Abstract
Ribonuclease P (RNase P) is an essential endonuclease that acts early in the tRNA biogenesis pathway. This enzyme catalyzes cleavage of the leader sequence of precursor tRNAs (pre-tRNAs), generating the mature 5′ end of tRNAs. RNase P activities have been identified in Bacteria, Archaea, and Eucarya, as well as organelles. Most forms of RNase P are ribonucleoproteins, i.e. they consist of an essential RNA subunit and protein subunits, although the composition of the enzyme in mitochondria and chloroplasts is still under debate. The recent purification of the eukaryotic nuclear RNase P has demonstrated a significantly larger protein content compared to the bacterial enzyme. Moreover, emerging evidence suggests that the eukaryotic RNase P has evolved into at least two related nuclear enzymes with distinct functions, RNase P and RNase MRP. Here we review current information on RNase P, with emphasis on the composition, structure, and functions of the eukaryotic nuclear holoenzyme, and its relationship with RNase MRP.
Keywords: tRNA processing, RNase MRP, rRNA processing, nucleolus, ribozyme
INTRODUCTION
Ribozyme Activity of RNase P RNA
The ribonucleoprotein nature of ribonuclease P (RNase P) has been proven for the holoenzymes in bacteria, archaea, and eukaryotic nuclei. Because the holoenzymes for RNase P and its closely related offshoot, RNase MRP, are highly complex nucleoproteins in eukaryotes (1–5), bacterial RNase P is composed of a large RNA subunit, usually ranging from 350 to 400 nucleotides, and one small protein subunit that contributes about 10% of the mass of the holoenzyme (reviewed in 6, 7); see also the RNase P database maintained by JW Brown at North Carolina State University, http://www.mbio.ncsu.edu/RNaseP/main.html (8). All bacterial RNase P RNAs that have been tested are ribozymes, i.e. they can recognize and cleave substrates of precursor tRNA (pre-tRNA) in the absence of the protein subunit under high ionic strength in vitro. However, the small protein subunit binds near the catalytic core of RNase P RNA and interacts directly with the pre-tRNA substrates, thus facilitating pre-tRNA recognition and modulating RNase P RNA structure (9–13). The protein component is essential for RNase P function in vivo, but it is not known whether this absolute requirement is caused by these known interactions with substrates and RNase P RNA, or whether the protein plays additional roles in the cell.
In contrast to the situation in bacteria, eukaryotic RNase P RNA subunits by themselves have not yet been shown to cleave pre-tRNA substrates even though they contain the five most conserved “critical regions” that are postulated to form the catalytic center of the bacterial ribozymes (14, 15). One possible reason is an increased dependence on the more extensive protein content of the eukaryotic holoenzyme for correct three-dimensional folding of the RNA subunit (16). However, it is also possible that essential functions of substrate binding and catalysis have devolved to the protein subunits.
An intermediate level of protein dependence is found in archaea. RNA subunits from some Methanobacteria, Thermococci, Pyrococci, and halobacteria display traces of catalytic activity under extremely high salt concentrations in the absence of protein (17). Recently, it has been shown that the RNase P purified from Methanothermobacter thermoautotrophicus has at least four proteins that are apparent homologs of eukaryotic nuclear RNase P protein subunits (Table 1) (18). The RNase P RNA from this species is a ribozyme at very high salt levels, and the activity can be partially restored at lower salt levels by addition of the Bacillus subtilis RNase P protein (17). This last result is surprising, since neither the archaeal nor the eukaryotic proteins have any obvious sequence homology to the small bacterial protein subunits. This suggests that the RNase P protein can enhance catalytic activity either in a relatively nonspecific fashion or by interacting with the conserved catalytic core of RNase P RNA.
TABLE 1.
Subunit composition of nuclear RNase P and RNase MRP from yeast (S. cerevisiae) and human cells
| Yeast gene | Human gene | |||||||
|---|---|---|---|---|---|---|---|---|
| RNase P | RNase MRP |
Subunit type |
Molecular weight (kDa)a |
Isoelectric point (pI)a |
RNase P | RNase MRP |
Archaeal RNase P |
Reference |
| RPR1 | — | RNA | 120 | — | H1 | — | — | 73, 79 |
| — | NME1 | RNA | 112 | — | — | 7-2 | — | 168, 169 |
| POP1 | POP1 | Protein | 100.5 | 9.84 | hPOP1 | hPOP1 | — | 80, 86 |
| POP3 | POP3 | Protein | 22.6 | 9.57 | — | — | — | 81 |
| POP4 | POP4 | Protein | 32.9 | 9.26 | RPP29b | RPP29b | MTH11 | 18, 82, 88 |
| POP5 | POP5 | Protein | 19.6 | 7.79 | hPOP5 | hPOP5 | MTH687 | 1, 18, 89 |
| POP6 | POP6 | Protein | 18.2 | 9.28 | — | — | — | 1 |
| POP7c | POP7c | Protein | 15.8 | 9.34 | RPP20 | — | — | 1, 85 |
| POP8 | POP8 | Protein | 15.5 | 4.57 | — | — | — | 1 |
| RPP1 | RPP1 | Protein | 32.2 | 9.76 | RPP30 | RPP30 | MTH688 | 18, 83, 85 |
| RPR2 | — | Protein | 16.3 | 9.99 | RPP21 | — | MTH1618 | 1, 18, 90 |
| — | SNM1 | Protein | 22.5 | 9.81 | — | — | — | 156 |
| — | — | Protein | — | — | RPP38d | RPP38d | — | 85 |
| — | — | Protein | — | — | RPP40d | — | — | 85 |
| — | — | Protein | — | — | RPP25d | — | — | 85 |
| — | — | Protein | — | — | RPP14d | — | — | 88 |
Functions of RNase P in Bacteria and Eukaryotes
The best-defined function of RNase P is catalysis of the hydrolysis of a specific phosphodiester bond in pre-tRNAs, leaving a phosphate group at the 5′ end of mature tRNA and a hydroxyl group at the 3′ end of the leader. In bacteria, RNase P can also recognize and cleave non-tRNA natural substrates, including the precursors for 4.5S rRNA (19), 10Sa rRNA (a.k.a. tmRNA) (20), and the polycistronic his operon mRNA (21). Emerging studies of the bacterial holoenzyme have shown that the protein subunit interacts directly with the single-stranded leader of substrates, which enhances the affinity of pre-tRNA (22, 23) and allows RNase P to more efficiently cleave a variety of non-tRNA substrates (12, 24, 25).
In the case of eukaryotic RNase P, pre-tRNAs are the only natural substrates that have been identified to date. However, the possibility has not been ruled out that nuclear RNase P cleaves non-tRNA substrates. Indeed, several pieces of data have suggested that nuclear RNase P might have a range of substrates. For instance, a temperature-sensitive mutation in the RNA subunit of yeast RNase P RNA results in accumulation of an unusual 5.8S rRNA species in vivo (26), although this accumulation could well be due to indirect effects. Furthermore, purified nuclear RNase P from Saccharomyces cerevisiae cleaves deproteinized pre-rRNA at a large number of discrete sites (26) that are not recognized by the bacterial RNase P ribozyme (J.R. Chamberlain & D.R. Engelke, unpublished observations). In eukaryotes an additional enzyme, RNase MRP, has also evolved from RNase P (see below). This enzyme cleaves pre-rRNA, and possibly other substrates.
Precursor tRNA Substrate Recognition
Since all pre-tRNAs in a cell appear to be processed by RNase P and sequences surrounding the cleavage site are not well conserved, RNase P must recognize the common structural elements of pre-tRNA substrates. Previous studies have shown that the major recognition determinants are localized to the mature domain of tRNA (7). The use of small model substrates revealed that the TφC and acceptor stems together form a minimal recognition determinant for the bacterial ribozyme (7). In contrast, efficient cleavage by eukaryotic RNase P requires both the TφC/acceptor stems and at least two of the other three tRNA domains, i.e. D-, anticodon, and variable arms (27).
In addition to the tertiary structure of the mature tRNA, the 5′ leader, 3′ trailer, and internal loops can participate in binding to RNase P in bacteria and eukaryotes. In the ribozyme-catalyzed reaction, recognition is facilitated by interactions between a specific internal loop (P15) in the ribozyme and the 3′-CCA sequence that exists in most of the bacterial pre-tRNA transcripts (reviewed in 7, 27). However, this interaction appears to be less crucial for substrate recognition by the holoenzyme (28). For the holoenzyme, the protein subunit of Bacillus subtilis RNase P contains a single-stranded RNA binding motif (29, 30) that directly contacts the substrate 5′ leader and enhances the pre-tRNA affinity (22, 23, 31). Unlike the bacterial pre-tRNAs, eukaryotic pre-tRNAs have a polyuridine [poly(U)] stretch as their 3′-trailing sequence and normally lack an encoded 3′-CCA. The nuclear RNase P RNA subunit also lacks the P15 internal loop that would base-pair with the substrate 3′-CCA in the bacterial enzyme. Interestingly, the 3′ end in yeast pre-tRNA still seems to be recognized by the yeast nuclear RNase P. A tRNA containing single-stranded 3′-trailer sequence binds to the holoenzyme more strongly than does the mature tRNA (32).
There is some question as to whether recognition of pre-tRNA by the nuclear RNase P holoenzyme might be controlled in vivo by the structure of the 5′ and 3′ ends, or by possible association of the pre-tRNA with other proteins. The 5′ leaders of eukaryotic pre-tRNAs are often purine-rich and can form base pairs to varying degrees with the U-rich 3′ trailer, giving an extension of the amino acyl stem. When this base pairing in the amino acyl stem is strong in yeast pre-tRNAs, an unpaired bulge at the cleavage site is needed to enhance efficient cleavage by RNase P (33). However, it is not yet clear whether the leader/trailer stem extension must be fully unwound before cleavage by nuclear RNase P. Nor is it yet clear whether the pre-tRNA substrates are complexed with one or more additional proteins as they are cleaved by nuclear RNase P. Pre-tRNAs and other RNA polymerase III transcripts are thought to associate during transcription with the La protein antigen (34–36). In yeast it has been shown that nuclear pre-tRNAs are found associated with the La-like protein Lhp1p both before and after RNase P cleavage (37; S.L. Wolin, personal communications). Lhp1p, like human La antigen, binds primarily to the 3′ poly(U) and would be expected to denature any structure between the 5′-leader and 3′-trailing sequences. Therefore, it is possible that inside the cell, La or other bound proteins serve as part of the pre-tRNA recognition mechanism by RNase P.
Mechanism for the Pre-tRNA Cleavage Reaction
The pre-tRNA cleavage reaction catalyzed by RNase P has been studied in Bacteria, Archaea, and Eucarya; the best characterized of these is the bacterial ribozyme reaction. The most likely mechanism for the ribozyme reaction, which is proposed mainly by analogy with other, better studied reactions, is an SN2 attack of a solvent nucleophile on the phosphate diester at the scissile bond with a trigonal bipyramidal transition state (38, 39).
The bacterial RNase P reaction consists of three basic steps: (a) pre-tRNA substrate binding; (b) scissile bond cleavage; and (c) product release. For bacterial RNase P, the affinity of RNase P for substrate and product is very dependent on the ionic conditions. However, at optimal salt conditions, the Kd of the interaction between the B. subtilis RNase P RNA alone and mature tRNA is about 3 nM. The addition of the protein component only modestly enhances the tRNA affinity, but increases the pre-tRNA affinity 104-fold (22). Under many conditions, the steady state kinetics for pre-tRNA cleavage, catalyzed by either RNase P RNA alone or the holoenzyme, are dominated by substrate binding and product dissociation steps. At low substrate concentrations, kcat/Km often reflects the substrate association step, which is nearly diffusion controlled, whereas at saturating substrate concentrations, tRNA dissociation is the main rate-limiting step (22, 40). The rate constant of the cleavage step can be measured directly using transient kinetics or altered solution conditions (low pH or substitution of Ca2+ for Mg2+). The rate constant for the scissile bond cleavage is pH dependent, increasing at higher pH (7). At pH 7, the rate constant for the cleavage catalyzed by B. subtilis RNase P RNA is 6 s−1 (40), and this is only modestly enhanced (<10-fold) by addition of the protein subunit (22).
Studies of the eukaryotic RNase P reaction have been performed primarily with partially purified holoenzymes from nuclei and organelles (32, 41–44). Initial characterizations of the purified yeast nuclear RNase P reaction by steady state analyses give a value for Km of 9 nM, and a value for kcat of 1.3 s−1 for cleavage of a specific yeast pre-tRNATyr (32), although the kinetic parameters vary for different substrates. The second-order rate constant, kcat/Km, for the yeast nuclear holoenzyme is 1.3 × 108M−1 s−1. This result is close to the predicted rate constant for diffusion (32), which suggests that this is a very efficient enzyme.
Divalent metal ions are important cofactors for the RNase P reaction. The roles of divalent metal ions in RNase P RNA folding, substrate recognition, and catalysis of the ribozyme reaction have recently been reviewed (7, 45). Divalent metal ions enhance the affinity of B. subtilis RNase P RNA with tRNA more than 103-fold (46), although cross-linking data suggest that RNase P RNA/tRNA association under some conditions may occur in the absence of metal ions (7). To date, all of the data indicate that divalent metal ions, preferably magnesium, are absolutely required for cleavage catalyzed by RNase P, although the exact functions of these metal ions have not yet been elucidated. Several possible metal binding sites important for catalysis or substrate binding have been proposed in both the pre-tRNA substrate and the RNase P RNA by phosphorothioate modification experiments (reviewed in 45). In the case of yeast nuclear RNase P, studies of the utilization of metal ions by the holoenzyme reveal an even higher specificity for magnesium as the divalent metal ion, but the precise role of the divalent cations is currently unknown. Substitution of divalent cations in the holoenzyme has been difficult to document owing to irreversible inactivation of the enzyme by prolonged exposure to EDTA (47). Phosphorothioate substitutions at the cleavage site in pre-tRNA decrease the cleavage activity of yeast nuclear RNase P in a fashion similar to that seen with bacterial RNase P, which suggests a similar mechanism (41, 43).
VARIOUS FORMS OF EUKARYOTIC RNASE P ACTIVITIES
In eukaryotes the RNase P functions have been partitioned into several different enzymes. The nuclear enzyme functions have been split into at least two distinct enzymes, RNase P and RNase MRP (see below). Pre-tRNA processing in mitochondria and chloroplasts is carried out by activities that, in most studies, appear to be distinct from the nuclear holoenzymes. Characterization of RNase P–like activities from both mitochondria and chloroplasts have identified RNA-based enzymes similar to the nuclear and bacterial forms, as well as purely protein-based enzymes. The physiological relevance of these activities is currently under discussion (48–50).
Mitochondrial RNase P
Mitochondrial RNase P activities have been characterized to various extents from yeasts, plants, vertebrates, and parasitic protozoa (7, 27, 51). The most detailed information on mitochondrial RNase P to date has been obtained by the studies of the budding yeast, Saccharomyces cerevisiae. The S. cerevisiae mitochondrial holoenzyme consists of an essential RNA subunit, Rpm1r (490 nucleotides, but varies in different strains) that is encoded in the mitochondrial genome, and a protein subunit, Rpm2p [105 kilodaltons (kDa)] that is encoded by a nuclear gene (52–55). Homologs of Rpm1r have also been identified in a number of yeasts with significant size variation compared to S. cerevisiae (56, 57), and a phylogenetic structure has been proposed for the RNA subunits that conforms to the bacterial consensus structure (58; the RNase P database, 8).
The Rpm2p protein subunit is required for mitochondrial RNase P activity although it, like the nuclear holoenzyme proteins, does not show significant sequence similarity to the bacterial RNase P proteins. Mutant alleles of Rpm2p cause accumulation of mitochondrial pre-tRNAs with the 5′ end (54). In addition to pre-tRNA cleavage, Rpm2p is needed for the processing of the mitochondrial RNase P RNA subunit (59, 60). Furthermore, a complete deletion of theRPM2 gene prevents fermentative growth of yeast cells (61), which suggests that Rpm2p has one or more unidentified functions that are essential for life.
The mitochondrial RNase P activities from other species are less well understood at this point. In the case of human mitochondrial RNase P, early reports identified an RNase P–like activity from HeLa cell mitochondrial fractions that appeared to be entirely protein based, and to have a mitochondrial-specific substrate specificity (50, 62–64). On the other hand, an RNase P activity has recently been partially purified from a human HeLa cell mitochondrial fraction that contains the same nuclear-encoded H1 RNA as the human nuclear RNase P (65). Because this enzyme was identified by assays using heterologous pre-tRNA substrates of a type not cleaved by the protein-based activity, it remains possible that the H1-containing enzyme is a contaminant from a nonmitochondrial cellular compartment. It seems unlikely, though possible, that human mitochondria have two activities that cleave pre-tRNA. Since neither enzyme type has yet been shown to play a relevant role in mitochondrial pre-tRNA biosynthesis, the question of whether the mitochondrial RNase P activity requires an RNA subunit remains controversial.
Chloroplast RNase P
The debate over RNase P RNA content in chloroplasts mirrors the discussion for the human mitochondrial enzymes. Chloroplast RNase P activities have been isolated from tobacco and spinach chloroplasts (7). Several lines of evidence suggest that spinach chloroplast RNase P might be a solely protein enzyme (reviewed in 7, 66): (a) the buoyant density of the enzyme in CsCl is 1.28 g/ml, which is coincident with the total chloroplast protein; (b) spinach chloroplast RNase P activity is insensitive to micrococcal nuclease digestion; (c) no RNA is detectable in the most purified enzyme preparations, even using sensitive assays. Moreover, spinach chloroplast activity seems to have a different cleavage mechanism than that of bacterial RNase P ribozyme (42), although the reaction is still very efficient. Spinach chloroplast RNase P binds to a substrate pre-tRNA with a Km of 16 nM (42). Substitution of the pro-Rp nonbridging oxygen with sulfur at the scissile bond of pre-tRNA dramatically decreases the activity of bacterial ribozyme, but the activity of spinach chloroplast RNase P is hardly affected (reviewed in 7; 42, 67). A cautionary note concerning this RNase P activity:it has not yet been proven essential for pre-tRNA maturation in vivo.
In contrast, the ribonucleoprotein nature of RNase P has been functionally proven in the cyanelle of the primitive alga Cyanophora paradoxa; the cyanelle is a photosynthetic organelle derived from cyanobacteria and belonging to a different phylogenetic branch than the chloroplasts of green alga and higher plants (68). Although the RNA subunit alone is not catalytically active (69, 70), it is essential for RNase P activity, as indicated by the sensitivity of the holoenzyme to micrococcal nuclease treatment (27). The protein subunits of cyanelle RNase P have yet to be identified, but they constitute about 80% of the mass of the holoenzyme (68), an extensive protein content. Footprinting analysis of cyanelle RNase P RNA in the native holoenzyme also reveals extensive protection of the RNA by proteins, with a pattern similar to that of the yeast nuclear RNase P (68). In addition to cyanelle RNase P, sequences putatively like RNase P RNA have been identified in several chloroplast genomes, including those from maize (71), Porphyra, and Nephroselmis olivacea (the RNase P database, 8).
Eukaryotic Nuclear RNase P
Composition of Nuclear RNase P
Nuclear RNase P has been purified or partially purified from several eukaryotes, primarily from yeasts and vertebrates. The buoyant densities of eukaryotic holoenzymes are around 1.28 –1.4 g/ml in Cs2SO4, which are less than those of the bacterial enzymes but between the densities of protein and RNA (7, 72). The protein complement contributes about 50% of the mass of the eukaryotic complex, as compared to 10% in the bacterial holoenzyme (73–77). These data, together with the ribonuclease sensitivity of eukaryotic RNase P activity, originally suggested the ribonucleoprotein nature of the nuclear holoenzymes.
In the yeast S. cerevisiae, the RNA subunit of nuclear RNase P, RPR1 RNA, was first identified about a decade ago (78, 79). Genetic and immunoprecipitation studies identified the first four proteins associated with yeast nuclear RNase P: Pop1p, Pop3p, Pop4p, and Rpp1p (80–83). Biochemical purification (1) has confirmed the presence of the RPR1 RNA, the above four proteins, and five additional polypeptides (Pop5p, Pop6p, Pop7p, Pop8p, and Rpr2p) (Table 1). Pop7p was also identified as a protein subunit because of homology to one of the human RNase P proteins (84). In vivo depletion studies have shown that all 10 subunits are essential for yeast viability and for RNase P activity (1, 79–83).
Purification of human RNase P from HeLa cells has revealed the existence of an RNA subunit, H1 RNA (73), and at least seven protein subunits that copurify with enzymatic activity, namely Rpp14, Rpp20, Rpp29, Rpp30, Rpp38, and Rpp40 (Table 1) (85). In addition, the human homolog of yeast Pop1p, hPop1, has been discovered independently by database searches, although the overall sequence similarity between the two proteins is low (86). Despite the lack of strong sequence similarity in POP1 genes, antibody raised against hPop1 can immunoprecipitate the H1 RNA and RNase P activity from HeLa cell extracts (86). Thus, hPop1 is likely to be an integral component of human RNase P. In a separate study, Rpp29 has been identified as the homolog of yeast Pop4p, and is also called hPop4 (87, 88). Database searches have recently identified additional human RNase P protein subunits based on sequence homology to other yeast nuclear RNase P proteins. hPop5 (the homolog of yeast Pop5p) and Rpp21 (the homolog of yeast Rpr2p) have proven to be integral subunits of human RNase P (89, 90). The relationship of other human RNase P proteins to yeast RNase P proteins remains unclear.
Recently, bioinformatic and biochemical studies have identified archaeal homologs of several yeast RNase P proteins, which suggests a eukaryotic-like protein content in archaeal RNase P. In Methanothermobacter thermoautotrophicus, the open reading frames MTH11 (POP4 homolog), MTH687 (POP5 homolog), MTH688 (RPP1 homolog), and MTH1618 (RPR2 homolog) have proven to be associated with RNase P activity (Table 1) (18).
Structure and Functions of the Nuclear RNA Subunit
The RNA component in eukaryotic nuclear RNase P has been isolated in several organisms (7, 8, 15). The corresponding genes appear to be transcribed by RNA polymerase III in yeast and vertebrates, but most of the information on the structure and function of the RNA subunit comes from the studies on the yeast enzyme. The RPR1 RNA gene from S. cerevisiae is transcribed as a 486-nucleotide primary transcript (pre-RPR1 RNA) (91). This large precursor, which might be a peculiarity of Saccharomyces, undergoes a single cleavage at the 5′ end to remove the 84-nucleotide leader sequence and multiple cleavages to remove the 33-nucleotide 3′-trailing sequence to give the mature RPR1 RNA (79). In normal S. cerevisiae strains, the ratio of the cellular level of pre-RPR1 RNA and RPR1 RNA is variable, but in actively growing cells it is about 1:9. Both pre-RPR1 RNA and RPR1 RNA can be immunoprecipitated using affinity-tagged protein subunits (1, 80–83). Moreover, ribonucleoprotein complexes containing the pre-RPR1 RNA and mature RPR1 RNA have similar chromotographic characteristics during most of the biochemical purification steps (26). These data imply that the pre-RPR1 RNA may be processed after the assembly into a ribonucleoprotein complex. Consistent with this, recent studies have shown that the pre-RPR1 complex has catalytic activity (C Srisawat, personal communication), which is not surprising considering that each of the protein subunits in the mature holoenzyme can immuno-precipitate the pre-RPR1 RNA. It is also possible that the pre-RPR1 RNA has transiently associated proteins not found in the mature enzyme. For example, Lsm proteins have been found associated with the precursor, but not the mature, RPR1 RNA (92).
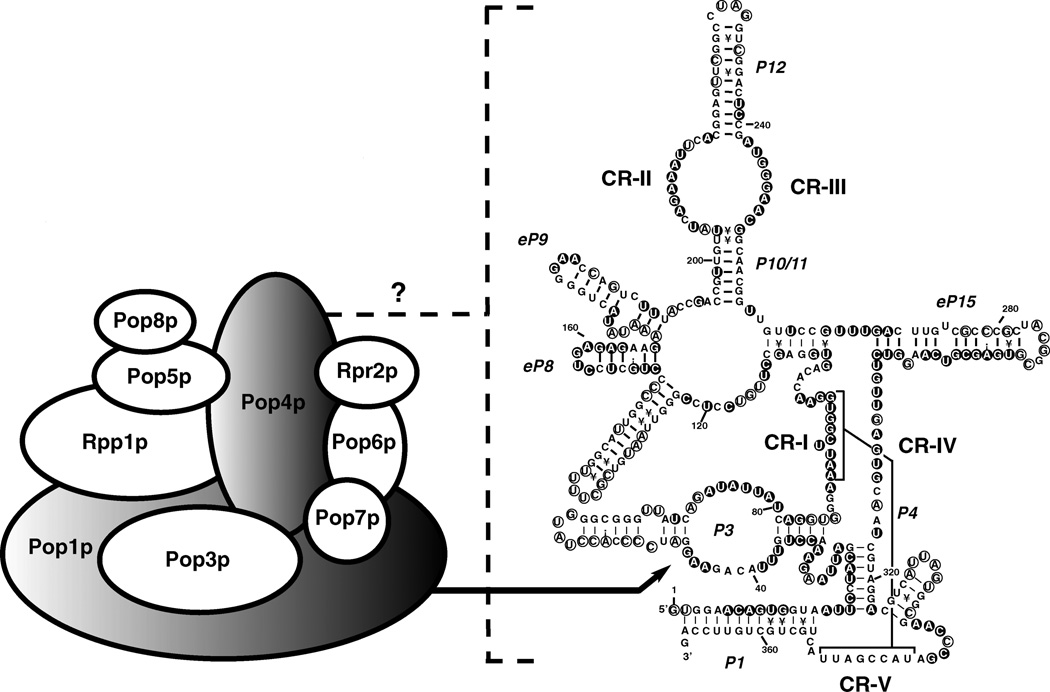
A secondary structure for RPR1 RNA has been proposed based on structure-sensitive RNA footprinting and phylogenetic studies (Figure 1) (15, 16, 93, 94). Comparison of the RPR1 RNA structure with the bacterial consensus structure and other eukaryotic RNase P RNA structures has revealed both similarities and variability. A core structure is conserved among RNase P RNAs from bacteria and eukaryotes (14, 15, 95, 96). The conserved elements include five “critical regions” (CR-I through CR-V) carrying conserved nucleotides and several stems (P1, P2, P3, P4, P7, P10/11, and P12) at similar positions in different RNA structures (Figure 1). CR-I, CR-IV, and CR-V are also easily recognizable in the mitochondrial RNase P RNA from S. cerevisiae, but no obvious candidate sequence for CR-II or CR-III has been found (97). At this time no crystal structure for any RNase P RNA subunit has yet appeared, so information on structure and function of conserved regions comes mainly from mutagenesis, cross-linking, chemical probing, and computer modeling.
Figure 1.
Summary of the yeast two-hybrid and three-hybrid test results of yeast (S. cerevisiae) nuclear RNase P. Ovals represent the protein subunits of yeast nuclear RNase P (Table 1). Overlaps of the ovals indicate a positive result in the two-hybrid test (141a). The shaded ovals of Pop1p and Pop4p indicate that they are the only protein subunits that interact specifically with the RPR1 RNA in a three-hybrid test. One of the recognition sites in the RPR1 RNA for Pop1p binding is the P3 loop, as indicated by the arrow (116). The binding sites for Pop4p are currently unknown, which is represented by the dotted bracket and a question mark. The predicted secondary structure of RPR1 RNA is adapted from the model proposed by Frank et al (15). Five critical regions, CR-I to CR-V, are numbered based on the Chen & Pace nomenclature (14). P represents helical regions, with numbers assigned according to the bacterial structure (101). eP indicates the eukaryotic paired regions whose homology to particular bacterial structures is uncertain, but which occupy the same positions as in the bacterial structure (15). Nucleotides in filled circles show protection from chemical modification and nuclease attack in the holoenzyme, whereas the nucleotides in open circles indicate exposure to solution (16).
The P4 helix, which is formed by the base pairing of CR-I and CR-V, is postulated to be the catalytic center of the bacterial enzymes by site-directed mutagenesis, phosphorothioate modification-interference studies, and deletional analysis (96, 98–103). It is also suggested to be a potential binding site for the bacterial RNase P protein subunit (13; S. Niranjanakumari & C.A. Fierke, personal communication). For yeast nuclear RNase P, mutagenesis studies of the conserved nucleotides within and near P4 have suggested an important role in pre-tRNA binding, catalysis, and RPR1 RNA maturation (103). Several substitutions of the conserved nucleotides cause more than a 10-fold reduction in the catalytic efficiency (kcat/Km) of the holoenzyme, and/or a severe decrease in the cellular level of the mature RPR1 RNA, which is thought to be caused by an RPR1 RNA maturation defect (103). Previous RNA footprinting studies have shown that P4 helix is protected from nuclease attacks in the RNase P holoenzyme (16), which suggests that it is not on the surface of the holoenzyme.
CR-II and CR-III, together with P10/P11 and P12, form a domain whose function and accessibility to chemical reagents is magnesium dependent in yeast RNase P (104, 105). Deletional studies of the yeast RNase P RNA have shown that the distal P12 stem is dispensable in vivo, although the internal loop containing the CR-II and CR-III regions is essential for yeast viability and RNase P activity (106). The CR-II region contains a consensus sequence AGARA (R = purine), which is conserved in all yeast RNase P RNAs and similar to the bacterial CR-II consensus (15, 104). Mutational studies of the AGAAA sequence in CR-II of the S. cerevisiae RNase P RNA indicate a function in magnesium utilization as measured by an increase in optimum Mg2+ concentration for the mutants (104). In addition, steady state kinetic studies of the mutant holoenzymes show effects primarily on turnover (kcat), with modest changes in Km (104). In contrast, it has been shown that the analogous CR-II/III region in the bacterial ribozyme is not absolutely essential for cleavage, rather playing a role in substrate discrimination (107–111). Previous studies on bacterial RNase P RNA have shown that substrate binding requires divalent cation-mediated interactions with the CR-II and CR-III conserved loop, and that the P10/11-P12 domain is subject to lead-induced hydrolysis and Mg2+ cleavage at high pH (98, 112). Cross-linking studies on the Escherichia coli and B. subtilis ribozymes also place CR-II and CR-III in proximity to the substrate aminoacyl stem (JM Nolan, personal communication).
Extensive mutagenesis of the CR-IV region of the RPR1 RNA gives large reduction in kcat, with little effect on Km (103). In addition, the maturation of RPR1 RNA does not seem to be affected in the strains containing the RNA mutated in this region (103). Studies on the bacterial enzyme have demonstrated the importance of this region in tRNA binding (28, 113, 114). Thus, CR-IV might also be located in the proximity of the active site of the eukaryotic holoenzyme.
Structural elements distinct from the bacterial RNase P RNA subunits also exist in the nuclear RNAs; the most obvious are the P3 and P15 regions. Although the RNA species from both kingdoms contain a P3 stem, the eukaryotic P3 has a more complex helix-loop-helix structure (Figure 1) (14, 15, 93, 95). P3 has been proposed as a protein binding site in bacterial RNase P holoenzyme, as indicated by deletional analysis and protein-directed RNA cleavage (13, 115; S. Niranjanakumari & C.A. Fierke, personal communication). In yeast RNase P, a mutated P3 loop causes defects in pre-tRNA processing and RPR1 RNA maturation in vivo (116). Changes of the conserved nucleotides in the P3 loop also disrupt the specific interaction between RPR1 RNA and one of the protein subunits (Pop1p) (116). This may cause a defect in the assembly of a ribonucleoprotein complex in vivo, which in turn may cause the loss of maturation of the RNA subunit. The P3 region in human RNase P RNA is also a polypeptide-binding site (117, 118), which suggests that both the bacterial and eukaryotic P3 regions are important for the assembly of RNase P holoenzyme. In addition to (or as a result of) its role in assembly, the eukaryotic P3 domain appears to be required for the proper subcellular localization of the RNase P RNA. In human RNase P, deletion of the P3 region causes the RNA component to fail to localize to the nucleolus (119).
In contrast to P3, the P15 region is often more complex in bacteria than in yeast, and completely disappears in human RNase P RNA. Because it is not clear whether the eukaryotic structure in this region plays a role similar to the bacterial P15, it is termed eP15 (Figure 1) (15). The function of the P15 region is better understood in the bacterial RNase P ribozyme reaction than that of the holoenzyme. In the ribozyme reaction, the P15 loop has been shown to base pair with the 3′-CCA sequence of the substrate pre-tRNA (and product) (28, 120). Kinetic and biophysical analysis suggested that there might be a metal ion– binding site in the bacterial P15 loop (121–123). However, this interaction may be less important in the cleavage catalyzed by the bacterial holoenzyme (123). The function of the eP15 loop, if any, is not known.
A consensus secondary structure of eukaryotic RNase P RNA has recently been proposed (15). In addition to the conserved and variant regions mentioned above, there are elements in the eukaryotic consensus structure that might have counterparts in the bacterial consensus structure. Helices eP8 and eP9 (Figure 1) could be the homologs of the corresponding helices in bacterial RNase P RNA, although the functional equivalence requires further evidence. The fungal eP8 stem-loop structures have the NUGAG sequence (N = A, U, C, or G), whereas most of the eP9 hairpins contain GNRA (R = purine) tetraloops (15). Studies of hairpins from ribosomal RNA and other sources have suggested that a tetraloop structure could increase the thermodynamic stability of an RNA duplex, or act as a docking site for intramolecular or intermolecular RNA-RNA interactions (124–130). In E. coli RNase P RNA (M1 RNA), a mutation in the L9 loop results in a reduction in Km and kcat of the holoenzyme-catalyzed reaction with pre-4.5S RNA as substrate (131), but little effect on pre-tRNA processing in vitro.
The presence of the conserved catalytic core structure of bacterial RNase P RNA (CR-I through V) in the eukaryotic nuclear RNA subunit strongly suggests that the nuclear RNA is serving functions similar to the bacterial counterpart, despite the presence of a much larger protein contingent. The importance of the critical regions in the yeast RPR1 RNA for RNase P activity supports the hypothesis that nuclear RNase P is an RNA-based enzyme.
Functions of the Protein Subunits
The protein complement of eukaryotic RNase P is much more complex than that of the bacterial holoenzyme. Bacterial RNase P has a small, highly basic protein subunit, with a molecular mass of 12–14 kDa. In contrast, RNA footprinting of the yeast holoenzyme has shown that most of the RNA subunit is covered by proteins (Figure 1) (16). The purified nuclear RNase P from S. cerevisiae has nine tightly associated, essential protein subunits, with molecular masses ranging from 15.5 to 100.5 kDa (Table 1). Except for Pop8p and Pop5p, the yeast nuclear RNase P protein components are very basic, with pI values higher than 9. The pI values of Pop8p and Pop5p are 4.6 and 7.8, respectively. Sequence analysis has revealed several putative signals for nuclear localization in some of the yeast protein subunits, but defined RNA-binding motifs are lacking, other than clusters of basic residues. A motif containing repetitive KKD/E sequences is found in most of the protein subunits (1). The functions of the basic clusters, including the KKD/E motif, are not clear, and it is plausible that these might participate in RNA-protein interaction, protein-protein interaction, and/or nuclear localization.
In fact, very little is known about any of the functions of individual protein subunits of nuclear RNase P, although several of the proteins expressed in bacteria exhibit nonspecific RNA-binding capability in vitro (F. Houser-Scott, C.E. Millikin & D.R. Engelke, unpublished observations). Depletion of most of the yeast protein subunits results in a drastic reduction in the cellular level of mature RPR1 RNA (1, 82–84). This might reflect incorrect assembly of the yeast holoenzyme, so that pre-RPR1 RNA cannot be processed, or destabilization of the mature RPR1 RNA. The sole exception to this appears to be Pop3p, which is essential but does not dramatically affect the ratio of precursor to mature RPR1 RNA (81). Recent studies on Pop3p have revealed that it can bind to RPR1 RNA, pre-tRNA, and single-stranded RNAs (132). At this point, it is not clear whether the proteins bind to the RNA subunit as a preformed complex, or are added to the RNA one at a time.
In the case of human RNase P protein subunits, a recent study has shown that the recombinant Rpp20 displays ATPase activity (133). However, the ATPase activities of Rpp20 and RNase P holoenzyme do not have the same optimal reaction conditions (133). The generality of this ATPase activity is unclear at present since the apparent yeast homolog of Rpp20, Pop7p, does not have the ATPase signature motif. Thus, the in vivo ATPase activity of Rpp20 and its importance to RNase P function await further analysis. In addition, a two-hybrid screen suggested that Rpp20 interacts with Hsp27, a small heat shock protein 27 (134). The function of Hsp27 in RNase P activity might be regulatory, since the addition of Hsp27 stimulates RNase P activity in a concentration-dependent manner (134).
It is likely that one or more protein subunits of eukaryotic RNase P mimic the functions of the bacterial protein in enhancing substrate binding and pre-tRNA discrimination (23). In human RNase P, Rpp21 and Rpp14 might interact directly with substrates, since they have been shown to bind to pre-tRNA in a gel mobility shift assay (90). However, the specificity for the sites of binding on the tRNA have not yet been defined. A labeled pre-tRNA substrate has been reported to form cross-links with protein components from the Tetrahymena thermophila and partially purified human RNase P (135). The yeast nuclear holoenzyme appears to have additional binding sites for single-stranded RNAs: unlike the bacterial enzyme, it strongly binds and is inhibited by single-stranded homoribopolymers [poly(G) and poly(U) > poly(A) >> poly(C)] (32). One role for such single-stranded binding sites in the proteins might be recognition of the 3′ trailer or the 5′ leader of pre-tRNAs (32). A much wider role for the protein components in substrate discrimination still awaits discovery, however. Although the full range of physiological substrates of RNase P has not been tested, preliminary studies suggest that the nuclear holoenzyme is relatively promiscuous in cleaving naked RNA (26). Since nuclear RNAs are expected to be complexed with proteins in most cases in vivo, the protein components of nuclear RNase P could help discriminate among potential ribonucleoprotein substrates, rather than simply recognizing RNA determinants. Indeed, part of the reason for the increased protein complexity of the nuclear enzyme could be to specify which RNAs are not to be cleaved.
The additional eukaryotic protein subunits do not appear to substantially alter the chemistry of the cleavage reactions. Studies on cleavage of phosphorothioate modified substrates have suggested that the protein-based spinach chloroplast activity uses different chemistry than the RNase P ribozyme (42), but that nuclear holoenzymes mirror the RNA-based cleavage (41, 43). It is also possible that entirely distinct enzymatic activities, such as RNA modification enzymes, might be coordinated with RNase P cleavage by being part of the same complex, or recruited to the complex through protein-protein interaction. Previous work has linked a tRNA methyltransferase activity to the stability of RPR1 RNA (136), although none of the identified subunits have any detectable sequence homology to known tRNA processing or modification enzymes.
The subnuclear localization of several pre-tRNA processing enzymes (137–139; see below) has led to the hypothesis that the nuclear pre-tRNA pathway is spatially organized. It is entirely possible that one or more protein subunits are important for localization of RNase P within the nucleus. This might be required for the spatial organization of pre-tRNA biosynthesis, the maturation of RNase P holoenzyme, or participation of the enzyme in other undiscovered processing pathways.
Architecture of the Eukaryotic Nuclear Holoenzyme
Although the crystal structure of eukaryotic RNase P holoenzyme will be difficult to obtain because of its low natural abundance and subunit complexity, a preliminary map of how the subunits fit together has been proposed by yeast two-hybrid and three-hybrid tests (140, 141), and by cross-linking studies. The presence of both RNA and protein components in RNase P suggests that some proteins may make direct contacts with the RNA moiety, and other proteins might be recruited through protein-protein interactions.
In the case of the S. cerevisiae nuclear RNase P, the yeast two-hybrid assay has revealed that all the protein components interact with at least one other protein subunit (Figure 1) (141a). Pop1p, Pop4p, Rpp1p, Pop5p, Pop6p, and Rpr2p are involved in multiple protein-protein interactions; Pop4p and Rpr2p are able to self-associate. By the use of the yeast three-hybrid system, the RNA-protein interactions within yeast nuclear RNase P have been at least partially delineated (Figure 1). Pop1p and Pop4p are the only protein subunits that specifically bind to the RPR1 RNA in the three-hybrid test (141a). A Pop1p binding site has been mapped to the P3 region of RPR1 RNA (116). The RNA binding site(s) for Pop4p remain to be discovered. It is worth noting that other protein subunits might make direct contacts with the RNA, but the interactions could be dependent on the formation of a multiprotein complex.
Using a similar genetic analysis and cross-linking experiments, the subunit interaction map of human nuclear RNase P has been examined. Eight of the ten known protein subunits of human nuclear RNase P were tested in a yeast two-hybrid assay for their abilities to interact with each other (134). Rpp21 (human Rpr2p homolog), Rpp29/hPop4, Rpp30 (human Rpp1p homolog), Rpp38, Rpp40, and hPop1 are involved in extensive, but weak, protein-protein interactions among the RNase P subunits. Rpp21, Rpp29, Rpp30, and Rpp38 bind to the H1 RNA as indicated by both a yeast three-hybrid test and UV cross-linking (142), although the RNA recognition elements for these proteins are currently unknown. Furthermore, the sera from some patients with autoimmune diseases react with a 40-kDa polypeptide, designated as To antigen, within human RNase P holoenzyme. The To antigen can be cross-linked to nucleotides 20–75 of human RNase P RNA (the To binding site), which encompasses the P3 domain of the RNA component (118). However, the relationship between the To/Th antigen and the known protein subunits of RNase P is unclear at present.
The protein-protein interactions and protein-RNA interactions in the yeast nuclear RNase P are not identical to those observed in the human holoenzyme, although there is significant similarity. Considering the low identity between the protein homologs and the variation in the RNA structures, it is possible that the subunit interaction surfaces are different in these organisms, resulting in subtle changes in the strength of binary subunit contacts and the spatial organization of RNase P holoenzymes.
RELATIONSHIP BETWEEN RNASE P AND RNASE MRP
Nuclear RNase P is closely related to another ribonucleoprotein complex, RNase MRP, which has been found only in eukaryotes. RNase MRP was originally identified as an RNA-containing endoribonuclease that cleaves the mitochondrial RNA primers for DNA replication in vitro (143, 144). However, most of the RNase MRP RNA is observed in the nucleolus (145–148), where the enzyme plays an important role in pre-rRNA processing (3, 149, 150). In yeast, RNase MRP has been shown to cleave pre-rRNA at site A3 within the first internal transcribed spacer (ITS 1), which is essential for generating the mature 5.8S rRNA in vivo (80, 151–153). Both the RNA and protein subunits of RNase MRP are essential for yeast viability, even though neither the cleavage of pre-rRNA at site A3 nor the mitochondrial DNA replication is required for life (149). Therefore, RNase MRP may have additional essential functions that have not yet been discovered. Indeed, a yeast strain carrying a temperature-sensitive mutant of RNase MRP RNA displays a defect in pre-rRNA processing at sites other than A3 at nonpermissive temperature (149). However, the essential role of RNase MRP might not involve the pre-rRNA pathway. Mutagenesis of yeast RNase MRP has shown that the enzyme plays a role in plasmid segregation (154), and it is entirely possible that RNase MRP has additional substrates that have not yet been identified.
Characterization of RNase MRP primarily comes from yeast and human cells. Yeast nucleolar RNase MRP has an RNA component, NME1 RNA, and at least nine protein subunits identified by genetics and immunoprecipitation (Table 1). Eight of these nine proteins are also tightly associated with the purified yeast nuclear RNase P. This protein subunit overlap and the similarity of the RNA subunits to the RNase P RNAs (155; see below) strongly indicate that RNases P and MRP are closely related descendants of an ancestral eukaryotic enzyme in which the RNA subunit has diverged, and unique protein subunits have evolved accordingly.
The one known unique protein subunit of yeast RNase MRP, Snm1p, is clearly distinct from the unique protein subunit of RNase P, Rpr2p, yet the two proteins have some properties in common. In two-hybrid tests, both proteins self-associate and bind to Pop4p (141a). One would predict that the Snm1p subunit might perform functions that set MRP aside from RNase P, such as substrate discrimination or enzyme localization. Indeed, a putative zinc-cluster domain has been identified in Snm1p, and the protein can bind to NME1 RNA in gel mobility shift and Northwestern assays (156). In contrast, Rpr2p has not been found to bind the RPR1 RNA (141a). It is worth noting that since RNase MRP has not yet been purified from any source, the holoenzyme could contain additional subunits not found in RNase P.
Secondary structures of RNase MRP RNA have been proposed based on phylogenetic studies (157–160). RNase MRP RNA can fold into a cage-shaped secondary structure similar to that of the RNase P RNA. RNase MRP has at least three of the critical regions (CR-I, CR-IV, and CR-V) that are conserved in all RNase P RNAs, and also shares the eukaryotic P3 loop. It is not known at present whether the equivalents of CR-II and CR-III are present in modified form in RNase MRP RNA.
The P3 internal loop sequences of yeast RNase P RNA and RNase MRP RNA appear to be functionally interchangeable (161). The P3 region is thought to be a specific binding site for a common protein of RNase P and RNase MRP, although different types of experiments done with yeast and human enzymes have identified different probable binding partners. In a yeast three-hybrid test, Pop1p binds to the P3 region, alone or in the context of RPR1 RNA, in a sequence-specific fashion (116). In human nuclear RNase P and RNase MRP, several polypeptides have been shown to require the P3 domain for binding, or bind to a region close to P3 (117, 118, 162).
Information concerning the arrangement of proteins on the RNA subunit gives different answers for yeast versus human RNase MRP. Although the yeast P3 domain (equivalent to the Th/To binding site in human RNase MRP RNA) binds directly to Pop1p, the human Pop1 does not cross-link to MRP RNA (162). However, the association of hPop1 with the holoenzyme complex requires the To/Th binding site in the RNA (162). Instead of hPop1, cross-linking experiments have shown that three polypeptides, with molecular masses of 20, 25, and 40 kDa, are in close contact with the To/Th-binding site (nucleotides 15–87) of the human RNase MRP RNA (117, 162). A 40-kDa protein is also cross-linked to the To/Th region (nucleotides 20–75) of human RNase P RNA (118). However, it is not known whether the 40-kDa polypeptides in these studies are identical, or whether they correspond to any of the identified yeast RNase P/MRP subunits. Another 40-kDa protein, which is identical to Rpp38, has been found to bind to an RNA site that is on the 3′ side of the To/Th-binding site (162).
SUBNUCLEAR LOCALIZATION OF RNASE P AND THE QUESTION OF COORDINATED RNA PATHWAYS
In bacteria, RNase P is involved in the processing of tRNAs, rRNAs, and in specialized cases, mRNAs. The existence of tRNAs within the large, ribosomal RNA precursors creates a particularly intimate association between the tRNA and rRNA pathways, since tRNA processing by RNase P is occurring as part of rRNA processing. In eukaryotes tRNA sequences are no longer found in the pre-rRNA transcription units, and cleavage between the large rRNA subunits is accomplished by a derivative of RNase P, RNase MRP, that has adapted to this task in the absence of a tRNA substrate structure. Given that nuclear tRNA and rRNA cleavage is accomplished by distinct enzymes, the question persists: Is there still any physical linkage between the two pathways?
In eukaryotes, most ribosome biogenesis, including the processing of prer-RNA by RNase MRP, takes place in dense, subnuclear regions called nucleoli. Pre-tRNA biosynthesis had been assumed to be distributed throughout the nucleoplasm, but emerging data on the localization of nuclear RNase P suggest this might not be the case. In the yeast S. cerevisiae, the RNase P cleavage is an early step in the nuclear tRNA maturation pathway. Most nuclear pre-tRNAs and both the precursor and mature RNase P RNAs are localized primarily to the nucleolus, with additional foci in the nucleoplasm (139, 163; E. Bertrand & D.R. Engelke, unpublished observations). Although one long-lived pre-tRNA, the intron-containing pre-tRNAIle (164), has been found throughout the nucleus (138), the predominantly nucleolar localization results clearly suggest that the early steps in nuclear tRNA processing pathway are compartmentalized, rather than distributed evenly in the nucleoplasm. The partial colocalization of pretRNA and pre-rRNA maturation pathways in the nucleolus might reflect the possible interplay between tRNA and rRNA biogenesis. Considering that RNase P and RNase MRP share eight out of nine protein subunits, it is not surprising that they might have at least partially common subcellular localization.
In mammalian cells, in situ hybridization shows that endogenous RNase MRP RNA is predominantly nucleolar, but that RNase P H1 RNA is localized throughout the nucleus (119). Microinjection of labeled RNase P RNA demonstrated that the RNA is first localized in the dense fibrillar component of the nucleolus, and subsequently redistributed throughout the nucleus (119). This would be consistent with some aspect of RNase P assembly taking place in the nucleolus, and would also be consistent with the observation that precursors to yeast RPR1 RNA are nucleolar (E. Bertrand & D.R. Engelke, unpublished observations). The To/Th antigen-binding site (nucleotides 25–75 in H1 RNA) is required for the initial nucleolar localization of the RNA, and the more 3′ region of the RNA (nucleotides 89–341 in H1 RNA) is necessary for its nucleoplasmic redistribution (119). The location of RNase P in eukaryotic nuclei is somewhat controversial, however, since earlier reports suggested that concentrated foci of RNase P RNA were localized on the fringes of some HeLa cell nucleoli, termed the perinucleolar compartments (PNCs) (165).
In addition to detection of the RNA subunit, subcellular localization of the human RNase P protein components has also been studied. These experiments generally give answers that are difficult to interpret, however. Since many of the subunits are common to both RNases P and MRP, it is never clear whether the protein being visualized is associated with one of the enzymes, with both, or with neither. Using indirect immunostaining, most of the endogenous hPop1 is found in the fibrillar compartment of the nucleolus, although a weak, homogeneous staining in the nucleoplasm is also observed (86). Rpp29, Rpp38, Rpp14, and hPop5 are mainly detected in the nucleolus (89, 166). Rpp29 and Rpp38 have also been located in Cajal bodies (166). However, localization should not necessarily be considered static, since Rpp29 has been shown to rapidly shuttle between nucleolus and nucleoplasm in human cells (167).
Rpp21, the human homolog of the yeast RNase P-specific protein subunit Rpr2p, is primarily nucleoplasmic with a small amount in the nucleoli (90). Whether Rpp21 is associated only with RNase P activity in human cells has yet to be confirmed. An alternatively spliced form of human Rpp21, which does not associate with RNase P RNA, is detected primarily in the nucleoli (90). The significance of this alternatively spliced Rpp21 form is not immediately clear, but its existence serves to emphasize how little we know about the functions of these proteins. For the yeast subunits, we know only that they are essential for life, for RNase P activity, and in most cases, for RNase MRP activity. These proteins could also play additional, completely unknown roles in the cell.
SUMMARY
Ribonuclease P is one of two ancient ribonucleoprotein enzymes that are found in all living organisms (the other is the ribosome). The existence of a “ribozyme” RNA subunit in the bacterial enzymes initially caused much excitement, contributing to the creation of an RNA World hypothesis in which RNAs were the primordial macromolecules that both carried genetic information and catalyzed reactions. In eukaryotic nuclei the RNA subunit still has most of the features that are conserved in the bacterial ribozymes, but the holoenzyme has gained nearly 20 times as much protein and has simultaneously lost the ability to bind and cut pre-tRNA substrates without the protein complement. Although the pre-tRNA cleavage mechanism and functions of the nuclear RNase P RNA subunit are thought to be similar or identical to the bacterial counterpart, the reasons for the huge increase in protein content of eukaryotic holoenzymes are largely mysterious. The nuclear enzyme clearly has to deal with issues that are not likely to be seen by the bacterial enzymes—subcellular localization during biogenesis, possible spatial organization of the pre-tRNA pathway, and a potential need to recognize more complex substrates that ribonucleoprotein contacts.
ACKNOWLEDGMENTS
We are grateful to T.A. Hall, J.W. Brown, S.L. Wolin, S. Niranjanakumari, W. Rossmanith, and J.M. Nolan for communicating results prior to publication, and to N.C. Martin and J.W. Brown in particular for helpful suggestions on the manuscript. This work is supported by the National Institutes of Health grants GM 34869 (to DRE) and GM 55387 (to CAF), the Anthony and Lilian Lu Fellowship (to SX), and postdoctoral fellowships from the UNCF-Pfizer and the Ford Foundation (to FHS).
Contributor Information
Shaohua Xiao, Email: shxiao@umich.edu.
Felicia Scott, Email: fhscott@umich.edu.
Carol A. Fierke, Email: fierke@umich.edu.
David R. Engelke, Email: engelke@umich.edu.
LITERATURE CITED
- 1.Chamberlain JR, Lee Y, Lane WS, Engelke DR. Genes Dev. 1998;12:1678–1690. doi: 10.1101/gad.12.11.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reddy R, Shimba S. Mol. Biol. Rep. 1995;22:81–85. doi: 10.1007/BF00988710. [DOI] [PubMed] [Google Scholar]
- 3.Reilly TH, Schmitt ME. Mol. Biol. Rep. 1995;22:87–93. doi: 10.1007/BF00988711. [DOI] [PubMed] [Google Scholar]
- 4.van Eenennaam H, Jarrous N, van Venrooij WJ, Pruijn GJM. IUBMB Life. 2000;49:265–272. doi: 10.1080/15216540050033113. [DOI] [PubMed] [Google Scholar]
- 5.Xiao SH, Houser-Scott F, Engelke DR. J. Cell. Physiol. 2001;187:11–21. doi: 10.1002/1097-4652(200104)187:1<11::AID-JCP1055>3.0.CO;2-K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pace NR, Brown JW. J. Bacteriol. 1995;177:1919–1928. doi: 10.1128/jb.177.8.1919-1928.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frank DN, Pace NR. Annu. Rev. Biochem. 1998;67:153–180. doi: 10.1146/annurev.biochem.67.1.153. [DOI] [PubMed] [Google Scholar]
- 8.Brown JW. Nucleic Acids Res. 1999;27:314. doi: 10.1093/nar/27.1.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niranjanakumari S, Kurz JC, Fierke CA. Nucleic Acids Res. 1998;26:3090–3096. doi: 10.1093/nar/26.13.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vioque A, Arnez J, Altman S. J. Mol. Biol. 1988;202:835–848. doi: 10.1016/0022-2836(88)90562-1. [DOI] [PubMed] [Google Scholar]
- 11.Talbot SJ, Altman S. Biochemistry. 1994;33:1399–1405. doi: 10.1021/bi00172a016. [DOI] [PubMed] [Google Scholar]
- 12.Loria A, Niranjanakumari S, Fierke CA, Pan T. Biochemistry. 1998;37:15466–15473. doi: 10.1021/bi9816507. [DOI] [PubMed] [Google Scholar]
- 13.Biswas R, Ledman DW, Fox RO, Altman S, Gopalan V. J. Mol. Biol. 2000;296:19–31. doi: 10.1006/jmbi.1999.3443. [DOI] [PubMed] [Google Scholar]
- 14.Chen JL, Pace NR. RNA. 1997;3:557–560. [PMC free article] [PubMed] [Google Scholar]
- 15.Frank DN, Adamidi C, Ehringer MA, Pitulle C, Pace NR. RNA. 2000;6:1895–1904. doi: 10.1017/s1355838200001461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tranguch AJ, Kindelberger DW, Rohlman CE, Lee JY, Engelke DR. Biochemistry. 1994;33:1778–1787. doi: 10.1021/bi00173a022. [DOI] [PubMed] [Google Scholar]
- 17.Pannucci JA, Haas ES, Hall TA, Harris JK, Brown JW. Proc. Natl. Acad. Sci. USA. 1999;96:7803–7808. doi: 10.1073/pnas.96.14.7803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall TA, Brown JW. RNA. 2002. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bothwell ALM, Garber RL, Altman S. J. Biol. Chem. 1976;251:7709–7716. [PubMed] [Google Scholar]
- 20.Komine Y, Kitabatake M, Yokogawa T, Nishikawa K, Inokuchi H. Proc. Natl. Acad. Sci. USA. 1994;91:9223–9227. doi: 10.1073/pnas.91.20.9223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alifano P, Rivellini F, Piscitelli C, Arraiano CM, Bruni CB, Carlomagno MS. Genes Dev. 1994;8:3021–3031. doi: 10.1101/gad.8.24.3021. [DOI] [PubMed] [Google Scholar]
- 22.Kurz JC, Niranjanakumari S, Fierke CA. Biochemistry. 1998;37:2393–2400. doi: 10.1021/bi972530m. [DOI] [PubMed] [Google Scholar]
- 23.Niranjanakumari S, Stams T, Crary SM, Christianson DW, Fierke CA. Proc. Natl. Acad. Sci. USA. 1998;95:15212–15217. doi: 10.1073/pnas.95.26.15212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gopalan V, Baxevanis AD, Landsman D, Altman S. J. Mol. Biol. 1997;267:818–829. doi: 10.1006/jmbi.1997.0906. [DOI] [PubMed] [Google Scholar]
- 25.Hartmann RK, Heinrich J, Schlegl J, Schuster H. Proc. Natl. Acad. Sci. USA. 1995;92:5822–5826. doi: 10.1073/pnas.92.13.5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chamberlain JR, Pagan R, Kindelberger DW, Engelke DR. Nucleic Acids Res. 1996;24:3158–3166. doi: 10.1093/nar/24.16.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schon A. FEMS Microbiol. Rev. 1999;23:391–406. doi: 10.1111/j.1574-6976.1999.tb00406.x. [DOI] [PubMed] [Google Scholar]
- 28.Oh BK, Pace NR. Nucleic Acids Res. 1994;22:4087–4094. doi: 10.1093/nar/22.20.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stams T, Niranjanakumari S, Fierke CA, Christianson DW. Science. 1998;280:752–755. doi: 10.1126/science.280.5364.752. [DOI] [PubMed] [Google Scholar]
- 30.Spitzfaden C, Nicholson N, Jones JJ, Guth S, Lehr R, et al. J. Mol. Biol. 2000;295:105–115. doi: 10.1006/jmbi.1999.3341. [DOI] [PubMed] [Google Scholar]
- 31.Crary SM, Niranjanakumari S, Fierke CA. Biochemistry. 1998;37:9409–9416. doi: 10.1021/bi980613c. [DOI] [PubMed] [Google Scholar]
- 32.Ziehler WA, Day JJ, Fierke CA, Engelke DR. Biochemistry. 2000;39:9909–9916. doi: 10.1021/bi000603n. [DOI] [PubMed] [Google Scholar]
- 33.Lee Y, Kindelberger DW, Lee JY, McClennen S, Chamberlain J, Engelke DR. RNA. 1997;3:175–185. [PMC free article] [PubMed] [Google Scholar]
- 34.Maraia RJ, Intine RV. Mol. Cell. Biol. 2001;21:367–379. doi: 10.1128/MCB.21.2.367-379.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolin SL, Matera AG. Genes Dev. 1999;13:1–10. doi: 10.1101/gad.13.1.1. [DOI] [PubMed] [Google Scholar]
- 36.Pannone BK, Xue D, Wolin SL. EMBO J. 1998;17:7442–7453. doi: 10.1093/emboj/17.24.7442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoo CJ, Wolin SL. Cell. 1997;89:393–402. doi: 10.1016/s0092-8674(00)80220-2. [DOI] [PubMed] [Google Scholar]
- 38.Mildvan AS. Proteins. 1997;29:401–416. [PubMed] [Google Scholar]
- 39.Steitz TA, Steitz JA. Proc. Natl. Acad. Sci. USA. 1993;90:6498–6502. doi: 10.1073/pnas.90.14.6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beebe JA, Fierke CA. Biochemistry. 1994;33:10294–10304. doi: 10.1021/bi00200a009. [DOI] [PubMed] [Google Scholar]
- 41.Pfeiffer T, Tekos A, Warnecke JM, Drainas D, Engelke DR, et al. J. Mol. Biol. 2000;298:559–565. doi: 10.1006/jmbi.2000.3655. [DOI] [PubMed] [Google Scholar]
- 42.Thomas BC, Li XQ, Gegenheimer P. RNA. 2000;6:545–553. doi: 10.1017/s1355838200991465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomas BC, Chamberlain J, Engelke DR, Gegenheimer P. RNA. 2000;6:554–562. doi: 10.1017/s1355838200991477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tekos A, Tsagla A, Stathopoulos C, Drainas D. FEBS Lett. 2000;485:71–75. doi: 10.1016/s0014-5793(00)02190-6. [DOI] [PubMed] [Google Scholar]
- 45.Kurz JC, Fierke CA. Curr. Opin. Chem. Biol. 2000;4:553–558. doi: 10.1016/s1367-5931(00)00131-9. [DOI] [PubMed] [Google Scholar]
- 46.Beebe JA, Kurz JC, Fierke CA. Biochemistry. 1996;35:10493–10505. doi: 10.1021/bi960870m. [DOI] [PubMed] [Google Scholar]
- 47.Lee J-Y. PhD thesis. Univ. Mich.: Ann Arbor; 1991. Characterization of Saccharomyces cerevisiae nuclear RNase P and its RNA subunit; p. 133. [Google Scholar]
- 48.Altman S, Gopalan V, Vioque A. RNA. 2000;6:1689–1694. doi: 10.1017/s1355838200001783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gegenheimer P. RNA. 2000;6:1695–1697. doi: 10.1017/s1355838200001850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rossmanith W, Potuschak T. Mol. Cell. Biol. 2001;21:8236–8237. doi: 10.1128/MCB.21.23.8236-8237.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salavati R, Panigrahi AK, Stuart KD. Mol. Biochem. Parasitol. 2001;115:109–117. doi: 10.1016/s0166-6851(01)00273-0. [DOI] [PubMed] [Google Scholar]
- 52.Miller DL, Martin NC. Cell. 1983;34:911–917. doi: 10.1016/0092-8674(83)90548-2. [DOI] [PubMed] [Google Scholar]
- 53.Underbrink-Lyon K, Miller DL, Ross NA, Fukuhara H, Martin NC. Mol. Gen. Genet. 1983;191:512–518. doi: 10.1007/BF00425771. [DOI] [PubMed] [Google Scholar]
- 54.Morales MJ, Dang YL, Lou YC, Sulo P, Martin NC. Proc. Natl. Acad. Sci. USA. 1992;89:9875–9879. doi: 10.1073/pnas.89.20.9875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dang YL, Martin NC. J. Biol. Chem. 1993;268:19791–19796. [PubMed] [Google Scholar]
- 56.Shu HH, Wise CA, Clark-Walker GD, Martin NC. Mol. Cell. Biol. 1991;11:1662–1667. doi: 10.1128/mcb.11.3.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wise CA, Martin NC. J. Biol. Chem. 1991;266:19154–19157. [PubMed] [Google Scholar]
- 58.Wise CA. PhD thesis. Univ. Tex.: Southwestern, Dallas; 1991. Structural and functional studies of the yeast mitochondrial RNase P RNA; p. 185. [Google Scholar]
- 59.Stribinskis V, Gao GJ, Sulo P, Dang YL, Martin NC. Mol. Cell. Biol. 1996;16:3429–3436. doi: 10.1128/mcb.16.7.3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stribinskis V, Gao GJ, Sulo P, Ellis SR, Martin NC. Nucleic Acids Res. 2001;29:3631–3637. doi: 10.1093/nar/29.17.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kassenbrock CK, Gao GJ, Groom KR, Sulo P, Douglas MG, Martin NC. Mol. Cell. Biol. 1995;15:4763–4770. doi: 10.1128/mcb.15.9.4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rossmanith W, Tullo A, Potuschak T, Karwan R, Sbisa E. J. Biol. Chem. 1995;270:12885–12891. doi: 10.1074/jbc.270.21.12885. [DOI] [PubMed] [Google Scholar]
- 63.Rossmanith W, Karwan RM. FEBS Lett. 1998;433:269–274. doi: 10.1016/s0014-5793(98)00928-4. [DOI] [PubMed] [Google Scholar]
- 64.Rossmanith W, Karwan RM. Biochem. Biophys. Res. Commun. 1998;247:234–241. doi: 10.1006/bbrc.1998.8766. [DOI] [PubMed] [Google Scholar]
- 65.Puranam RS, Attardi G. Mol. Cell. Biol. 2001;21:548–561. doi: 10.1128/MCB.21.2.548-561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gegenheimer P. Mol. Biol. Rep. 1995;22:147–150. doi: 10.1007/BF00988720. [DOI] [PubMed] [Google Scholar]
- 67.Chen Y, Li XQ, Gegenheimer P. Biochemistry. 1997;36:2425–2438. doi: 10.1021/bi9620464. [DOI] [PubMed] [Google Scholar]
- 68.Cordier A, Schon A. J. Mol. Biol. 1999;289:9–20. doi: 10.1006/jmbi.1999.2762. [DOI] [PubMed] [Google Scholar]
- 69.Baum M, Cordier A, Schon A. J. Mol. Biol. 1996;257:43–52. doi: 10.1006/jmbi.1996.0145. [DOI] [PubMed] [Google Scholar]
- 70.Pascual A, Vioque A. FEBS Lett. 1999;442:7–10. doi: 10.1016/s0014-5793(98)01621-4. [DOI] [PubMed] [Google Scholar]
- 71.Collins LJ, Moulton V, Penny D. J. Mol. Evol. 2000;51:194–204. doi: 10.1007/s002390010081. [DOI] [PubMed] [Google Scholar]
- 72.True HL, Celander DW. J. Biol. Chem. 1996;271:16559–16566. doi: 10.1074/jbc.271.28.16559. [DOI] [PubMed] [Google Scholar]
- 73.Bartkiewicz M, Gold H, Altman S. Genes Dev. 1989;3:488–499. doi: 10.1101/gad.3.4.488. [DOI] [PubMed] [Google Scholar]
- 74.Doria M, Carrara G, Calandra P, Tocchini-Valentini GP. Nucleic Acids Res. 1991;19:2315–2320. doi: 10.1093/nar/19.9.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kline L, Nishikawa S, Soll D. J. Biol. Chem. 1981;256:5058–5063. [PubMed] [Google Scholar]
- 76.Stathopoulos C, Kalpaxis DL, Drainas D. Eur. J. Biochem. 1995;228:976–980. doi: 10.1111/j.1432-1033.1995.tb20349.x. [DOI] [PubMed] [Google Scholar]
- 77.Jayanthi GP, Van Tuyle GC. Arch. Biochem. Biophys. 1992;296:264–270. doi: 10.1016/0003-9861(92)90571-d. [DOI] [PubMed] [Google Scholar]
- 78.Lee JY, Engelke DR. Mol. Cell. Biol. 1989;9:2536–2543. doi: 10.1128/mcb.9.6.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee JY, Rohlman CE, Molony LA, Engelke DR. Mol. Cell. Biol. 1991;11:721–730. doi: 10.1128/mcb.11.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lygerou Z, Mitchell P, Petfalski E, Seraphin B, Tollervey D. Genes Dev. 1994;8:1423–1433. doi: 10.1101/gad.8.12.1423. [DOI] [PubMed] [Google Scholar]
- 81.Dichtl B, Tollervey D. EMBO J. 1997;16:417–429. doi: 10.1093/emboj/16.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chu S, Zengel JM, Lindahl L. RNA. 1997;3:382–391. [PMC free article] [PubMed] [Google Scholar]
- 83.Stolc V, Altman S. Genes Dev. 1997;11:2926–2937. doi: 10.1101/gad.11.21.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stolc V, Katz A, Altman S. Proc. Natl. Acad. Sci. USA. 1998;95:6716–6721. doi: 10.1073/pnas.95.12.6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Eder PS, Kekuda R, Stolc V, Altman S. Proc. Natl. Acad. Sci. USA. 1997;94:1101–1106. doi: 10.1073/pnas.94.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lygerou Z, Pluk H, van Venrooij WJ, Seraphin B. EMBO J. 1996;15:5936–5948. [PMC free article] [PubMed] [Google Scholar]
- 87.van Eenennaam H, Pruijn GJM, van Venrooij WJ. Nucleic Acids Res. 1999;27:2465–2472. doi: 10.1093/nar/27.12.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jarrous N, Eder PS, Wesolowski D, Altman S. RNA. 1999;5:153–157. doi: 10.1017/s135583829800185x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van Eenennaam H, Lugtenberg D, Vogelzangs JHP, van Venrooij WJ, Pruijn GJM. J. Biol. Chem. 2001;276:31635–31641. doi: 10.1074/jbc.M103399200. [DOI] [PubMed] [Google Scholar]
- 90.Jarrous N, Reiner R, Wesolowski D, Mann H, Guerrier-Takada C, Altman S. RNA. 2001;7:1153–1164. doi: 10.1017/s1355838201010469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee JY, Evans CF, Engelke DR. Proc. Natl. Acad. Sci. USA. 1991;88:6986–6990. doi: 10.1073/pnas.88.16.6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Salgado-Garrido J, Bragado-Nilsson E, Kandels-Lewis S, Seraphin B. EMBO J. 1999;18:3451–3462. doi: 10.1093/emboj/18.12.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tranguch AJ, Engelke DR. J. Biol. Chem. 1993;268:14045–14055. [PubMed] [Google Scholar]
- 94.Zimmerly S, Gamulin V, Burkard U, Soll D. FEBS Lett. 1990;271:189–193. doi: 10.1016/0014-5793(90)80403-6. [DOI] [PubMed] [Google Scholar]
- 95.Pitulle C, Garcia-Paris M, Zamudio KR, Pace NR. Nucleic Acids Res. 1998;26:3333–3339. doi: 10.1093/nar/26.14.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Altman S, Wesolowski D, Puranam RS. Genomics. 1993;18:418–422. doi: 10.1006/geno.1993.1488. [DOI] [PubMed] [Google Scholar]
- 97.Martin NC, Lang BF. Nucleic Acids Symp. Ser. 1997;36:42–44. [PubMed] [Google Scholar]
- 98.Kazakov S, Altman S. Proc. Natl. Acad. Sci. USA. 1991;88:9193–9197. doi: 10.1073/pnas.88.20.9193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Darr SC, Zito K, Smith D, Pace NR. Biochemistry. 1992;31:328–333. doi: 10.1021/bi00117a003. [DOI] [PubMed] [Google Scholar]
- 100.Guerrier-Takada C, Altman S. Biochemistry. 1993;32:7152–7161. doi: 10.1021/bi00079a012. [DOI] [PubMed] [Google Scholar]
- 101.Haas ES, Brown JW, Pitulle C, Pace NR. Proc. Natl. Acad. Sci. USA. 1994;91:2527–2531. doi: 10.1073/pnas.91.7.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Harris ME, Pace NR. RNA. 1995;1:210–218. [PMC free article] [PubMed] [Google Scholar]
- 103.Pagan-Ramos E, Lee Y, Engelke DR. RNA. 1996;2:441–451. [PMC free article] [PubMed] [Google Scholar]
- 104.Pagan-Ramos E, Lee Y, Engelke DR. RNA. 1996;2:1100–1109. [PMC free article] [PubMed] [Google Scholar]
- 105.Ziehler WA, Yang J, Kurochkin AV, Sandusky PO, Zuiderweg ER, Engelke DR. Biochemistry. 1998;37:3549–3557. doi: 10.1021/bi972886y. [DOI] [PubMed] [Google Scholar]
- 106.Pagan-Ramos E, Tranguch AJ, Kindelberger DW, Engelke DR. Nucleic Acids Res. 1994;22:200–207. doi: 10.1093/nar/22.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Loria A, Pan T. RNA. 1996;2:551–563. [PMC free article] [PubMed] [Google Scholar]
- 108.Loria A, Pan T. Nucleic Acids Res. 2001;29:1892–1897. doi: 10.1093/nar/29.9.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Massire C, Jaeger L, Westhof E. J. Mol. Biol. 1998;279:773–793. doi: 10.1006/jmbi.1998.1797. [DOI] [PubMed] [Google Scholar]
- 110.Fang XW, Pan T, Sosnick TR. Biochemistry. 1999;38:16840–16846. doi: 10.1021/bi991700n. [DOI] [PubMed] [Google Scholar]
- 111.Mobley EM, Pan T. Nucleic Acids Res. 1999;27:4298–4304. doi: 10.1093/nar/27.21.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ciesiolka J, Hardt WD, Schlegl J, Erdmann VA, Hartmann RK. Eur. J. Biochem. 1994;219:49–56. doi: 10.1111/j.1432-1033.1994.tb19913.x. [DOI] [PubMed] [Google Scholar]
- 113.Burgin AB, Pace NR. EMBO J. 1990;9:4111–4118. doi: 10.1002/j.1460-2075.1990.tb07633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Harris ME, Nolan JM, Malhotra A, Brown JW, Harvey SC, Pace NR. EMBO J. 1994;13:3953–3963. doi: 10.1002/j.1460-2075.1994.tb06711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Morse DP, Schmidt FJ. J. Mol. Biol. 1993;230:11–14. doi: 10.1006/jmbi.1993.1120. [DOI] [PubMed] [Google Scholar]
- 116.Ziehler WA, Morris J, Scott FH, Millikin C, Engelke DR. RNA. 2001;7:565–575. doi: 10.1017/s1355838201001996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yuan Y, Tan E, Reddy R. Mol. Cell. Biol. 1991;11:5266–5274. doi: 10.1128/mcb.11.10.5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu MH, Yuan Y, Reddy R. Mol. Cell. Biochem. 1994;130:75–82. doi: 10.1007/BF01084270. [DOI] [PubMed] [Google Scholar]
- 119.Jacobson MR, Cao LG, Taneja K, Singer RH, Wang YL, Pederson T. J. Cell Sci. 1997;110:829–837. doi: 10.1242/jcs.110.7.829. [DOI] [PubMed] [Google Scholar]
- 120.Kirsebom LA, Svard SG. EMBO J. 1994;13:4870–4876. doi: 10.1002/j.1460-2075.1994.tb06814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Glemarec C, Kufel J, Foldesi A, Maltseva T, Sandstrom A, et al. Nucleic Acids Res. 1996;24:2022–2035. doi: 10.1093/nar/24.11.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kufel J, Kirsebom LA. RNA. 1998;4:777–788. doi: 10.1017/s1355838298970923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Oh BK, Frank DN, Pace NR. Biochemistry. 1998;37:7277–7283. doi: 10.1021/bi973100z. [DOI] [PubMed] [Google Scholar]
- 124.Tuerk C, Gauss P, Thermes C, Groebe DR, Gayle M, et al. Proc. Natl. Acad. Sci. USA. 1988;85:1364–1368. doi: 10.1073/pnas.85.5.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cheong C, Varani G, Tinoco I., Jr Nature. 1990;346:680–682. doi: 10.1038/346680a0. [DOI] [PubMed] [Google Scholar]
- 126.Heus HA, Pardi A. Science. 1991;253:191–194. doi: 10.1126/science.1712983. [DOI] [PubMed] [Google Scholar]
- 127.Jaeger L, Michel F, Westhof E. J. Mol. Biol. 1994;236:1271–1276. doi: 10.1016/0022-2836(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 128.Murphy FL, Cech TR. J. Mol. Biol. 1994;236:49–63. doi: 10.1006/jmbi.1994.1117. [DOI] [PubMed] [Google Scholar]
- 129.Cate JH, Gooding AR, Podell E, Zhou K, Golden BL, et al. Science. 1996;273:1696–1699. doi: 10.1126/science.273.5282.1696. [DOI] [PubMed] [Google Scholar]
- 130.Gluck A, Endo Y, Wool IG. J. Mol. Biol. 1992;226:411–424. doi: 10.1016/0022-2836(92)90956-k. [DOI] [PubMed] [Google Scholar]
- 131.Krummel DAP, Altman S. Proc. Natl. Acad. Sci. USA. 1999;96:11200–11205. doi: 10.1073/pnas.96.20.11200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Brusca EM, True HL, Celander DW. J. Biol. Chem. 2001;276:42543–42548. doi: 10.1074/jbc.M107293200. [DOI] [PubMed] [Google Scholar]
- 133.Li Y, Altman S. Proc. Natl. Acad. Sci. USA. 2001;98:441–444. doi: 10.1073/pnas.021555498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jiang T, Altman S. Proc. Natl. Acad. Sci. USA. 2001;98:920–925. doi: 10.1073/pnas.021561498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.True HL, Celander DW. J. Biol. Chem. 1998;273:7193–7196. doi: 10.1074/jbc.273.13.7193. [DOI] [PubMed] [Google Scholar]
- 136.Calvo O, Cuesta R, Anderson J, Gutierrez N, Garcia-Barrio MT, et al. Mol. Cell. Biol. 1999;19:4167–4181. doi: 10.1128/mcb.19.6.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Clark MW, Abelson J. J. Cell. Biol. 1987;105:1515–1526. doi: 10.1083/jcb.105.4.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sarkar S, Hopper AK. Mol. Biol. Cell. 1998;9:3041–3055. doi: 10.1091/mbc.9.11.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bertrand E, Houser-Scott F, Kendall A, Singer RH, Engelke DR. Genes Dev. 1998;12:2463–2468. doi: 10.1101/gad.12.16.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fields S, Song O. Nature. 1989;340:245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- 141.SenGupta DJ, Zhang B, Kraemer B, Pochart P, Fields S, Wickens M. Proc. Natl. Acad. Sci. USA. 1996;93:8496–8501. doi: 10.1073/pnas.93.16.8496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141a.Houser-Scott F, Xiao S, Millikin CE, Zengel JM, Lindahl L, Engelke DR. Proc. Natl. Acad. Sci. USA. 2002 doi: 10.1073/pnas.052586299. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jiang T, Guerrier-Takada C, Altman S. RNA. 2001;7:937–941. doi: 10.1017/s1355838201010299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chang DD, Clayton DA. EMBO J. 1987;6:409–417. doi: 10.1002/j.1460-2075.1987.tb04770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chang DD, Clayton DA. Science. 1987;235:1178–1184. doi: 10.1126/science.2434997. [DOI] [PubMed] [Google Scholar]
- 145.Reimer G, Raska I, Scheer U, Tan EM. Exp. Cell Res. 1988;176:117–128. doi: 10.1016/0014-4827(88)90126-7. [DOI] [PubMed] [Google Scholar]
- 146.Kiss T, Marshallsay C, Filipowicz W. EMBO J. 1992;11:3737–3746. doi: 10.1002/j.1460-2075.1992.tb05459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li K, Smagula CS, Parsons WJ, Richardson JA, Gonzalez M, et al. J. Cell Biol. 1994;124:871–882. doi: 10.1083/jcb.124.6.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jacobson MR, Cao LG, Wang YL, Pederson T. J. Cell Biol. 1995;131:1649–1658. doi: 10.1083/jcb.131.6.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tollervey D. Mol. Biol. Rep. 1995;22:75–79. doi: 10.1007/BF00988709. [DOI] [PubMed] [Google Scholar]
- 150.Lindahl L, Zengel JM. Mol. Biol. Rep. 1995;22:69–73. doi: 10.1007/BF00988708. [DOI] [PubMed] [Google Scholar]
- 151.Schmitt ME, Clayton DA. Mol. Cell. Biol. 1993;13:7935–7941. doi: 10.1128/mcb.13.12.7935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lygerou Z, Allmang C, Tollervey D, Seraphin B. Science. 1996;272:268–270. doi: 10.1126/science.272.5259.268. [DOI] [PubMed] [Google Scholar]
- 153.Chu S, Archer RH, Zengel JM, Lindahl L. Proc. Natl. Acad. Sci. USA. 1994;91:659–663. doi: 10.1073/pnas.91.2.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cai T, Reilly TR, Cerio M, Schmitt ME. Mol. Cell. Biol. 1999;19:7857–7869. doi: 10.1128/mcb.19.11.7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sbisa E, Pesole G, Tullo A, Saccone C. J. Mol. Evol. 1996;43:46–57. doi: 10.1007/BF02352299. [DOI] [PubMed] [Google Scholar]
- 156.Schmitt ME, Clayton DA. Genes Dev. 1994;8:2617–2628. doi: 10.1101/gad.8.21.2617. [DOI] [PubMed] [Google Scholar]
- 157.Forster AC, Altman S. Cell. 1990;62:407–409. doi: 10.1016/0092-8674(90)90003-w. [DOI] [PubMed] [Google Scholar]
- 158.Karwan R. FEBS Lett. 1993;319:1–4. doi: 10.1016/0014-5793(93)80025-p. [DOI] [PubMed] [Google Scholar]
- 159.Schmitt ME, Bennett JL, Dairaghi DJ, Clayton DA. FASEB J. 1993;7:208–213. doi: 10.1096/fasebj.7.1.7678563. [DOI] [PubMed] [Google Scholar]
- 160.Schmitt ME. J. Mol. Biol. 1999;292:827–836. doi: 10.1006/jmbi.1999.3116. [DOI] [PubMed] [Google Scholar]
- 161.Lindahl L, Fretz S, Epps N, Zengel JM. RNA. 2000;6:653–658. doi: 10.1017/s1355838200992574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pluk H, van Eenennaam H, Rutjes SA, Pruijn GJM, van Venrooij WJ. RNA. 1999;5:512–524. doi: 10.1017/s1355838299982079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kendall A, Hull MW, Bertrand E, Good PD, Singer RH, Engelke DR. Proc. Natl. Acad. Sci. USA. 2000;97:13108–13113. doi: 10.1073/pnas.240454997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.O’Connor JP, Peebles CL. Mol. Cell. Biol. 1991;11:425–439. doi: 10.1128/mcb.11.1.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Matera AG, Frey MR, Margelot K, Wolin SL. J. Cell Biol. 1995;129:1181–1193. doi: 10.1083/jcb.129.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jarrous N, Wolenski JS, Wesolowski D, Lee C, Altman S. J. Cell Biol. 1999;146:559–572. doi: 10.1083/jcb.146.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chen D, Huang S. J. Cell Biol. 2001;153:169–176. doi: 10.1083/jcb.153.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Schmitt ME, Clayton DA. Genes Dev. 1992;6:1975–1985. doi: 10.1101/gad.6.10.1975. [DOI] [PubMed] [Google Scholar]
- 169.Gold HA, Topper JN, Clayton DA, Craft J. Science. 1989;245:1377–1380. doi: 10.1126/science.2476849. [DOI] [PubMed] [Google Scholar]