoside triphosphates. This observation suggests that there is a single enzymatic site in vaccinia that is able to react with any ribonucleoside triphosphate.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Galasso G. J., Sharp D. G. Effect of particle aggregation on the survival of irradiated vaccinia virus. J Bacteriol. 1965 Oct;90(4):1138–1142. doi: 10.1128/jb.90.4.1138-1142.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford G. E., Klapper D. G. Enhancement of vaccinia virus plaque formation by trypsin. Proc Soc Exp Biol Med. 1967 Nov;126(2):515–517. doi: 10.3181/00379727-126-32492. [DOI] [PubMed] [Google Scholar]
- Gold P. H., Dales S. Localization of nucleotide phosphohydrolase activity within vaccinia. Proc Natl Acad Sci U S A. 1968 Jul;60(3):845–852. doi: 10.1073/pnas.60.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOKLIK W. K. The purification fo four strains of poxvirus. Virology. 1962 Sep;18:9–18. doi: 10.1016/0042-6822(62)90172-1. [DOI] [PubMed] [Google Scholar]
- Kates J. R., McAuslan B. R. Poxvirus DNA-dependent RNA polymerase. Proc Natl Acad Sci U S A. 1967 Jul;58(1):134–141. doi: 10.1073/pnas.58.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Munyon W., Paoletti E., Grace J. T., Jr RNA polymerase activity in purified infectious vaccinia virus. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2280–2287. doi: 10.1073/pnas.58.6.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munyon W., Paoletti E., Ospina J., Grace J. T., Jr Nucleotide phosphohydrolase in purified vaccinia virus. J Virol. 1968 Mar;2(3):167–172. doi: 10.1128/jvi.2.3.167-172.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp D. G., Kim K. S. Multiplicity reactivation and radiation survival of aggregated vaccinia virus. Calculation of plaque titer based on MR and particle aggregation seen in the electron microscope. Virology. 1966 Jul;29(3):359–366. doi: 10.1016/0042-6822(66)90211-x. [DOI] [PubMed] [Google Scholar]
- ULRICH F. KINETIC STUDIES OF THE ACTIVATION OF MITOCHONDRIAL ADENOSINE TRIPHOSPHATASE BY MG++. J Biol Chem. 1964 Oct;239:3532–3536. [PubMed] [Google Scholar]
- WALLIS C., YANG C. S., MELNICK J. L. Effect of cations on thermal inactivation of vaccinia, herpes simplex, and adenoviruses. J Immunol. 1962 Jul;89:41–46. [PubMed] [Google Scholar]


