Abstract
Haber-Bosch nitrogen (N) has been increasingly used in industrial products, e.g., nylon, besides fertilizer. Massive numbers of species of industrial reactive N (Nr) have emerged and produced definite consequences but receive little notice. Based on a comprehensive inventory, we show that (1) the industrial N flux has increased globally from 2.5 to 25.4 Tg N yr−1 from 1960 through 2008, comparable to the NOx emissions from fossil fuel combustion; (2) more than 25% of industrial products (primarily structural forms, e.g., nylon) tend to accumulate in human settlements due to their long service lives; (3) emerging Nr species define new N-assimilation and decomposition pathways and change the way that Nr is released to the environment; and (4) the loss of these Nr species to the environment has significant negative human and ecosystem impacts. Incorporating industrial Nr into urban environmental and biogeochemical models could help to advance urban ecology and environmental sciences.
Human activities have more than doubled the annual production of Nr to satisfy human needs for food, energy, fiber and other products1. This doubling has serious ecological and societal consequences2,3,4,5. Industrial Nr (e.g., polyacrylonitrile) uses (N-containing products, e.g., nylon and explosives, but not including synthetic fertilizer) have contributed significantly to this problem6,7. The fate and impact of Nr used in food production and Nr created by fossil fuel combustion has received considerable attention8,9; however, little attention has been given to the Nr used in industrial production on global10 and national scales other than quantifying the magnitude of Nr creation for industrial uses (e.g., EPA-SAB11, European N Assessment12). The absence of this component from the study of human alteration of the N cycle is a serious concern7,10. Accordingly, the way in which industrial Nr contributes to the alteration of the global N cycle, which further affects the environment and human health, has become a crucial topic. To address this issue, we study the role of industrial Nr in the global N cycle by first considering Nr species, turnover rate and flux, and we analyze the emissions of industrial Nr to the environment and the effects of industrial Nr on human health and climate change. Finally, we discuss the policy implications of mitigating the negative effects of industrial Nr.
Results
Emerging Nr species
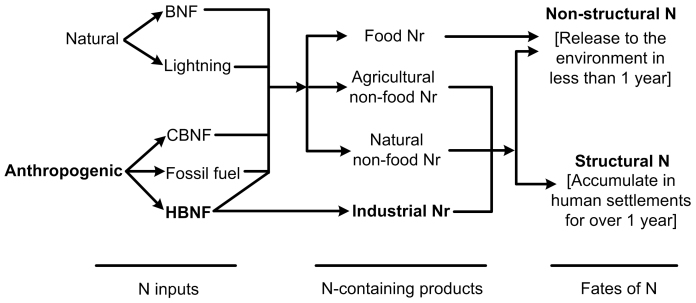
Before Haber-Bosch N fixation (HBNF), Nr in food and non-food products (e.g., leather, cotton lint, wood furniture, Fig. 1) was primarily provided by biological N fixation in agricultural and natural ecosystems. However, due to HBNF, there are now 7 principal categories and 18 subcategories of Nr species, such as azo and diazo compounds, which are produced as intermediates in industrial processes. More than 50% of these intermediates belong to emerging Nr species rather than the Nr species present in the natural environment (Table 1). The end products of industrial processes have also contributed new Nr species, e.g., polyamide fiber (nylon) has approximately 140 or more species of N-containing compounds estimated on the basis of their molecular weights, including aliphatic, aliphatic-aromatic and aromatic nylon. N-containing pesticides comprise more than 60 species, namely, nitrile, amidine, heterocyclic, amine, cyanide, nitro and urea pesticides13. At least 118 species of azo dyes have been banned by the German government due to their carcinogenicity14.
Figure 1. Sources and fates of industrial, natural and agricultural Nr products.
BNF, biological nitrogen fixation; CBNF, cultivated biological nitrogen fixation; HBNF, Haber-Bosch nitrogen fixation.
Table 1. Intermediates and end products of industrial products.
| Category | Subcategory | Toxicity | End products | Service life | Existing in nature |
|---|---|---|---|---|---|
| Nitrate ester (-ONOx)a | Nitrate | low | fuel, drug, explosive | < 1 yr | no |
| Nitrite | low | drug, reagent | < 1 yr | no | |
| Amines (-NHyR2-y)b | Aliphatic | low | reagent, drug | < 1 yr | many |
| Aromatic | high | dye, resin, drug, pesticide | > 1 yr | no | |
| Amides (-OCNH2) | Amide | low | reagent, fiber, plastic, rubber, resin | > 1 yr | few |
| Urea | low | drug | < 1 yr | many | |
| Hydrazone | low | dye | > 1 yr | few | |
| Amidine | low | pesticide | < 1 yr | no | |
| Nitriles (-C≡N) | Nitrile | high | fiber, plastic, rubber | > 1 yr | some |
| Isonitrile | high | pesticide, drug | < 1 yr | some | |
| Isocyanate | high | pesticide, drug, fiber, plastic, rubber | > 1 yr | no | |
| Oxime | high | drug, fiber, reagent | > 1 yr | no | |
| Nitro (-NOx) | Nitro | high | explosive, drug, paint, dye, reagent | < 1 yr | few |
| Nitroso | high | drug, reagent | < 1 yr | few | |
| Azo (-N = N-) | Azo | high | dye, reagent | > 1 yr | no |
| Diazo | high | dye | > 1 yr | no | |
| Hydrazine | high | fuel, drug, rubber, resin | < 1 yr | some | |
| Heterocyclic | N Heterocyclic | high | dye, drug, pesticide | < 1 yr | many |
a, x = 1 or 2.
b, y = 0, 1 or 2.
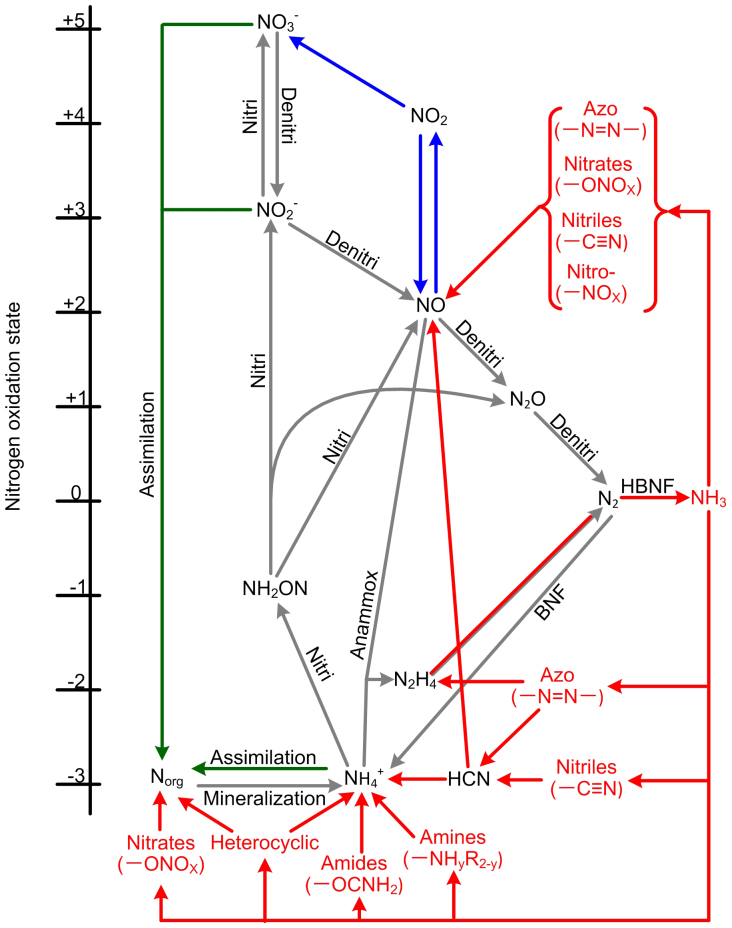
These new Nr species create new pathways of N assimilation by combining synthetic NH3 and its derivative products with different carbon skeletons (Fig. 2). These new pathways are supported by catalyzers, energy inputs and appropriate air pressure and temperature in factories1,15. On the basis of intermediates and their end products, a conservative estimate for the number of new N assimilation pathways in industrial production is greater than 400; these pathways can be examined in comparison to biological N assimilation pathways (Fig. 2, Table 1). The decomposition of industrial products follows various pathways that are determined by the processes and uses associated with these products16,17,18. For example, the burning of polyurethane releases hydrogen cyanide (HCN) and other N-containing compounds to the air19, and landfills containing industrial products release Nr to the hydrosphere20.
Figure 2. Industrial Nr involved in the major transformation pathways of the global N cycle.
Red arrows represent the pathways of the industrial N cycle; grey arrows represent the pathways dominated by microbial activities; green arrows represent the pathways dominated by plants; blue arrows represent the pathways dominated by atmospheric chemical reactions. Due to the difficulty of determining the N oxidation states of industrial Nr compounds, their vertical positions do not follow the order of N oxidation states from −3 to + 5. Abbreviations: BNF, biological N fixation; Denitri, denitrification; HBNF, Haber-Bosch N fixation; Nitri, nitrification.
Slowing N turnover rates
The emerging Nr species and their complex biogeochemical cycling pathways are a difficult aspect of the study of the turnover of industrial products (Fig. 2, Table 2). The study of the emerging Nr species and their pathways is important because it provides insights into the environmental fates of industrial products. To address these issues, we classified industrial Nr into two categories: structural and non-structural N.
Table 2. Quantities and Nr fluxes of structural industrial products worldwide circa 2008.
| Species | Quantity (Tg) | N content (%) | N flux (Tg) | Reference |
|---|---|---|---|---|
| Synthetic fiber | ||||
| Polyamide fiber | 3.9 | 12.4–32.6 | 0.5–1.3 | Ou59 |
| Acrylic fibers | 2.0 | 26.4 | 0.5 | |
| Polyurethane fiber | 0.3 | 23.7 | 0.1 | |
| Plastic* | ||||
| AS (SAN) | 7.5 | 8.9 | 0.7 | CPIEO36 |
| ABS | 12.3 | 4–9 | 0.5–1.1 | |
| PU | 13.5 | 23.7 | 3.2 | |
| Synthetic rubber | ||||
| Nitrile rubber | 0.8 | 13 | 0.1 | Li & Wu60 |
| Acryl-nitrile rubber | 4.7–12.1 | |||
| Dye | 1.3 | ** | ** | Zhang61 |
| Paint | 36.6 | ** | ** | CPIA34 |
| Resin | ** | ** | ** | |
| Total | > 78.2 | > 5.6–7.0# | ||
*AS (SAN): styrene-acrylonitrile copolymers; ABS: acrylonitrile-butadiene-styrene copolymers; PU: polyurethanes.
**, no data available.
#, the average value is 6.3.
The category of structural N is defined as the set of N-containing products that offer physical support services (e.g., housing, furniture, clothing, transportation) or combine with physical support services (e.g., dye, paint). Based on their functions, structural industrial products are divided into 6 principal categories: fiber, plastic, rubber, resin, dye and paint. Each category includes hundreds to thousands of Nr species. Structural N has a low N turnover rate because of its long service life, the length of time over which an industrial product can maintain its function. For example, the service life of nitrile rubber is approximately 10 years; the service life of decorations and furniture (e.g., synthetic fiber and paints) is 10–20 years; and construction materials (e.g., resins, glues) could have a service life of more than 50 years21,22. The long service life of structural industrial Nr leads to the persistent accumulation of Nr in human settlements prior to its release to the environment. The current Nr release from structural N is from materials produced years to decades ago, not from recently produced materials. Hence, the current patterns and fluxes of structural industrial Nr release may reflect the historical industrial N cycle more accurately than they reflect the contemporary industrial N cycle. The accumulation of industrial Nr in human settlements, especially in urban areas21, contributes to changes in the global Nr pool9. The use of structural industrial Nr has established urban areas as an important Nr pool (Supplementary Fig. S5), especially in terms of the cumulative total amount of structural industrial Nr over decades. If the structural N from agricultural production (2.5 Tg N yr−1, e.g., leather, cotton lint, and wood furniture23) is included, the structural N accumulated in human settlements (total 8.9 Tg N yr−1) is almost equal to the anthropogenic Nr accumulated in natural ecosystems (approximately 9 Tg N yr−1, ref. 24).
In contrast, non-structural industrial Nr generally does not involve physical support services but is associated with chemical reactions or biological metabolism. The principal non-structural industrial products are explosives, rocket fuel, drugs, pesticides and reagents. The turnover rate of non-structural industrial Nr, like that of food and fuel Nr, is relatively high because the N contained in these products is usually released to the environment within one year. The N contained in explosives and rocket fuel is released to the environment after use6. Pesticide N can persist in the environment for a relatively long time, but this duration is still less than one year13. The half-lives of nitrile and amidine pesticides are as high as ~ 60 days; for heterocyclic and amine pesticides, the half-life is approximately 45 days; whereas for cyanide, nitro- and urea pesticides, the half-life ranges from 15 to 30 days prior to decomposition to simple N-containing compounds (Supplementary Fig. S1).
Increasing N fluxes
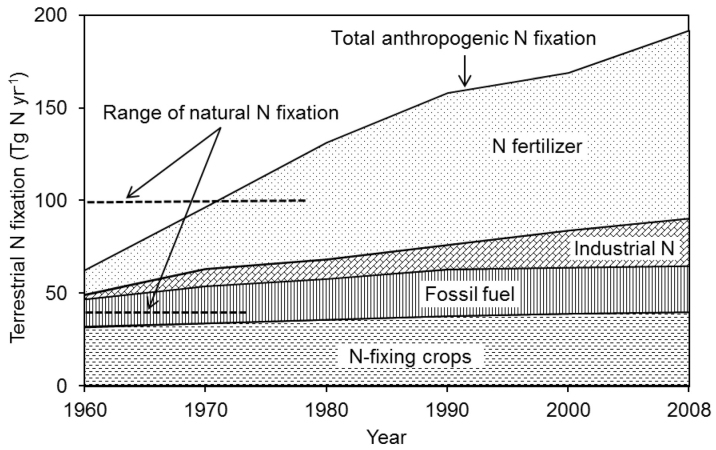
Human activities resulted in the fixation of 192 Tg N yr−1 in 2008. Industrial Nr represented 13% of this total, approximately the same magnitude as fossil fuel combustion (Fig. 3). More recently, several studies have estimated the current global industrial Nr flux, ranging from 22.8 to 28.5 Tg N yr−1 (ref. 10, 23, 25). Through a comprehensive inventory, we estimated that the global industrial Nr flux increased rapidly, from 2.5 to 25.4 Tg N yr−1, from 1960 through 2008. This estimate falls within the range of previous estimates10,23,25. Industrial Nr use represents 20% of global HBNF (the remaining amount is used in N fertilizer). Through category-based flux estimation, we first attempt to quantify the global structural industrial Nr flux at > 6.3 Tg N yr−1 in 2008 (Table 2), representing > 25% of total industrial Nr flux. The remaining quantity is considered non-structural industrial N. The rate of natural N fixation to land ranges between 40 and 100 Tg N yr−1 through lightning and biological N fixation26; thus, if industrial Nr flux is included, human activities have more than tripled the terrestrial N fixation (Fig. 3).
Figure 3. Anthropogenic fixation of N in terrestrial ecosystems over time in comparison with the range of estimates of natural biological N fixation on land.
Data in the figure are updated from Vitousek et al.2. The range of natural N fixation is from Vitousek et al26.
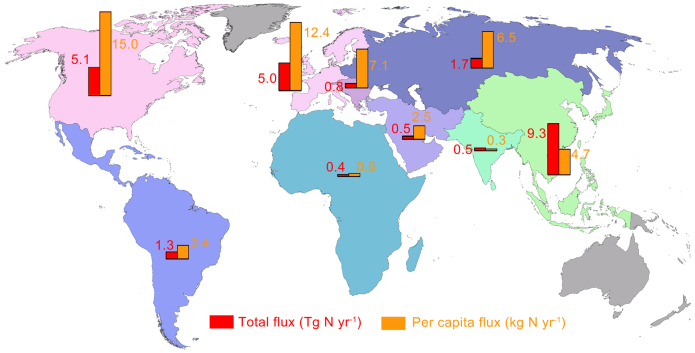
On a regional scale, industrial Nr fluxes vary substantially. The per capita industrial Nr flux reaches 12–15 kg N yr−1 in Western Europe and North America, but it is only 0.3–0.6 kg N yr−1 in Africa and South Asia (Fig. 4). Industrial Nr flux represents ~ 30% of HBNF in developed countries8,11 but generally less than 15% in developing countries7,25. In contrast to the global per capita food Nr fluxes, which have increased slowly and stably, industrial Nr fluxes have fluctuated markedly with economic development over the past half century (Fig. 5). Given the large differences in per capita industrial fluxes between developed and developing countries (Fig. 4), an enormous potential increase of industrial Nr is expected in developing countries as economic development proceeds (Supplementary Fig. S2, S3). If the global per capita consumption of industrial Nr reaches the current level found in developed countries, approximately 12–15 kg N yr−1, the total global industrial Nr flux would increase at least 3–4 times.
Figure 4. Industrial Nr fluxes in different continental regions.
Colored bars represent industrial Nr fluxes. The global map was created with the GMAP function in SAS 9.1 (SAS Institute Inc., Cary, NC, USA).
Figure 5. Temporal dynamics of global industrial Nr and food Nr consumption, 1960–2008.
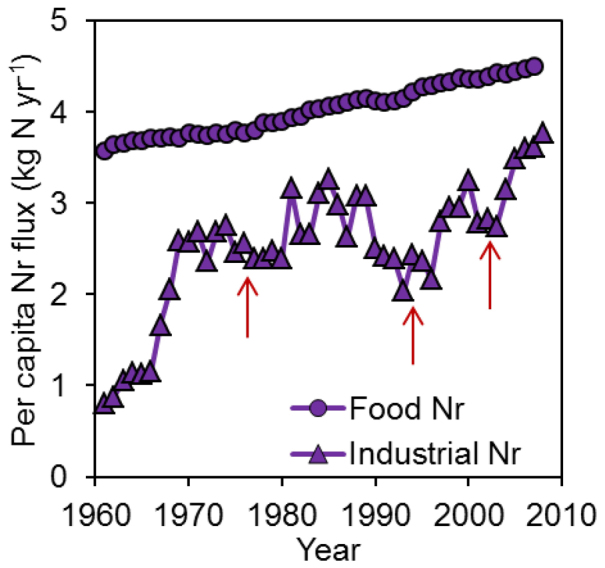
Red arrows indicate global economic crises. Modified from Gu et al.7.
Discussion
Industrial production shows a relatively low proportion of N loss (Nr input for production but not contained in the final products, divided by total Nr input for production), e.g., ~ 2% for the US in 19966 and ~ 5% for China in 20087. Compared with the high initial proportion of N loss in agricultural production, 50% to 80%27,28,29, the low proportion of N loss in industrial production only resulted in 0.5–1.3 Tg N yr−1 released to the environment in 2008 worldwide (based on a 2–5% N loss rate). However, the Nr release during industrial production belongs to the category of point emissions that usually have an extremely high Nr concentration and cause deterioration in the local environment30. The groundwater nitrate concentration beneath industrial land use is much higher than that beneath other land uses, e.g., cropland and human settlements (Supplementary Fig. S4). Moreover, the composition of the Nr emissions is complex due to the massive contribution of Nr species during industrial production, and approximately one-half of the intermediates and their end products are toxic (Table 1). The emissions of these complex toxic Nr species have serious effects on the health of people who work in or live near the factories. The leakage of hydrazine can irritate eyes and cause delayed inflammation and can also have strong corrosive effects on skin if the exposure involves a concentration greater than 0.06 mg m−3 (ref. 31). The emitted Nr species, such as NOx emissions from the production of nitric acid32, can react with other components (e.g., volatile organic compounds, VOCs) to form near ground-level ozone and fine particulate matter that penetrate and are deposited in the lungs, causing serious harm to human health3,33.
The structural industrial Nr accumulated in human settlements annually, approximately 8.9 Tg N yr−1, could be one of the explanations for the global unknown Nr sink cited by Schlesinger24, with a size of approximately 26 Tg N yr−1. Nevertheless, the accumulated structural industrial N can be inadvertently released into the environment. Concrete admixtures, plates, furniture and decorative materials can release NH3 and toluene diisocyanate (TDI) to produce indoor air pollution34. Toxic Nr can also be released during fires or other emergencies. The burning of polyacrylonitrile materials can release 0.06 g HCN per gram polyacrylonitrile18, and HCN is highly toxic and sometimes fatal to humans18,35. More than 50 people were killed in a fire disaster in Shanghai in November 2010. These deaths were largely attributed to the emission of HCN from the burning of polyacrylonitrile and polyurethane foam used in the redecoration of the building35,36. Burning for energy recycling is an important type of waste treatment involving structural industrial Nr, and it could cause direct environmental and health risks for local or downwind residents37. This process results in aggravated reactive airways disease (RAD), coughs, asthma and chronic respiratory disease if no steps are taken to reduce the N contained in the burning structural industrial products38. In landfills containing structural industrial Nr, the groundwater quality around the sites has been seriously impacted by the leaching of pollutants16, especially in developing countries with rapid urbanization, e.g., China, India, Mexico17,39.
Nevertheless, we can say little about the quantity of Nr released to air, soil or water from non-structural industrial Nr. This quantity depends on the processes and uses associated with these products. However, the total Nr released to the environment from non-structural industrial Nr should be smaller than its total flux, ~ 19.1 Tg N yr−1. Generally, the use of explosives will cause rapid emissions of N2 and Nr (NO, N2O, NH3) to the atmosphere, and the emitted Nr would photochemically or chemically react with other chemicals (e.g., VOCs) and may be further deposited on land and water6. Drugs decompose or are discharged directly into domestic wastewater through the human metabolic system. However, pesticides, depending on their relatively long half-lives (Supplementary Fig. S1), volatility and fat-solubility, disperse globally. They can bioaccumulate in food chains and affect human health and the health of other species far from the point of release and for many years after release40,41. In addition to the effects of Nr released to the environment, non-structural industrial Nr can also affect the environment and human health via direct usage. Drugs related to industrial Nr have made a profound and substantial contribution to the improvement of human health42, although drug resistance and the side effects of certain drugs may also damage human health.
Anthropogenic Nr release to the environment has both a warming and cooling effect on the climate, primarily through changes in the greenhouse gas balance38,43,44. Currently, the importance of the industrial N cycle in regulating climate is gaining increasing attention32,43. However, the net effect of industrial Nr on climate is highly uncertain due to the substantial increase in Nr species and the complex cycling pathways (Fig. 2, Table 1). Industrial production, such as the production of nylon43, nitric acid, adipic acid and glyoxal, releases N2O, which acts as a significant source of global warming4. The European N assessment has identified the chemical industry as an important source of N2O emissions, representing 20% of total European N2O emissions32. Industrial Nr reductive or oxidative pathways could also release N2O. For example, the explosion of 1 kg of nitroglycerin releases approximately 97 g of N2O6. As an important non-structural industrial Nr end product, NH3 is regaining its position as a refrigerant, replacing chlorofluorocarbons, which can potentially have a substantial effect on global warming45.
Trade reshapes the global pattern of Nr fluxes and would affect human health through the distribution loss and transfer of Nr emissions during production and after consumption10. For example, China exported a large quantity of industrial products, e.g., apparel (80 Mt), chemical, rubber, and plastic products (44 Mt), to the United States and Europe in 200446. Imports can contribute to the environmental damage associated with distribution loss and N loss during production in the exporting countries10. For structural industrial products, environmental damage is associated not only with N loss during production but also with Nr release after consumption. This impact should be a concern due to the legacy effect6. The legacy effect has long-term consequences on human health because the Nr released from structural industrial Nr would continue to damage the environment16,20 until denitrification converted the Nr to N247.
Given the development of a global socio-economy, industrial Nr production is still accelerating in terms not only of Nr flux but also of new Nr species and cycling pathways, a trend unlikely to change in the near future (Fig. 5, Supplementary Fig. S2). By understanding the environmental and health effects of industrial Nr, we focus on the intervention points in the industrial N cycle. This approach can better support the public and decision makers and ultimately yield more sustainable policies that both reduce negative effects and optimize N-related services in the future. First, the rates of reuse of structural industrial Nr should be increased to reduce the legacy effects. The legacy effect of structural N may cause unexpected changes in the environment and produce irreversible consequences, such as the garbage siege of large cities and the severe pollution of their groundwater17. Given the difficulties of monitoring and quantifying the legacy effects of structural N, an increase in reuse rates to reduce the fluxes of newly produced structural N is essential. Second, the environmental effects of industrial N should be reduced through better product designs. Nr emissions, especially toxic Nr, during the usage of industrial products and after the abandonment of structural products should be reduced. For example, fire retardant should be added to polyacrylonitrile and polyurethane products to reduce the HCN emissions when these products are burned48. Third, industrial Nr should be integrated into urban biogeochemistry models to help manage the N problem, given that more than 50% of the global population lives in urban areas49. The absence of industrial Nr from large-scale N studies, particularly in the case of urban areas50, would result in substantial uncertainties in the understanding of regional to global N cycles and the interactions of N with other elements8,51. Further studies serving to reduce these uncertainties are extremely important for understanding the nature of the global system. In addition, modeling tools and novel approaches must be made available to researchers in the fields of N studies, engineering, environmental sciences, social sciences and ecological modeling.
Methods
Industrial Nr species
We first collected information on N-containing industrial products from previous studies6,7,12,19, chemical statistics yearbooks34,52,53 and the Global Chemical Network54. All these industrial products were categorized based on their function and chemical structure, e.g., synthetic fiber, rubber, plastics, explosives. The intermediates of these categorized products during production were retrieved from Pan55 and the Global Chemical Network54. The industrial Nr species were then further categorized into seven groups based on their N functional group, e.g., nitriles, nitro, azo (Table 2).
Industrial Nr turnover rate
We estimated the service lives of all the industrial products from previous studies6,13,25,26 and chemical statistics yearbooks34,52,53 to conduct the analysis of the N turnover rate. Based on their service lives (longer or not longer than 1 year) and their principal functions, all these industrial products were divided into two groups: structural and non-structural.
Industrial Nr flux
We estimated the N fluxes of global structural industrial products via total production and the N concentrations (Table 2). However, we could not obtain information on the production and N concentration of non-structural industrial Nr because many more Nr species were involved in this group than in structural industrial Nr. For this reason, it is difficult to estimate the global N fluxes of non-structural industrial Nr. To investigate the historical trends in the global industrial Nr flux, we therefore assumed that the industrial flux was approximately equal to the difference between total HBNF and N fertilizer production and distribution loss. Data on global HBNF from 1960 to 2008 were taken from the USGS Minerals Yearbook56. Nitrogen fertilizer production data were retrieved from FAO57. To verify the accuracy and reduce the uncertainties of estimation, HBNF in previous years was validated and revised for the subsequent 2–3 years to bring it nearer the real-world value56. Our estimates may introduce variation due to uncertainties about the distribution loss during transportation. Fortunately, this source contributes less than 5% of the total industrial Nr flux25 and would not change the increasing trend in the global industrial Nr flux.
The continental breakdown of industrial Nr consumption in 2008 was estimated based on Prud'homme25. Data on the global and regional production and consumption of agricultural Nr products from 1960 to 2008 were retrieved from FAO57. Data on the global population, urbanization and GDP were retrieved from the World Bank58.
Author Contributions
J.C., C.P., B.G. and J.G. contributed to the development of project ideas and design. B.G., Y.M., Q.Z. and Y.G. conducted the data collection and performed the analyses. All authors substantially contributed to interpreting the results and writing the paper.
Supplementary Material
Supplementary information
Acknowledgments
We would like to thank Dr. William H. Schlesinger for his valuable comments and suggestions on discussions of the early version of this manuscript and Dr. Hongliang Fan for her help with the analysis of chemical forms of industrial products. We gratefully acknowledge funding provided by the National Science Foundation of China (Grant No. 41201502 and 31170305), the China Postdoctoral Science Special Foundation (Grant No. 2012T50508), the Y.C. Tang Disciplinary Development Fund, a NSERC Discovery Grant and the QianRen Program.
References
- Erisman J. W., Sutton M. A., Galloway J., Klimont Z. & Winiwarter W. How a century of ammonia synthesis changed the world. Nature Geosci. 1, 636–639 (2008). [Google Scholar]
- Vitousek P. M. et al. Human alteration of the global nitrogen cycle: sources and consequences. Ecol. Appl. 7, 737–750 (1997). [Google Scholar]
- Compton J. E. et al. Ecosystem services altered by human changes in the nitrogen cycle: a new perspective for US decision making. Ecol. Lett. 14, 804–815 (2011). [DOI] [PubMed] [Google Scholar]
- Sutton M. A. et al. Too much of a good thing. Nature 472, 159–161 (2011). [DOI] [PubMed] [Google Scholar]
- Galloway J. N. et al. The nitrogen cascade. Bioscience 53, 341–356 (2003). [Google Scholar]
- Domene L. A. F. & Ayres R. U. Nitrogen's role in industrial systems. J. Ind. Ecol. 5, 77–103 (2001). [Google Scholar]
- Gu B. et al. Rapid growth of industrial nitrogen fluxes in China: driving forces and consequences. Sci. China Earth Sci. 56, 662–670 (2013). [Google Scholar]
- Gruber N. & Galloway J. N. An earth-system perspective of the global nitrogen cycle. Nature 451, 293–296 (2008). [DOI] [PubMed] [Google Scholar]
- Canfield D. E., Glazer A. N. & Falkowski P. G. The evolution and future of Earth's nitrogen cycle. Science 330, 192–196 (2010). [DOI] [PubMed] [Google Scholar]
- Galloway J. N. et al. Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320, 889–892 (2008). [DOI] [PubMed] [Google Scholar]
- EPA-SAB (Environmental Protection Agency Science Advisory Board). Reactive Nitrogen in the United States: An Analysis of Inputs, Flows, Consequences and Management Options, EPA-SAB-11-013. (EPA-SAB, 2011). [Google Scholar]
- Jensen L. S. et al. in The European Nitrogen Assessment (eds Sutton A., et al.) Ch. 3, 32–61 (Cambridge University Press, 2011). [Google Scholar]
- Lin Y. Pesticides and Ecological Environment Protection. (Chemical Industry Press, 2000). [Google Scholar]
- MCI (Ministry of Chemistry Industry). 20 kinds of carcinogenic aromatic amine and 118 dye species banned by Germany. China Chem. Ind. 3, 61–62 (1995). [Google Scholar]
- Maxwell G. R. Synthetic Nitrogen Products: A Practical Guide to the Products and Processes. (Kluwer Academic Press, 2004). [Google Scholar]
- Mor S., Ravindra K., Dahiya R. & Chandra A. Leachate characterization and assessment of groundwater pollution near municipal solid waste landfill site. Environ. Monit. Assess. 118, 435–456 (2006). [DOI] [PubMed] [Google Scholar]
- Zhang D. Q., Tan S. K. & Gersberg R. M. Municipal solid waste management in China: Status, problems and challenges. J. Environ. Manage. 91, 1623–1633 (2010). [DOI] [PubMed] [Google Scholar]
- Wang Z., Jia F. & Galea E. R. A generalized relationship between the normalized yields of carbon monoxide and hydrogen cyanide. Fire Mater. 35, 577–591 (2011). [Google Scholar]
- Christian T. J. et al. Trace gas and particle emissions from domestic and industrial biofuel use and garbage burning in central Mexico. Atmos. Chem. Phys. 10, 565–584 (2010). [Google Scholar]
- Berge N., Reinhart D. & Townsend T. The fate of nitrogen in bioreactor landfills. Crit. Rev. Environ. Sci. Technol. 35, 365–399 (2005). [Google Scholar]
- Murakami S., Oguchi M., Tasaki T., Daigo I. & Hashimoto S. Lifespan of commodities, part I. J. Ind. Ecol. 14, 598–612 (2010). [Google Scholar]
- Law K. L. et al. Plastic accumulation in the North atlantic subtropical gyre. Science 329, 1185–1188 (2010). [DOI] [PubMed] [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations). Current World Fertilizer Trends and Outlook to 2012. (FAO, Communication division, Electronic publishing policy and support branch, 2008). [Google Scholar]
- Schlesinger W. H. On the fate of anthropogenic nitrogen. Proc. Natl. Acad. Sci. USA 106, 203–208 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prud'homme M. Global fertilizers and raw materials supply and supply/demand balances 2009 – 2013. (International Fertilizer Industry Association, 2009). [Google Scholar]
- Vitousek P. M., Menge D. N. L., Reed S. C. & Cleveland C. C. Biological nitrogen fixation: rates, patterns, and ecological controls in terrestrial ecosystems. P. Trans. Royal. Soc. Ser. B 368, 20130119 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. et al. A high-resolution assessment on global nitrogen flows in cropland. Proc. Natl. Acad. Sci. USA 107, 8035–8040 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassman K. G., Dobermann A. & Walters D. T. Agroecosystems, nitrogen-use efficiency, and nitrogen management. Ambio 31, 132–140 (2002). [DOI] [PubMed] [Google Scholar]
- Oenema O. & Tamminga S. Nitrogen in global animal production and management options for improving nitrogen use efficiency. Sci. China Ser. C-Life Sci. 48, 871–887 (2005). [PubMed] [Google Scholar]
- Kapley A. & Purohit H. J. Diagnosis of treatment efficiency in industrial wastewater treatment plants: a case study at a refinery ETP. Environ. Sci. Technol. 43, 3789–3795 (2009). [DOI] [PubMed] [Google Scholar]
- MHC (Ministry of Health of China). Occupational exposure limits for hazardous agents in the workplace, chemical hazardous agents. Vol. GBZ 2.1-2007 (MHC Press, 2007). [Google Scholar]
- Leip A. et al. in The European Nitrogen Assessment (eds Sutton A., et al.) Ch. 16, 345–376 (Cambridge University Press, 2011). [Google Scholar]
- Townsend A. R. et al. Human health effects of a changing global nitrogen cycle. Front. Ecol. Environ. 1, 240–246 (2003). [Google Scholar]
- CPIA (China Paint Industry Association). China Paint and Coatings Industry Annual. (CPIA Press, 2010). [Google Scholar]
- CWEO (Construction Worker Editorial Office). Thinking of the Shanghai fire disaster. Jianzhu Gongren 12, 14–15 (2010). [Google Scholar]
- CPIEO (China Plastics Industry Editorial Office). The progress of the world's plastics industry in 2009–2010. China Plast. Ind. 39, 1–42 (2011). [Google Scholar]
- CUDRD (Committee of Urban Domestic Refuge Disposal of CAEPI). China development report on urban domestic refuge disposal industry in 2010. China Environ. Protec. Ind. 4, 32–37 (2011). [Google Scholar]
- Davidson E. et al. Excess nitrogen in the U.S. environment: trends, risks, and solutions. Issues Ecol. 15, 1–16 (2012). [Google Scholar]
- Troschinetz A. M. & Mihelcic J. R. Sustainable recycling of municipal solid waste in developing countries. Waste Manage. 29, 915–923 (2009). [DOI] [PubMed] [Google Scholar]
- Tilman D. et al. Forecasting agriculturally driven global environmental change. Science 292, 281–284 (2001). [DOI] [PubMed] [Google Scholar]
- Starks S. E. et al. Peripheral nervous system function and organophosphate pesticide use among licensed pesticide applicators in the Agricultural Health Study. Environ. Health Perspect. 120, 515 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. T. J. et al. Linking environmental nutrient enrichment and disease emergence in humans and wildlife. Ecol. Appl. 20, 16–29 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson E. A. The contribution of manure and fertilizer nitrogen to atmospheric nitrous oxide since 1860. Nature Geosci. 2, 659–662 (2009). [Google Scholar]
- Pinder R. W. et al. Climate change impacts of US reactive nitrogen. Proc. Natl. Acad. Sci. USA 109, 7671–7675 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redwood I. Theoretical and Practical Ammonia Refrigeration. (Nabu Press, 2010). [Google Scholar]
- Davis S. J. & Caldeira K. Consumption-based accounting of CO2 emissions. Proc. Natl. Acad. Sci. USA 107, 5687–5692 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni M. V., Groffman P. M. & Yavitt J. B. Solving the global nitrogen problem: it's a gas!. Front. Ecol. Environ. 6, 199–206 (2008). [Google Scholar]
- Yang S. & Lu L. Effect of flame retardants on HCN from the thermal decomposition of soft PU foam. China Plastics 24, 77–83 (2010). [Google Scholar]
- Grimm N. B. et al. Global change and the ecology of cities. Science 319, 756–760 (2008). [DOI] [PubMed] [Google Scholar]
- Kaye J. P., Groffman P. M., Grimm N. B., Baker L. A. & Pouyat R. V. A distinct urban biogeochemistry? Trends Ecol. Evol. 21, 192–199 (2006). [DOI] [PubMed] [Google Scholar]
- Gu B. et al. The role of technology and policy in mitigating regional nitrogen pollution. Environ. Res. Lett. 6, 014011 (2011). [Google Scholar]
- CPCIF (China Petroleum and Chemical Industry Federation). China Chemical Industry Yearbook. (China National Chemical Information Center Press, 2011). [Google Scholar]
- CNCIC (China National Chemical Information Center). Dictionary of Chemical Products and Producers in China. (Chemical Industry Press, 2012). [Google Scholar]
- GCN (Global Chemical Network). Chemical industry directory. Dataset available at: http://www.chemnet.com/. Accessed Feb 20, 2013.
- Pan H. Organic Chemistry. (Chemical Industry Press, 2010). [Google Scholar]
- USGS (United States Geological Survey). Nitrogen statistics and information. Dataset available at: http://minerals.usgs.gov/minerals/pubs/commodity/nitrogen/index.html#myb. USGS, USA. Accessed Feb 20, 2013.
- FAO (Food and Agriculture Organization of the United Nations). FAOSTAT: FAO Statistical Databases. (Rome, Italy, 2012). [Google Scholar]
- TWB (The World Bank). World DataBank: world Development Indicators & Global Development Finance. (Washington, USA, 2012). [Google Scholar]
- Ou R. Global production of synthestic fiber in 2009 is over 7000 million tons. Synthetic Fiber 6, 51–52 (2010). [Google Scholar]
- Li Y. & Wu X. Production status and market prospect of nitrile rubber in China and worldwide. China Rubber Science and Technology Market 2, 7–12 (2011). [Google Scholar]
- Zhang J. Developing trends for dyestuff industry in the world. Chem. Tech.-Econ. 24, 46–52 (2006). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information