Abstract
Background
Natural killer (NK) cells derived from patients with cancer exhibit diminished cytotoxicity compared with NK cells from healthy individuals. We evaluated the tumor response and in vivo expansion of allogeneic NK cells in recurrent ovarian and breast cancer.
Methods
Patients underwent a lymphodepleting preparative regimen: fludarabine 25 mg/m2 × 5 doses, cyclophosphamide 60 mg/kg × 2 doses, and, in seven patients, 200 cGy total body irradiation (TBI) to increase host immune suppression. An NK cell product, from a haplo-identical related donor, was incubated overnight in 1000 U/mL interleukin (IL)-2 prior to infusion. Subcutaneous IL-2 (10 MU) was given three times/week × 6 doses after NK cell infusion to promote expansion, defi ned as detection of ≥ 100 donor-derived NK cells/µL blood 14 days after infusion, based on molecular chimerism and flow cytometry.
Results
Twenty (14 ovarian, 6 breast) patients were enrolled. The median age was 52 (range 30–65) years. Mean NK cell dose was 2.16 × 107 cells/kg. Donor DNA was detected 7 days after NK cell infusion in 9/13 (69%) patients without TBI and 6/7 (85%) with TBI. T-regulatory cells (Treg) were elevated at day +14 compared with pre-chemotherapy (P = 0.03). Serum IL-15 levels increased after the preparative regimen (P = <0.001). Patients receiving TBI had delayed hematologic recovery (P = 0.014). One patient who was not evaluable had successful in vivo NK cell expansion.
Conclusions
Adoptive transfer of haplo-identical NK cells after lymphodepleting chemotherapy is associated with transient donor chimerism and may be limited by reconstituting recipient Treg cells. Strategies to augment in vivo NK cell persistence and expansion are needed.
Keywords: breast cancer, immunotherapy, natural killer cells, ovarian cancer
Introduction
Human natural killer (NK) cells are a subset of peripheral blood lymphocytes defined by the expression of CD56 or CD16 and the absence of T-cell receptor (CD3) (1–10).NK cells play a role in tumor surveillance and can recognize unhealthy cells with decreased expression of class I major histocompatibility complex (MHC) molecules, referred to as ‘loss of self’ (11,12). In a number of tumor types generally considered unresponsive to chemotherapy, a series of well-publicized trials at the National Cancer Institute (NCI,Bethesda,MD,USA) has documented anti-tumor effects in patients using adoptive cellular immunotherapy (ACT). (13,14). This approach involves harvesting mononuclear cells from patients via lymphapheresis, incubation of the cells ex vivo using high concentrations of the lymphokine interleukin (IL)-2, and administration of the expanded and IL-2-activated cells (lymphokine-activated killer, or LAK,cells) to the patient along with IL-2 administration. Early clinical trials showed modest clinical success using autologous LAK with high-dose IL-2 in lymphoma, melanoma and renal cancers, with the majority of cytotoxicity attributed to NK cells (15). The rationale for this study was the potent function of IL-2-activated allogeneic NK cells, compared with autologous NK cells, against ovarian and breast cancer (16–20).We now understand that the failure of autologous NK therapy is partially because of the down-regulation of NK cell killing that occurs with recognition of self-class I MHC on tumor cells, making allogeneic cell transfer more attractive (11,12,21).
Murine models show that depletion of immune cells before ACT enhances the anti-tumor efficacy of transferred donor cells, with a direct correlation between the extent of lymphodepletion and in vivo anti-tumor effect of the transferred cells (22).Lymphodepletion has been shown to augment innate immunity by increasing exposure to homeostatic cytokines (IL-7 and IL-15),eliminating competing elements of the immune system (‘cytokine sinks’) and limiting the number of regulatory T lymphocytes (Treg) and myeloid-derived suppressor cells (23,24).Lymphodepletion followed by ACT has produced approximately 20% complete and partial responses in initial trials at the NCI, with responses occurring primarily in melanoma, renal cell cancers and non-Hodgkin lymphoma (15).A clinical trial assessing the safety and effi cacy of related donor, HLA-haplo-identical, allogeneic NK-enriched peripheral blood cell infusion in patients with poor prognosis acute myeloid leukemia (AML) has been completed at the University of Minnesota (25). We learned that infusion of related donor haploidentical allogeneic NK cell infusions is safe and that successful in vivo donor NK cell expansion, which correlates with efficacy in AML, requires a high-dose cyclophosphamide and fludarabine lymphodepleting preparative regimen (Hi-Cy/Flu). More recently, accumulating data in animal models suggest that further lymphodepletion may improve ACT persistence and efficacy (23,26).Dudley et al. (22) evaluated the efficacy and safety of adding total body irradiation (TBI) to a non-myeloablative chemotherapy preparative regimen in patients with metastatic melanoma. They reported an objective response rate of 50–70%. Additionally, they found increases in homeostatic cytokines IL-7 and IL-15, hypothesized to lead to the persistence, proliferation and activation of the adoptively transferred cells.
Based on the above findings, we evaluated the in vivo expansion and clinical efficacy of an adoptively transferred haplo-identical donor NK cell product in a solid tumor setting following a preparative regimen with and without total body irradiation (TBI).
Methods
Patient eligibility
Patients over the age of 18 years with refractory metastatic breast or ovarian cancer with adequate performance status, organ function [total bilirubin, Aspartate transaminase(AST)/Alanine transaminase(ALT) ≤5 times upper limits of normal, and creatinine <2.0 mg/dL, or calculated creatinine clearance ≥50 mL/ min for patients with creatinine levels above normal] and hematologic reserve (platelet count greater than 80,000/µL, hemoglobin level greater than 9 gm/ dL, and an absolute neutrophil count greater than 1000/µL) were eligible to participate. We treated 20 (14 ovarian and 6 breast) patients. All patients had failed at least four prior therapies for recurrent disease. Corticosteroids or other immunosuppressive medications were not allowed for 3 days prior to study entry or while participating in the study. All patients consented to participate in the study, approved by the Committee on the Use of Human Subjects in Research at the University of Minnesota (MN,USA) according to the Declaration of Helsinki.
Study design
We conducted a standard phase II study associated with Investigational New Drug (IND) application BB-IND 8847 (JSM, sponsor). Lymphodepleting chemotherapy (Hi-Cy/Flu regimen) given prior to the NK cell infusion (day 0) consisted of 60 mg/kg intravenous cyclophosphamide (days–5 and–4) and 25 mg/m2 fludarabine for 5 consecutive days (days–6 to–2). Haploidentical related donors were chosen, with preference given to killer immunoglobulin-like receptor (KIR) ligand-incompatible donors. They underwent lymphapheresis for 3–5 h on the day prior to cell infusion (day–1). Peripheral blood mononuclear cells (PBMC) were collected using a Fenwal CS-3000 Plus blood cell separator (number 4R4538) with a granulocyte separation chamber and small volume collection chamber (SVCC; Fenwal Division, Baxter Healthcare, Deerfield, IL,USA). Up to 2×1010 PBMC were incubated with magnetic-activated cell sorting (MACS) colloidal super-paramagnetic CD3 Micro-Beads (Miltenyi Biotec,Auburn,CA,USA),which consisted of monoclonal mouse anti-human CD3 antibodies conjugated to microspheres. T cells were depleted from the lymphopheresis product using the Miltenyi Biotec CliniMACS cell selection device under good manufacturing practice (GMP) conditions (AmCell, Sunnyvale, CA, USA) and the CD3-depleted products were prepared for infusion as described previously by McKenna et al. (27). All cell products contained more than 70% viable cells for infusion.
NK cells were administered by intravenous infusion 2 days after the last dose of fludarabine. On the evening of the cell infusion, patients began subcutaneous injections of IL-2 (10 MU) given three times weekly for a total of six doses, based on regimens known to expand autologous and allogeneic NK cells in vivo (25,28). Seven patients also received 200 cGy TBI on day–1. TBI was delivered with a linear accelerator using 6, 18 or 25 MV photons in a single fraction on day–1,with right and left lateral fields at a dose rate between 10 and 19 cGy/min prescribed to the midplane of the patient at the level of the umbilicus.
Laboratory methods
NK cell products and peripheral blood samples obtained from the 20 subjects at specified time-points were analyzed by four-color flow cytometry on a FACSCalibur (BD Biosciences, San Jose, CA, USA) using CELLQuest Pro acquisition software (BD Biosciences) and FlowJo analysis software (Tree Star Inc., Ashland, OR, USA). Cells were stained with the following monoclonal antibodies (MAb): peridinin chlorophyll A protein (PerCP)-conjugated SK7 (anti-CD3), allophycocyanin (APC)-conjugated NCAM16.2 (anti-CD56), APC-conjugated SK3 (anti-CD4), phycoerythrin (PE)-conjugated 2A3 (anti-CD25), APC-conjugated M-A251 (anti-CD25), PE-conjugated h-IL7R–M21 (anti-CD127) (BD Biosciences), fluorescein isothiocyanate (FITC)-conjugated eBioRDR5 (anti-CD127) (eBioscience, San Diego, CA, USA) and Alexa Fluor 488-conjugated 259D (anti-Foxp3) (Biolegend, San Diego, CA, USA), as indicated. IL-15 and IL-7 concentrations were determined on frozen serum and plasma, respectively, by a commercial enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN, USA) with an assay sensitivity of 2 pg/mL.
Donor chimerism was measured using a standard short-tandem repeat assay on unsorted mononuclear cells. This has proved to be a reliable assay in the setting of AML, where NK donor expansion is seen with 90–100% donor chimerism (29). The test was performed by the University of Minnesota clinical molecular diagnostics laboratory (30). The primary endpoint of the study was to evaluate the successful in vivo expansion of the infused allogeneic donor NK cell product. Successful NK cell expansion was defined as the detection of ≥100 donor-derived NK cells/µL whole blood 14 days after the NK cell infusion and was determined using the following formula: absolute donor-derived NK cells = (absolute lymphocyte count × percentage of donor chimerism × percentage of CD56+ CD3−(NK) lymphocytes by flow cytometry).
Statistics
Enrollment was targeted at 14 patients to provide 80% power at a 0.05 significance level to detect a successful donor NK cell expansion rate of at least 30%. Prospective stopping rules were applied in each cohort and included prolonged neutropenia, defined as an absolute neutrophil count of less than 500 on day +35, as well as a rate of any non-hematologic grade 4 toxicity, except fevers or grade 4 neutropenia, at day +28 exceeding 20%.
Secondary objectives for this trial were to characterize the toxicities and estimate disease response. Patients were assessed 28 days after completing therapy. Responses were evaluated as complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD), based on the Response Evaluation Criteria in Solid Tumors (RECIST) (31). Days to last platelet transfusion, last red blood cell transfusion and engraftment were measured from the study start date. Data from patients who did not receive transfusions were censored at the date of study completion or date of death. Time to event was estimated using the Kaplan–Meier method. Median days to events and their corresponding 95% confidence intervals are reported. The generalized Wilcoxon test was used to compare the survival curves between groups and a t-test was used to compare the differences between mean values of two groups.
Results
Characteristics of the NK cell products
The apheresis products contained a median of 9.5% CD56+ CD3−NK cells (range 4.0–29.6%) prior to manipulation. Following enrichment by CD3 depletion, the final products contained a median of 33% NK cells (range 23.1–55.5%), resulting in a median infused NK cell dose of 2.15 × 107 NK cells/kg (range 8.33 × 106–3.94 × 107). The products were signifi cantly T-cell depleted after processing, with a median of 0.11% CD3+ cells (range 0.03–1.9%), giving a median infused T-cell dose of 5.59×104 CD3+ cells/kg (range 2.35 × 104–5.69 × 105). Of the mononuclear cells in the fi nal product, 43.9 ± 3.4% were CD14+ and 47.1 ± 3.3% were lymphocytes (of which 51.9 ± 3.8% were NK cells and 45.0 ± 3.8% were CD19+ B cells, with minimal T and natural killer T-cells [NKT]). The lymphocyte contribution to the fi nal product included 25.0 ± 0.3% NK cells, 20.1 ± 0.2% CD19+ B cells and <0.001% T or NKT cells.
Haplo-identical NK cell infusion following Hi-Cy/Flu preparation: toxicity and response
Twenty patients with recurrent ovarian (n = 14) or breast cancer (n = 6) were treated with haplo-identical NK cell infusions (Table I). All received the Hi-Cy/ Flu preparative regimen as lymphodepletion and to prevent immunologic rejection of donor cells by the recipient. Seven received 200 cGy TBI for additional lymphodepletion (Hi-Cy/Flu/TBI). All patients were monitored at specified time-points throughout the study for toxicity, expected during treatment with high-dose chemotherapy and low-dose IL-2 (Table II). Expected low-grade (1 and 2) toxicities were observed in most patients. Eleven patients developed grade 3 toxicity, and none of the expected side-effects reached grade 4. Despite the known constitutional symptoms associated with IL-2, injections were tolerable, with 93% (111/120) of the planned IL-2 doses administered. Sixteen patients received all six planned doses. Fewer doses were given to one patient with high fever (five doses), one with hypoxia (five doses) and one with autoimmune hemolysis (four doses).
Table I.
Patient demographics and clinical characteristics.
| Ovarian (n = 14) |
Breast (n = 6) |
|
|---|---|---|
| Age (years) | ||
| 30–39 | 0 | 2 |
| 40–49 | 2 | 3 |
| 50–59 | 9 | 1 |
| 60–69 | 3 | 0 |
| Performance status | ||
| 0 | 11 | 4 |
| 1 | 3 | 2 |
| Ethnicity | ||
| Caucasian | 14 | 5 |
| Hispanic/Latino | 0 | 1 |
| Black | 0 | 0 |
| Median number of prior therapies (range) |
7 (4–15) | 10 (5–12) |
Table II.
Toxicity.
| Toxicity | Grade | Total by grade |
|---|---|---|
| Dyspnea | 3 | 3 |
| 4 | 0 | |
| Hypoxia | 3 | 0 |
| 4 | 0 | |
| Fever in absence of infection | 3 | 1 |
| 4 | 0 | |
| Chills | 3 | 0 |
| 4 | 0 | |
| Hypertension | 3 | 0 |
| 4 | 0 | |
| Hypotension | 3 | 0 |
| 4 | 0 | |
| Fatigue (lethargy, malaise) | 3 | 1 |
| 4 | 0 | |
| Edema | 3 | 0 |
| 4 | 0 | |
| Pneumonitis/pulmonary infiltrates | 3 | 2 |
| 4 | 0 | |
| Injection site reaction | 3 | 0 |
| 4 | 0 | |
| Rash/desquamation | 3 | 0 |
| 4 | 0 | |
| Nausea | 3 | 3 |
| 4 | 0 | |
| Myalgia | 3 | 1 |
| 4 | 0 | |
| Total | 3 | 11 |
| 4 | 0 |
Patients were monitored at specified time-points prior to therapy, after the Hi-Cy/Flu ± TBI regimen, after NK cell infusion and after each IL-2 dose, for expected toxicities attributed to the therapy. The number of patients who had at least 1 day with a grade 3 or grade 4 toxicity is shown.
There were 10 unanticipated severe adverse events (SAE) (four grade 3, five grade 4 and one grade 5) (Table III). Eight of these occurred following the addition of TBI. Two of the SAEs (decrease in cardiac ejection fraction and abdominal pain), although unexpected, resolved with no permanent sequelae. The grade 5 toxicity was a death attributed to tumor lysis syndrome (TLS) in a 51-year-old woman with significant ovarian cancer tumor burden (maximal size liver metastasis 6.8×4.8 cm). On admission, her white blood cell (WBC) count was 24 000; she had negative blood and urine cultures and was without obvious infection. She received her first IL-2 dose 4 h after NK cell infusion and experienced chills, rigors and temperature to 100.3°F. Within 24 h she became lethargic, weak, tachycardic and hypotensive. Despite fluid resuscitation and antibiotics, she became more unresponsive, hyperglycemic, hypoxic and acidotic and eventually went into cardiac arrest and died. At that time she was neutropenic (WBC 0.2) and had an elevated lactate dehydrogenase (LDH) and potassium. Cultures from her blood and the NK cell product subsequently came back negative. Autopsy revealed disease throughout the abdomen and pelvis. Metastatic lesions in the liver, spleen and diaphragm were necrotic. Sections of the metastatic carcinoma showed scattered CD56+ NK cells and CD3+T cells. Based on these results, death was believed to be the result of metabolic derangement consistent with TLS as a result of NK cell therapy.
Table III.
Severe adverse events associated with the Hi-Cy/Flu ± TBI regimen.
| Category | Toxicity | Grade 3 | Grade 4 | Grade 5 | Total |
|---|---|---|---|---|---|
| Blood/bone marrow | Hemolysis | 2 (TBI) | 0 | 0 | 2 |
| Blood/bone marrow | Neutropenia beyond day +28 | 0 | 5 (4 TBI) | 0 | 5 |
| Cardiac general | Left ventricular systolic dysfunction | 1 (TBI) | 0 | 0 | 1 |
| Pain | Pain, abdomen | 1 (TBI) | 0 | 0 | 1 |
| Syndromes | TLS | 0 | 0 | 1 | 1 |
| Total | 4 | 5 | 1 | 10 |
Another unanticipated toxicity that occurred in two patients was passenger lymphocyte syndrome (PLS). PLS is an autoimmune hemolytic anemia as a result of the production of antibodies by donor B ‘passenger lymphocytes’ in an immune response against the recipient’s red blood cell antigens (32). One patient with type B+ blood (donor 0+) had a drop in her hemoglobin to 5.7 (from 8.0) on day +8, after four doses of IL-2. This was associated with an elevated LDH, low haptoglobin and spherocytes on a peripheral smear. Serial direct anti-globulin testing (DAT) on her serum samples demonstrated development of an anti-B antibody 3 days prior to clinical hemolysis, with an increasing titer that peaked at 1:128. The IL-2 was discontinued and she was treated with high-dose methylprednisolone and rituximab, with resolution of the hemolysis within 48 h. A patient with type A blood had a drop in hemoglobin with an associated DAT positive for anti-A antibodies after an NK cell product from a type O donor. This event resolved spontaneously within 1 day and only in retrospect was it attributed to PLS.
Five patients had neutropenia beyond day +28, four of whom received TBI. There were no significant differences between the breast and ovarian cohorts with respect to time to neutrophil or platelet recovery or red blood cell (RBC) transfusion independence. However, patients who received TBI had signifi cantly longer median times to neutrophil recovery [TBI 32 days, 95% confi dence interval (CI) 15 – 34; no TBI 15 days, 95% CI 13–15; P = 0.014). Similarly, the median times to transfusion independence were longer after TBI: platelets (TBI 51 days, 95% CI 23–82; no TBI 18 days, 95% CI 13–36; P = 0.040) and RBC (TBI 43 days, 95% CI 37–76; no TBI 15 days, 95% CI 13–20; P = 0.001).
Re-evaluation of disease by computed tomography (CT) scan was performed 4–6 weeks following NK infusion and every 3 months thereafter. There were 4 patients with PR (all ovarian), 12 with SD (8 ovarian and 4 breast) and 3 with PD (1 ovarian and 2 breast) at a median of 36 days (range 31–109) following NK cell infusion. The median time to progression was 2 months (range 1–6).
In vivo expansion of haplo-identical NK cells after Hi-Cy/Flu and Hi-Cy/Flu/TBI
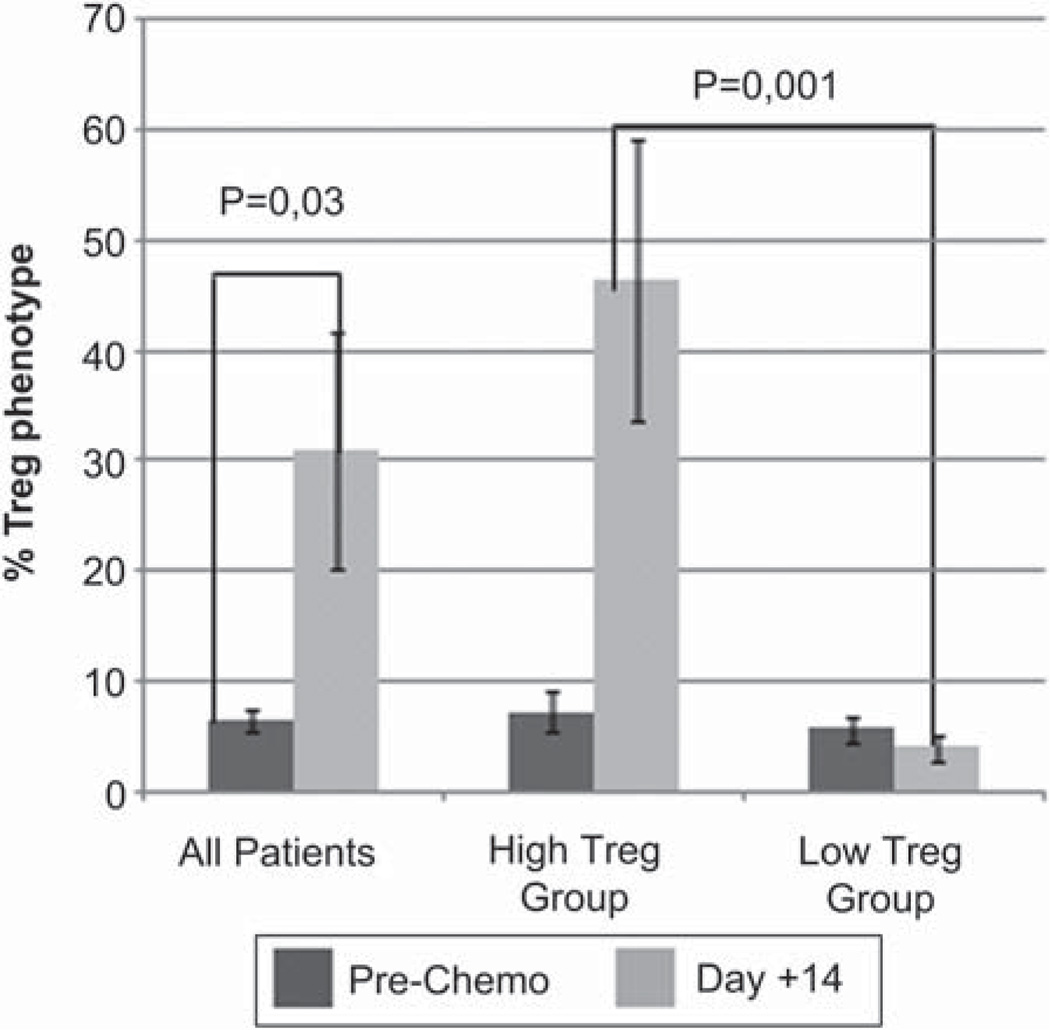
None of the 13 patients in the non-TBI Hi-Cy/Flu group met the pre-defined criterion for successful in vivo NK cell expansion at day +14, although nine (69%) had detectable donor chimerism at day +7 (mean 47 ± 9%; range 0–83%). At that time NK cells comprised 40.81 ±8.6 (range 1.0–86.6%) of circulating lymphocytes. By day +14, the percentage of circulating NK cells had dropped to 4.1 ± 6.8 (range 0–29.8%), replaced by a corresponding increase in T cells (85.3 ± 7.6%). All patients had similarly low Treg profiles at baseline (prior to starting chemotherapy) that were significantly elevated at day +14 compared with pre-chemotherapy counts (P = 0.03). Interestingly, the T-cell profile split into two clusters, with one group having a marked increase in a population with a Treg phenotype (CD4+, CD25 + and CD127−) at day +14, the other with mainly CD8+ T cells. By day +14 the percentage of lymphocytes with a Treg phenotype in the ‘low Treg ’ group was 4.9 ± 1.6% and in the ‘high Treg group’ 47 ± 10.4% (P = 0.001) (Figure 1). A representative flow cytometry plot from a high Treg patient is shown in Figure 2. The surface phenotype panel for Treg correlated closely with the percentage of true Treg, as defined by the expression of Foxp3 (see CD127 and Foxp3 panels). There was no variable that predicted the Treg group or NK cell persistence (KIR ligand mismatch, prior therapy, baseline phenotype, phenotype of the product [Table IV]). At day + 14, one of 13 patients had 8.1% donor DNA compared with 9 of 13 on day + 7 (P = 0.004), showing NK cell loss between days + 7 and +14. All donor DNA had disappeared by day +28.
Figure 1.
Treg increase 14 days after NK + IL-2 administration. PBMC were collected prior to chemotherapy and 14 days after haplo-identical NK cell infusion and IL-2 administration. When all the ovarian patients were considered, there was a significant increase in Treg, but two patterns were seen. Seven patients exhibited a high Treg profi le, defined by the presence of CD4+, CD25+ and CD127– cells at day +14. Six patients exhibited a low Treg pattern, where the post-therapy sample did not change from pre-therapy. There was no difference in Treg elevation based on presence or absence of TBI (P = 0.44). Error bars represent standard error of the mean.
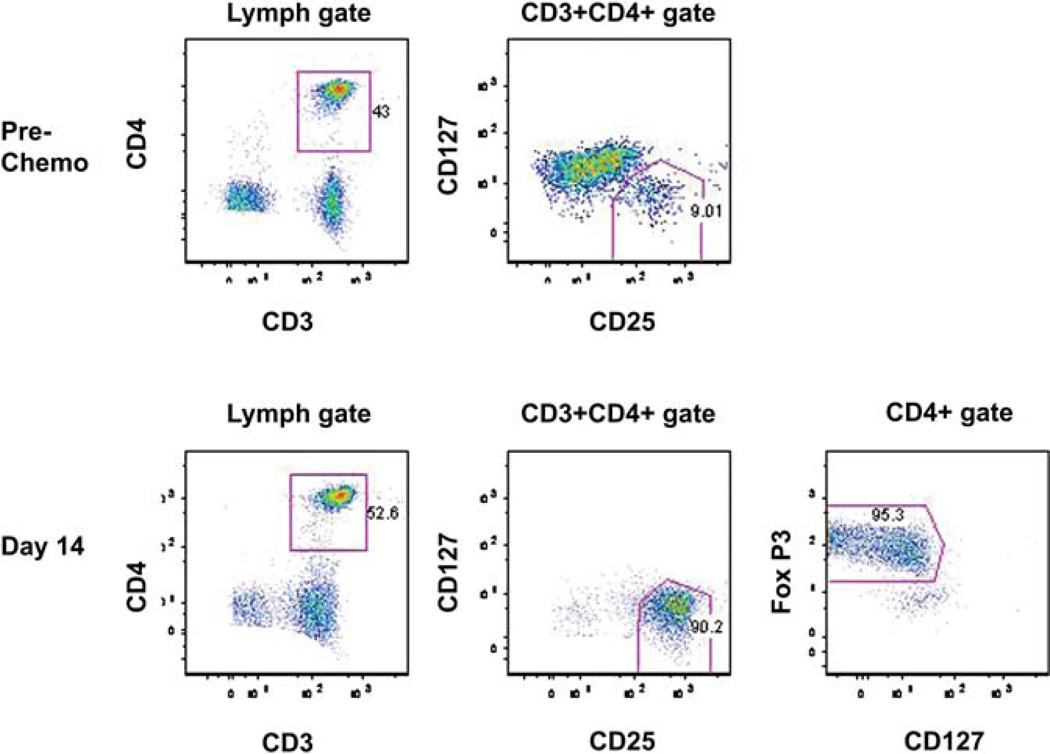
Figure 2.
Phenotypic Treg express Foxp3. PBMC were collected prior to chemotherapy and 14 days after haplo-identical NK cell infusion from a patient in the high Treg group. At day +14, 52.6% of circulating cells were CD3+ CD4+.Of those, 90% were consistent with circulating Treg. The surface phenotype panel for Treg CD25+ CD127– correlated closely with the percentage of true Treg (95%), as defined by the expression of Foxp3.
Table IV.
Treatment characteristics by Treg group.
| Patient number |
Breast/ ovarian |
KIR mismatch |
TBI | Treg group |
|---|---|---|---|---|
| 1 | Breast | Yes | No | Not tested |
| 2 | Breast | No | No | Not tested |
| 3 | Breast | Yes | No | Not tested |
| 4 | Breast | No | No | Not tested |
| 5 | Ovarian | Yes | No | High |
| 6 | Ovarian | Yes | No | Low |
| 7 | Ovarian | No | No | High |
| 8 | Ovarian | No | No | High |
| 9 | Ovarian | Yes | No | High |
| 10 | Ovarian | No | No | High |
| 11 | Ovarian | No | No | Low |
| 12 | Ovarian | No | No | Dead |
| 13 | Ovarian | No | No | Low |
| 14 | Ovarian | Yes | Yes | Low |
| 15 | Ovarian | No | Yes | Low |
| 16 | Ovarian | No | Yes | Low |
| 17 | Ovarian | Yes | Yes | High |
| 18 | Ovarian | No | Yes | Low |
| 19 | Breast | No | Yes | Not tested |
| 20 | Breast | No | Yes | Not tested |
Hypothesizing that inadequate host lymphodepletion was contributing to rejection of the donor NK cells, 200 cGy TBI was added to achieve further immune suppression in seven patients. No significant differences in rates of donor chimerism (6/7 at day +7) (P= 0.61) or lymphocyte profiles at day +7, + 14 or +28 were seen between patients who did and did not receive TBI. In vivo NK cell expansion was detected at day +14 in one patient ineligible for that endpoint because she received only 4/6 doses of IL-2 and received high-dose steroids to treat the autoimmune hemolysis associated with PLS. Her donor-derived NK cells persisted from day +28 (91%; 180 donor derived NK cells/µL) to day +42.
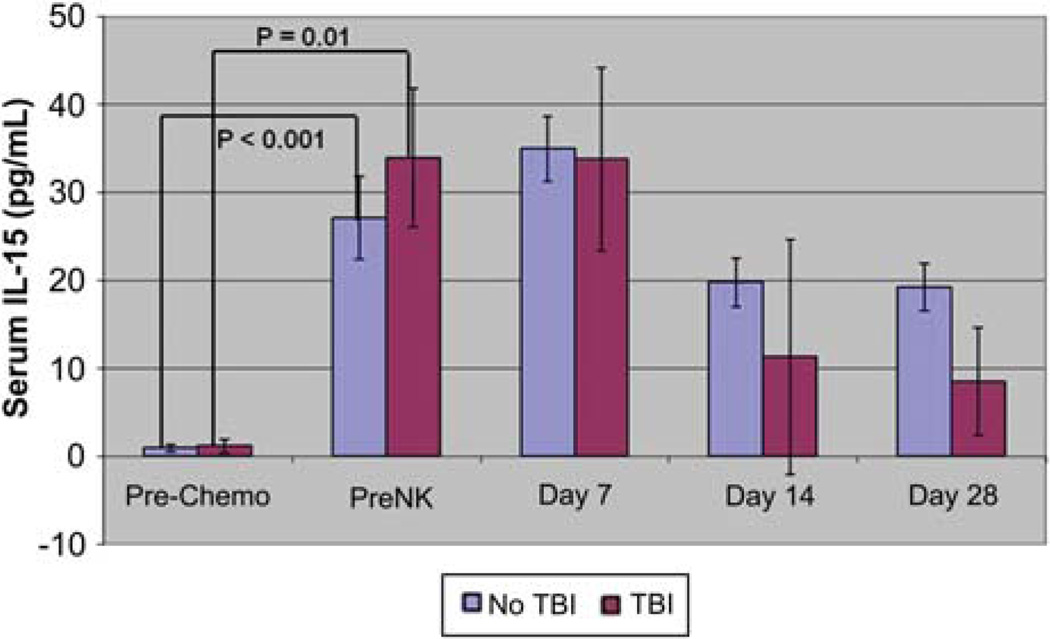
As IL-15 is an ideal candidate to drive NK-cell expansion, based on previous studies (25), we measured the level of endogenous IL-15 throughout therapy (Figure 3). All patients had increased IL-15 levels from baseline after the chemotherapy preparative regimen (1.2 ± 0.3 pg/mL versus 31.8 ± 4.0; P = <0.001) that was sustained at day +7 (mean 34.1 ± 3.6) and began to fall at day +14 (12.9 ± 3.8). The seven subjects who received TBI had higher IL-15 levels at days 14 (19.7 ± 0.4 versus 11.3 ± 2.7) and 28 (19.2 ± 0.3 versus 8.6 ± 2.7) that did not reach statistical significance. IL-7 levels also rose after the lymphodepleting chemotherapy, and remained elevated until at least day + 28 (data not shown).
Figure 3.
IL-15 levels increase after lymphodepleting therapy. Plasma was collected from patients at the indicated time-points before and after the Hi-Cy/Flu ± TBI preparative regimen. A significant increase in IL-15 concentration was detected following the chemotherapy prior to donor NK infusion and at day + 7. By day +14 concentrations began to decrease. Error bars represent standard error of the mean.
Discussion
We tested the use of adoptively transferred haploidentical NK cells to treat patients with solid tumors. Following preparation with a Hi-Cy/Flu regimen, donor DNA was detectable 7 days after the NK cell infusion in 79% (15/19) of patients. Although most patients had high percentages of NK cells at day + 7, the absolute lymphocyte count was essentially immeasurable at that time-point. After completion of the IL-2 course 14 days after NK cell infusions, the small numbers of donor NK cells found earlier were replaced by recipient T cells, often with a Treg phenotype. IL-7 and IL-15 levels both increased after the preparative regimen, but did not correlate with the lymphocyte phenotype.
As no successful NK cell expansion was seen with the Hi-Cy/Flu chemotherapy alone, we investigated whether intensifying lymphodepletion by adding 200 cGy TBI was necessary to achieve successful NK expansion and possibly eliminate the suppressive effects of the Treg. Our hypothesis was supported by the work of Rosenberg et al. (33) showing that the addition 200–200 cGy TBI to a Hi-Cy/Flu preparative regimen promoted T-cell expansion and persistence in melanoma, and by the finding that 400 cGy TBI was associated with significantly better NK cell expansion in patients with AML (29).With the addition of TBI 200, we found a significantly greater time to hematopoietic recovery in our heavily pre-treated population, but no improvement in rates of NK cell expansion in evaluable patients. It is interesting that the only patient with expanded NK cells was not evaluable for this end-point because she developed PLS requiring high-dose steroids and interruption of her IL-2 administration after four doses. Steroids at high doses may have inadvertently contributed to the greater immunosuppression in this patient. Although this provided proof of the concept that NK cells can expand in solid tumor patients, the presumed inhibitory effects of steroids on NK cell function are contrary to the goals of maximizing NK cell function in vivo.
Our protocol was based on the findings of Miller et al. (25), who induced complete remission in 5 of 19 poor prognosis patients with AML. They were able to demonstrate successful expansion and persistence of haplo-identical NK cells 14 days after NK infusion in patients following the Hi-Cy/Flu preparative regimen. Higher numbers of circulating donor-derived NK cells were seen in the patients who achieved complete remission. While tumor response was not a primary outcome of this study, in Miller’s study 26% achieved complete remissions following haplo-identical NK cell therapy. Although our current trial corroborates the fi ndings of Arai et al. (34), who reported SD in 4 of 12 advanced renal cell carcinoma and melanoma patients receiving NK-92 cells as allogeneic immunotherapy, the contribution towards therapeutic efficacy played by the NK cells is difficult to differentiate from the high-intensity chemotherapy regimen. It remains unknown whether there is any absolute threshold for NK cell persistence or expansion that may correlate with efficacy. It is also possible that in vivo-expanded NK cells home to tumor and are not found circulating in blood at the time of sample collection. Although tracking cells in vivo could address this issue, clinically applicable methods have been hampered by lack of resolution to allow whole body imaging of cells that might divide in vivo.
During the lymphopenic window induced by the preparative regimen in our study, there was evidence of transient donor NK cell persistence, but by day + 14 patients had reconstituted with host T cells, suggesting that suppressive factors induced in the tumor microenvironment may be inhibiting donor NK cell expansion. The proliferation of adoptively transferred NK cells could be limited by host factors, including immune rejection by effector T cells, suppression by myeloid-derived suppressor cells (MDSC), which are widely studied for their suppressive properties in tumor models (24,35,36), and suppression by Treg, which are known to maintain tolerance to self/tumor antigens (37–42) and induce immune tolerance against tumors in both mouse models and humans (38,39,43). In support of this, Treg depletion before adoptive cell transfer of T cells in a murine AML model signifi cantly increased the efficacy in the setting of substantial preexisting tumor burden (44). As half the patients in our study had significant Treg expansion, the ability to deplete Treg appears critical for effective adoptive cellular therapy. As others have shown an inverse relationship between the accumulation of Treg in human ovarian cancer and survival (39), eradicating this cell population for this disease may not only enhance the effectiveness of adoptively transferred NK cells but may also be of therapeutic benefit.
Successful adoptive cell transfer requires the creation of a lymphopenic environment to ‘make space’ and change the competitive balance between endogenous lymphocytes and proliferating donor lymphocytes. In addition, lymphopenia may induce survival or deplete inhibitory factors (reviewed in 45,46) to promote cellular expansion. Despite their ability to decrease Treg (37,47,48), in our study cyclophosphamide and fludarabine alone, or with 200 cGy TBI, were inadequate to promote NK cell expansion. Our use of exogenous IL-2 to promote in vivo NK cell expansion may have had the unintended effect of promoting host Treg expansion (49), supporting the use of IL-15 as a more NK-selective cytokine when it becomes available for use in humans. Cyclosporine (CsA), a calcineurin inhibitor, inhibits Treg activity by blocking the induction of the nuclear factor of activated T cells (NFAT), leading to a decrease in IL-2 and IL-2 receptor gene expression. The lack of IL-2 or its functional receptor blocks the generation of Treg both in the thymus and in the periphery (50). More recently, CsA was found to be significantly more immunosuppressive to T cells than NK cells, and the anti-tumor effects of adoptively transfused in vitro-expanded NK cells were found to be maintained in the presence of CsA (51). The use of CsA for Treg depletion needs prospective clinical testing.
Haplo-identical donor NK cell infusions were well tolerated overall, with limited infusional or IL-2-related toxicity. However, two signifi cant unanticipated toxicities, TLS and PLS, add to our understanding of the possible consequences of allogeneic NK-cell therapy in heavily pre-treated patients with significant tumor burden. TLS occurs from massive necrosis of neoplastic cells after chemotherapy. This syndrome is infrequently encountered in the treatment of ovarian cancer, with only three separate reported incidences in the literature following carboplatin/cyclophosphamide, topotecan and weekly paclitaxel (52–54). In other solid tumors, TLS has been reported to occur 4–7 days following chemotherapy and most frequently in patients with widespread tumor highly sensitive to anti-neoplastic therapy (55). The ovarian cancer patient developed TLS 5 days following completion of her second infusion of high-dose cyclophosphamide, with her symptoms beginning within 6 h of the NK cell infusion. The timing of the event, together with the detection of NK cells in her necrotic liver specimen on autopsy, suggest that NK cells could have contributed to the tumor lysis. Since this event, patients have been given allopurinol as prophylaxis for TLS and no other episodes have occurred.
In addition, we identified a previously unreported event of adoptive-cell therapy-related PLS resulting in autoimmune hemolysis in two patients. In our trial, PBMC from related haplo-identical donors were enriched for NK cells by depletion of CD3+ T cells. Thus the final product contained monocytes and B cells. The potential toxicity of B-cell derived antibody production from ABO-incompatible cellular therapy donors should be noted and cellular products from ABO-incompatible donors should be B-cell depleted. Additionally, other selection strategies, such as CD56+ selection, could be used to enrich the NK cell product further, but this double-selection strategy leads to more cell loss.
In summary, the adoptive transfer of haploidentical NK cells after lymphodepleting chemotherapy in patients with ovarian and breast cancer is associated with transient donor chimerism that is not improved with the addition of low-dose TBI. Sustained in vivo NK cell expansion may be limited by host rejection, competition with host lymphocytes or suppression by recipient Treg or MDSC. More effective strategies to augment in vivo NK cell persistence and expansion are needed to test the clinical benefit of NK cells against solid tumors.
Acknowledgments
We would like to thank Dixie Lewis and Megan Whitmore for their excellence in study coordination and patient care during this trial, and Giordi Orreg-gio for his skills in data management. Additionally, we thank Jessica Kuehn-Hajder, MD, for her radiologic expertise in this trial.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Miller JS, Oelkers S, Verfaillie C, McGlave P. Role of monocytes in the expansion of human activated natural killer cells. Blood. 1992;80:2221–2229. [PubMed] [Google Scholar]
- 2.Miller JS, Verfaillie C, McGlave P. The generation of human natural killer cells from CD34+/DR-primitive progenitors in long-term bone marrow culture. Blood. 1992;80:2182–2187. [PubMed] [Google Scholar]
- 3.Pierson B, Miller J, Verfaillie B, McGlave P, Hu W-S. Population dynamics of human activated killer cells in culture. Biotech Bioeng. 1994;43:685–692. doi: 10.1002/bit.260430803. [DOI] [PubMed] [Google Scholar]
- 4.Miller JS, Alley KA, McGlave P. Differentiation of natural killer (NK) cells from human primitive marrow progenitors in a stroma-based long-term culture system: identification of a CD34+7+ NK progenitor. Blood. 1994;83:2594–2601. [PubMed] [Google Scholar]
- 5.Miller JS, Klingsporn S, Lund J, Perry EH, Verfaillie C, McGlave P. Large scale ex vivo expansion and activation of human natural killer cells for autologous therapy. Bone Marrow Transplant. 1994;14:555–562. [PubMed] [Google Scholar]
- 6.Pierson BA, McGlave PB, Hu WS, Miller JS. Natural killer cell proliferation is dependent on human serum and markedly increased utilizing an enriched supplemented basal medium. J Hematother. 1995;4:149–158. doi: 10.1089/scd.1.1995.4.149. [DOI] [PubMed] [Google Scholar]
- 7.Pierson BA, Gupta K, Hu WS, Miller JS. Human natural killer cell expansion is regulated by thrombospondin-mediated activation of transforming growth factor-beta 1 and independent accessory cell-derived contact and soluble factors. Blood. 1996;87:180–189. [PubMed] [Google Scholar]
- 8.Cervantes F, Pierson BA, McGlave PB, Verfaillie CM, Miller JS. Autologous activated natural killer cells suppress primitive chronic myelogenous leukemia progenitors in long-term culture. Blood. 1996;87:2476–2485. [PubMed] [Google Scholar]
- 9.Pierson BA, Europa AF, Hu WS, Miller JS. Production of human natural killer cells for adoptive immunotherapy using a computer-controlled stirred-tank bioreactor. J Hematother. 1996;5:475–483. doi: 10.1089/scd.1.1996.5.475. [DOI] [PubMed] [Google Scholar]
- 10.Pierson BA, Miller JS. CD56+bright and CD56+dim natural killer cells in patients with chronic myelogenous leukemia progressively decrease in number, respond less to stimuli that recruit clonogenic natural killer cells, and exhibit decreased proliferation on a per cell basis. Blood. 1996;88:2279–2287. [PubMed] [Google Scholar]
- 11.Raulet DH, Held W. Natural killer cell receptors: the offs and ons of NK cell recognition. Cell. 1995;82:697–700. doi: 10.1016/0092-8674(95)90466-2. [DOI] [PubMed] [Google Scholar]
- 12.Karre K. Express yourself or die: peptides, MHC molecules, and NK cells. Science. 1995;267:978–979. doi: 10.1126/science.7863341. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg SA. Karnofsky Memorial lecture. The immunotherapy and gene therapy of cancer. J Clin Oncol. 1992;10:180–199. doi: 10.1200/JCO.1992.10.2.180. [DOI] [PubMed] [Google Scholar]
- 14.Canevari S, Stoter G, Arienti F, Bolis G, Colnaghi MI, Di Re EM, et al. Regression of advanced ovarian carcinoma by intraperitoneal treatment with autologous T lymphocytes retargeted by a bispecific monoclonal antibody. J Natl Cancer Inst. 1995;87:1463–1469. doi: 10.1093/jnci/87.19.1463. [DOI] [PubMed] [Google Scholar]
- 15.Rosenberg SA, Lotze MT, Muul LM, Chang AE, Avis FP, Leitman S, et al. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med. 1987;316:889–897. doi: 10.1056/NEJM198704093161501. [DOI] [PubMed] [Google Scholar]
- 16.Carlsten M, Bjorkstrom NK, Norell H, Bryceson Y, van Hal T, Baumann BC, et al. DNAX accessory moleule-1 mediated recognition of freshly isolated ovarian carcinoma by resting natural killer cells. Cancer Res. 2007;67:1317–1325. doi: 10.1158/0008-5472.CAN-06-2264. [DOI] [PubMed] [Google Scholar]
- 17.Cooley S, Burns L, Repka T, Miller J. Natural killer cell cytotoxicity of breast cancer targets is enhanced by two distinct mechanisms of antibody-dependent cellular cytotoxicity against LFA-3 and HER2/neu. Exp Hematol. 1999;27:1533–1541. doi: 10.1016/s0301-472x(99)00089-2. [DOI] [PubMed] [Google Scholar]
- 18.Lotzova E, Savary CA, Freedman RS, Bowen JM. Natural killer cell cytotoxic potential of patients with ovarian carcinoma and its modulation with virus-modified tumor cell extract. Cancer Immunol Immunother. 1984;17:124–129. doi: 10.1007/BF00200048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lotzova E, Savary CA, Freedman RS, Edwards CL, Wharton JT. Recombinant IL-2-activated NK cells mediate LAK activity against ovarian cancer. Int J Cancer. 1988;42:225–231. doi: 10.1002/ijc.2910420214. [DOI] [PubMed] [Google Scholar]
- 20.Lai P, Rabinowich H, Crowley-Nowick PA, Bell MC, Mantovani G, Whiteside TL. Alterations in expression and function of signal-transducing proteins in tumor-associated T and natural killer cells in patients with ovarian carcinoma. Clin Cancer Res. 1996;2:161–173. [PubMed] [Google Scholar]
- 21.Moretta A, Vitale M, Bottino C, Orengo AM, Morelli L, Augugliaro R, et al. P58 molecules as putative receptors for major histocompatibility complex (MHC) class I molecules in human natural killer (NK) cells. Anti-p58 antibodies reconstitute lysis of MHC class I-protected cells in NK clones displaying different specifi cities. J Exp Med. 1993;178:597–604. doi: 10.1084/jem.178.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muranski P, Boni A, Wrzesinski C, Citrin DE, Rosenberg SA, Childs R, et al. Increased intensity lymphodepletion and adoptive immunotherapy: how far can we go? Nat Clin Pract Oncol. 2006;3:668–681. doi: 10.1038/ncponc0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watanabe S, Deguchi K, Zheng R, Tamai H, Wang LX, Cohen PA, et al. Tumor-induced CD11b + Gr-1+ myeloid cells suppress T cell sensitization in tumor-draining lymph nodes. J Immunol. 2008;181:3291–3300. doi: 10.4049/jimmunol.181.5.3291. [DOI] [PubMed] [Google Scholar]
- 25.Miller J, Soignier Y, Panoskaltsis-Mortari A, McNearney S, Yun G, Fautsch S, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 26.Wrzesinski C, Restifo NP. Less is more: lymphodepletion followed by hematopoietic stem cell transplant augments adoptive T-cell-based anti-tumor immunotherapy. Curr Opin Immunol. 2005;17:195–201. doi: 10.1016/j.coi.2005.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKenna DH, Jr, Sumstad D, Bostrom N, Kadidlo DM, Fautsch S, McNearney S, et al. Good manufacturing practice production of natural killer cells for immunotherapy: a six-year single-institution experience. Transfusion. 2007;47:520–528. doi: 10.1111/j.1537-2995.2006.01145.x. [DOI] [PubMed] [Google Scholar]
- 28.Miller JS, Tessmer-Tuck J, Pierson BA, Weisdorf D, McGlave P, Blazar BR, et al. Low dose subcutaneous interleukin-2 after autologous transplantation generates sustained in vivo natural killer cell activity. Biol Blood Marrow Transplant. 1997;3:34–44. [PubMed] [Google Scholar]
- 29.Cooley S, Gada P, McKenna D, McCullar V, Fautsch S, Verneris M, Blazar BR, et al. Successful haploidentical hematopoietic cell engraftment using a non-myeloablative preparative regimen including natural killer (NK) cells. Blood. 2008;112 Abstract 827. [Google Scholar]
- 30.Thyagarajan B, Young S, Floodman S, Peterson R, Wang X. Systematic analysis of interference due to stutter in estimating chimerism following hematopoietic cell transplantation. J Clin Lab Anal. 2009;23:308–313. doi: 10.1002/jcla.20336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Therasse P, Arbuck S, Eisenhauer E, Wanders J, Kaplan R, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 32.Reed M, Yearsley M, Krugh D, Kennedy MS. Severe hemolysis due to passenger lymphocyte syndrome after hematopoietic stem cell transplantation from an HLA-matched related donor. Arch Pathol Lab Med. 2003;127:1366–1368. doi: 10.5858/2003-127-1366-SHDTPL. [DOI] [PubMed] [Google Scholar]
- 33.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 2009;21:233–240. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arai S, Meagher R, Swearingen M, Myint H, Rich E, Martinson J, et al. Infusion of the allogeneic cell line NK-92 in patients with advanced renal cell cancer or melanoma: a phase I trial. Cytotherapy. 2008;10:625–632. doi: 10.1080/14653240802301872. [DOI] [PubMed] [Google Scholar]
- 35.Talmadge JE. Pathways mediating the expansion and immunosuppressive activity of myeloid-derived suppressor cells and their relevance to cancer therapy. Clin Cancer Res. 2007;13:5243–5248. doi: 10.1158/1078-0432.CCR-07-0182. [DOI] [PubMed] [Google Scholar]
- 36.Pandolfi F, Cianci R, Lolli S, Dunn IS, Newton EE, Haggerty TJ, et al. Strategies to overcome obstacles to successful immunotherapy of melanoma. Int J Immunopathol Pharmacol. 2008;21:493–500. doi: 10.1177/039463200802100302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol. 1999;163:5211–5218. [PubMed] [Google Scholar]
- 38.Turk MJ, Guevara-Patino JA, Rizzuto GA, Engelhorn ME, Sakaguchi S, Houghton AN. Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J Exp Med. 2004;200:771–782. doi: 10.1084/jem.20041130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 40.Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, et al. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766–4772. [PubMed] [Google Scholar]
- 41.Chen ML, Pittet MJ, Gorelik L, Flavell RA, Weissleder R, von Boehmer H, et al. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-beta signals in vivo. Proc Natl Acad Sci USA. 2005;102:419–424. doi: 10.1073/pnas.0408197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sakaguchi S, Sakaguchi N, Shimizu J, Yamazaki S, Sakihama T, Itoh M, et al. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182:18–32. doi: 10.1034/j.1600-065x.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- 43.Antony PA, Restifo NP. CD4+CD25+ T regulatory cells, immunotherapy of cancer, and interleukin-2. J Immunother. 2005;28:120–128. doi: 10.1097/01.cji.0000155049.26787.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou Q, Bucher C, Munger ME, Highfill SL, Tolar J, Munn DH, et al. Depletion of endogenous tumor-associated regulatory T cells improves the effi cacy of adoptive cytotoxic T-cell immunotherapy in murine acute myeloid leukemia. Blood. 2009;114:3793–3802. doi: 10.1182/blood-2009-03-208181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Theofi lopoulos AN, Dummer W, Kono DH. T cell homeostasis and systemic autoimmunity. J Clin Invest. 2001;108:335–340. doi: 10.1172/JCI12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maine GN, Mule JJ. Making room for T cells. J Clin Invest. 2002;110:157–159. doi: 10.1172/JCI16166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Sch lom J, Sabzevari H. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105:2862–2868. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- 48.Beyer M, Kochanek M, Darabi K, Popov A, Jensen M, Endl E, et al. Reduced frequencies and suppressive function of CD4+CD25hi egulatory T cells in patients with chronic lymphocytic leukemia after therapy with fludarabine. Blood. 2005;106:2018–2025. doi: 10.1182/blood-2005-02-0642. [DOI] [PubMed] [Google Scholar]
- 49.Zorn E, Mohseni M, Kim H, Porcheray F, Lynch A, Bellucci R, et al. Combined CD4+ donor lymphocyte infusion and low-dose recombinant IL-2 expand FOXP3+ regulatory T cells following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2009;15:382–388. doi: 10.1016/j.bbmt.2008.12.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bocian K, Borysowski J, Wierzbicki P, Wyzgal J, Klosowska D, Bialoszewska A, et al. Rapamycin, unlike cyclosporine A, enhances suppressive functions of in vitro-induced CD4+CD25+ Tregs. Nephrol Dial Transplant. 2009;25:710–717. doi: 10.1093/ndt/gfp586. [DOI] [PubMed] [Google Scholar]
- 51.Yokoyama H, Lundqvist A, Berg M, Ramanathan M, Lopez R, Smith A, Gormley N, et al. Adoptively infused NK cells maintain their antitumor effects in vivo in the presence of cyclosporin A (CSA) Blood. 2008;112 Abstract 2563. [Google Scholar]
- 52.Chan JK, Lin SS, McMeekin DS, Berman ML. Patients with malignancy requiring urgent therapy: CASE 3. Tumor lysis syndrome associated with chemotherapy in ovarian cancer. J Clin Oncol. 2005;23:6794–6795. doi: 10.1200/JCO.2005.08.115. [DOI] [PubMed] [Google Scholar]
- 53.Bilgrami SF, Fallon BG. Tumor lysis syndrome after combination chemotherapy for ovarian cancer. Med Pediatr Oncol. 1993;21:521–524. doi: 10.1002/mpo.2950210712. [DOI] [PubMed] [Google Scholar]
- 54.Yahata T, Nishikawa N, Aoki Y, Tanaka K. Tumor lysis syndrome associated with weekly paclitaxel treatment in a case with ovarian cancer. Gynecol Oncol. 2006;103:752–754. doi: 10.1016/j.ygyno.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 55.Drakos P, Bar-Ziv J, Catane R. Tumor lysis syndrome in non-hematologic malignancies. Report of a case and review of the literature. Am J Clin Oncol. 1994;17:502–505. doi: 10.1097/00000421-199412000-00010. [DOI] [PubMed] [Google Scholar]