Abstract
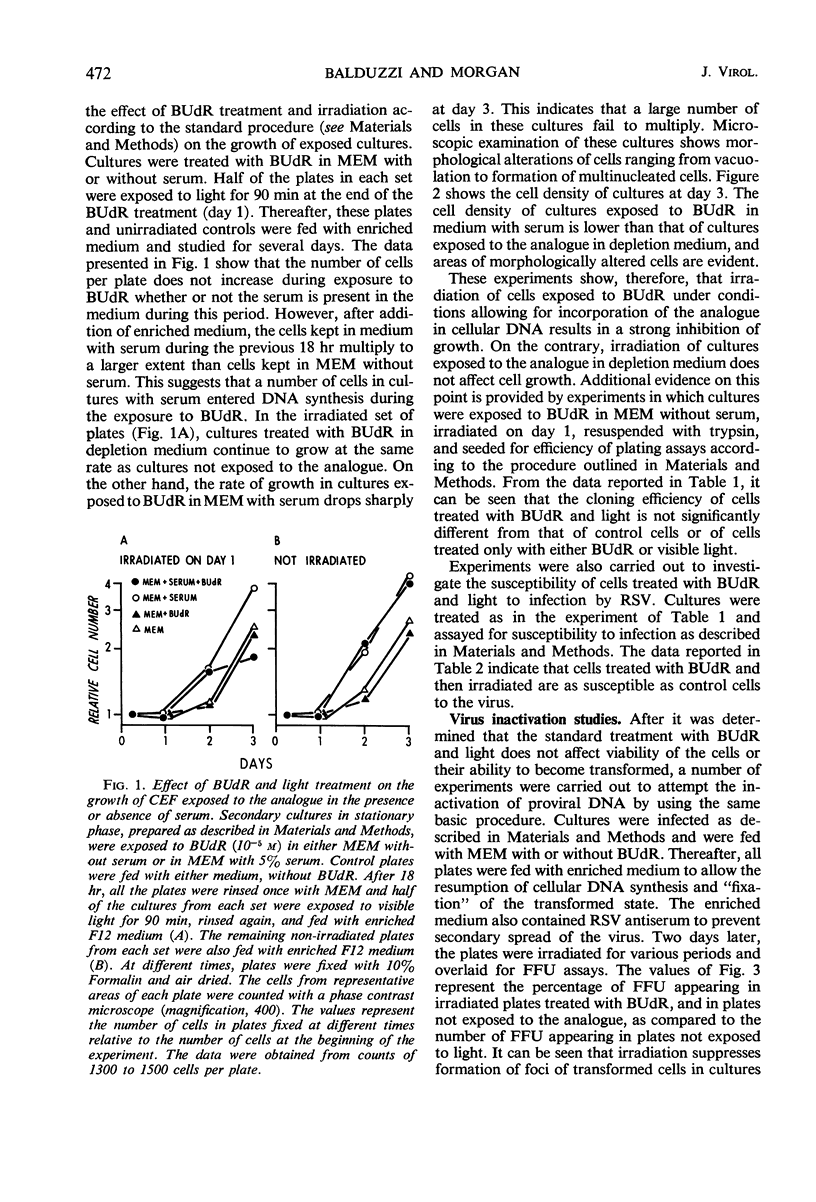
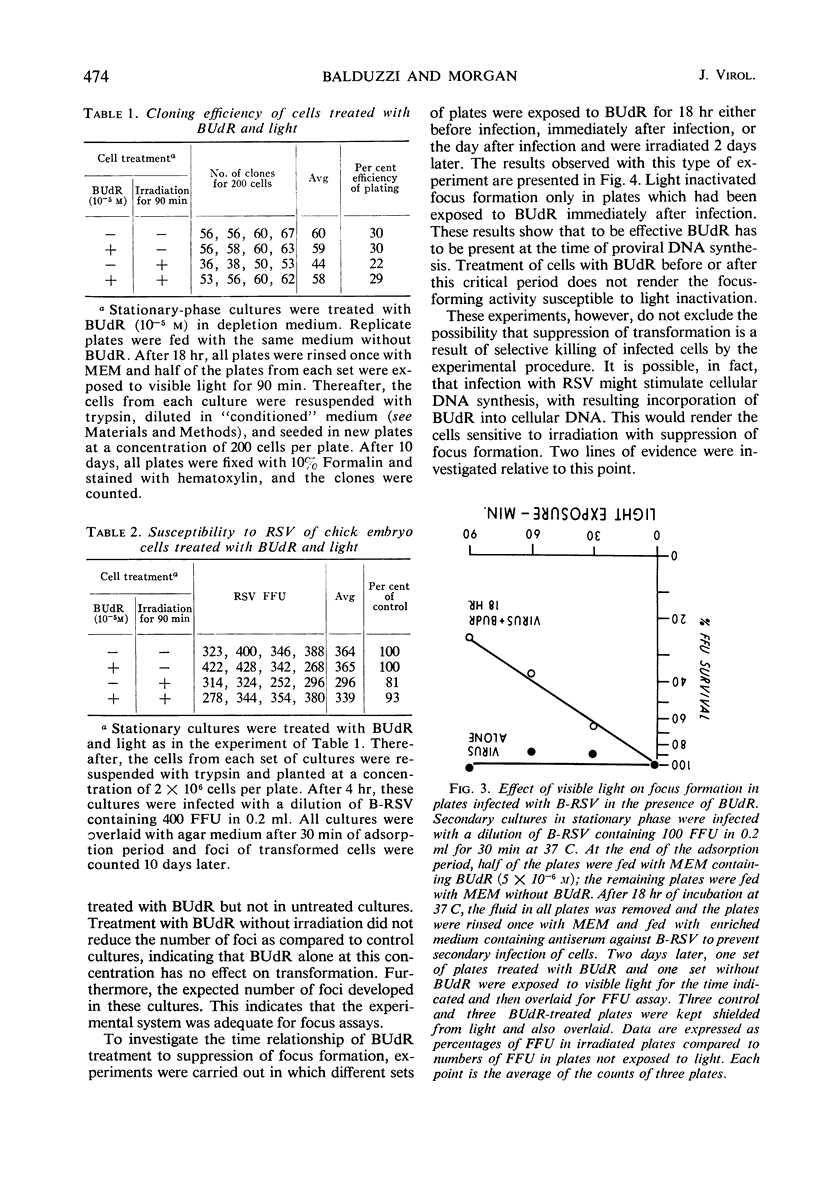
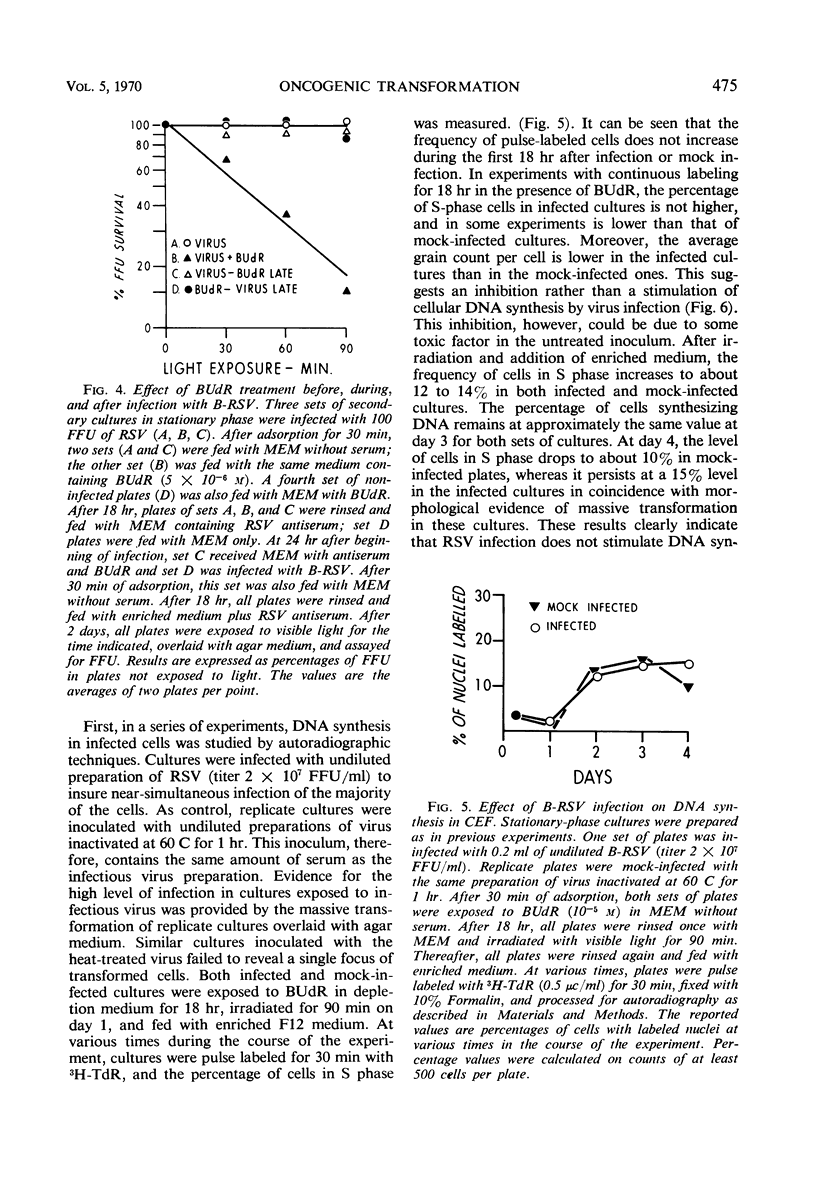
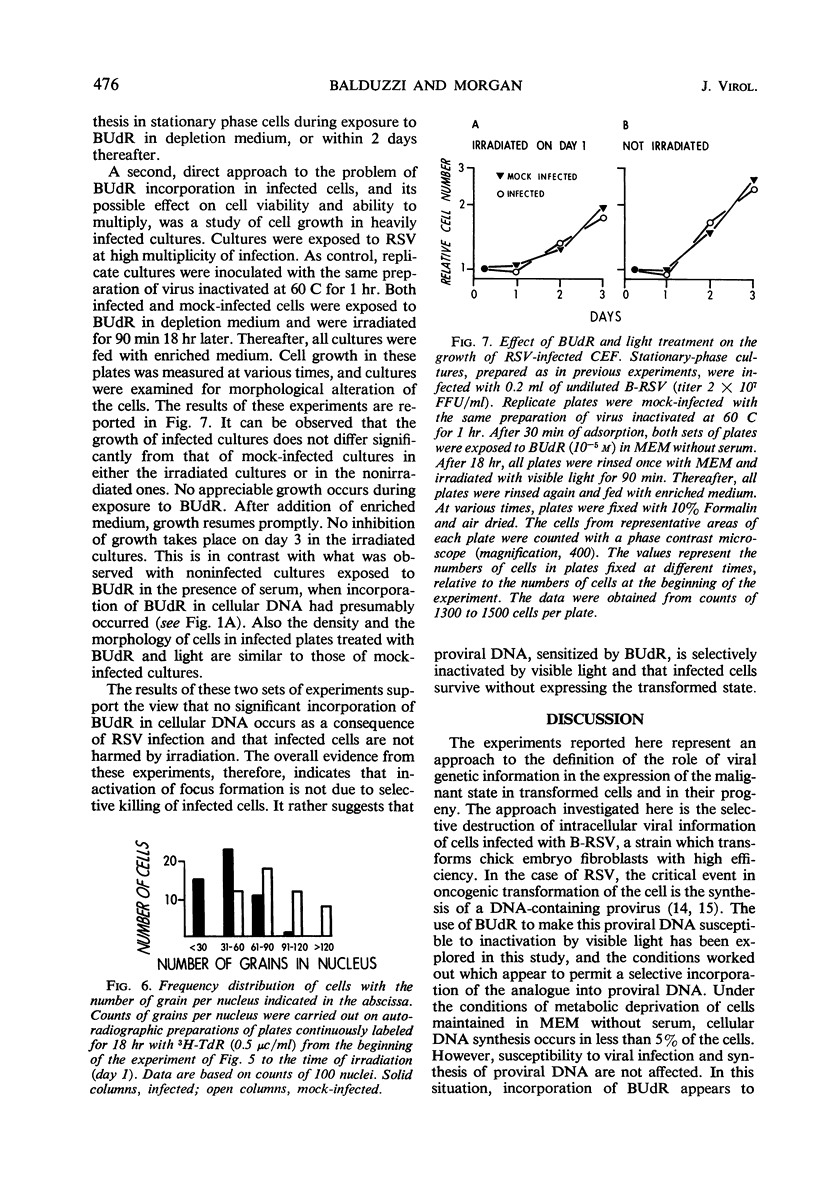
Chick embryo fibroblasts brought into stationary phase of growth by maintenance in serum-free Eagle's MEM medium were infected with the Bryan strain of Rous sarcoma virus (B-RSV) and incubated for 18 hr in the presence of 5-bromo-deoxyuridine (BUdR). The cells were then allowed to resume growth and deoxyribonucleic acid (DNA) synthesis by addition of an enriched F12 medium containing serum and RSV antibody to prevent spread of viral infection. After 48 hr, the cultures were exposed for various periods to visible light, overlaid with solid culture medium, and observed for the appearance of foci of transformed cells. In cultures treated with BUdR at the time of infection, exposure to light resulted in a suppression of focus formation of from 50 to 90% in various experiments. Treatment with BUdR for 18 hr before infection or on the day after infection, followed by exposure to light, had no effect on focus formation. In cultures in which almost all cells were infected, treatment with BUdR followed by exposure to light did not result in cell death. This suggests that suppression of transformation is not due to selective killing of infected cells by this treatment but rather to the intracellular inactivation of the transforming ability of Rous sarcoma proviral DNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- HAM R. G. CLONAL GROWTH OF MAMMALIAN CELLS IN A CHEMICALLY DEFINED, SYNTHETIC MEDIUM. Proc Natl Acad Sci U S A. 1965 Feb;53:288–293. doi: 10.1073/pnas.53.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa H. Rapid transformation of cells by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1969 Jun;63(2):318–325. doi: 10.1073/pnas.63.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare J. D. A simplified sampling technique for studying radio-isotope labelling of cells in culture. Proc Soc Exp Biol Med. 1966 Mar;121(3):774–777. doi: 10.3181/00379727-121-30883. [DOI] [PubMed] [Google Scholar]
- Kára J. Induction of cellular DNA synthesis in chick embryo fibroblasts infected with Rous sarcoma virus in culture. Biochem Biophys Res Commun. 1968 Sep 6;32(5):817–824. doi: 10.1016/0006-291x(68)90314-8. [DOI] [PubMed] [Google Scholar]
- Lee H. H., Kaighn M. E., Ebert J. D. Induction of thymidine-3H incorporation in multinucleated myotubes by Rous sarcoma virus. Int J Cancer. 1968 Jan 15;3(1):126–136. doi: 10.1002/ijc.2910030115. [DOI] [PubMed] [Google Scholar]
- Macieira-Coelho A., Hiu I. J., Garcia-Giralt E. Stimulation of DNA synthesis in resting stage human fibroblasts after infection with Rous sarcoma virus. Nature. 1969 Jun 21;222(5199):1172–1172. doi: 10.1038/2221172a0. [DOI] [PubMed] [Google Scholar]
- Morgan H. R. Antibodies for Rous sarcoma virus in fowl, animal, and human populations of East Africa. I. Antibodies in domestic chickens and wildfowl. J Natl Cancer Inst. 1965 Dec;35(6):1043–1045. [PubMed] [Google Scholar]
- Morgan H. R. Ultrastructure of the surfaces of cells infected with avian leukosis-sarcoma viruses. J Virol. 1968 Oct;2(10):1133–1146. doi: 10.1128/jvi.2.10.1133-1146.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata Y., Bader J. P. Studies on the fixation and development of cellular transformation by Rous sarcoma virus. Virology. 1968 Nov;36(3):401–410. doi: 10.1016/0042-6822(68)90165-7. [DOI] [PubMed] [Google Scholar]
- Puck T. T., Kao F. T. Genetics of somatic mammalian cells. V. Treatment with 5-bromodeoxyuridine and visible light for isolation of nutritionally deficient mutants. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1227–1234. doi: 10.1073/pnas.58.3.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin H., Colby C. Early release of growth inhibition in cells infected with Rous sarcoma virus. Proc Natl Acad Sci U S A. 1968 Jun;60(2):482–488. doi: 10.1073/pnas.60.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEMIN H. M., RUBIN H. A kinetic study of infection of chick embryo cells in vitro by Rous sarcoma virus. Virology. 1959 Jun;8(2):209–222. doi: 10.1016/0042-6822(59)90005-4. [DOI] [PubMed] [Google Scholar]
- TEMIN H. M. THE PARTICIPATION OF DNA IN ROUS SARCOMA VIRUS PRODUCTION. Virology. 1964 Aug;23:486–494. doi: 10.1016/0042-6822(64)90232-6. [DOI] [PubMed] [Google Scholar]
- TEMIN H. M. The control of cellular morphology in embryonic cells infected with rous sarcoma virus in vitro. Virology. 1960 Feb;10:182–197. doi: 10.1016/0042-6822(60)90038-6. [DOI] [PubMed] [Google Scholar]



