Abstract
Hosts have numerous defenses against parasites, of which behavioral immune responses are an important but under-appreciated component. Here we describe a behavioral immune response Drosophila melanogaster utilizes against endoparasitoid wasps. We found that when flies see wasps they switch to laying eggs in alcohol-laden food sources that protect hatched larvae from infection. This oviposition behavior change, mediated by neuropeptide F, is retained long after wasps are removed. Flies respond to diverse female larval endoparasitoids but not to pupal endoparasitoids or males, showing they maintain specific wasp search images. Furthermore, the response evolved multiple times across the genus Drosophila. Our data reveal a behavioral immune response based on anticipatory medication of offspring, and outline a non-associative memory paradigm based on innate parasite recognition by the host.
Although immune systems are often thought of as a set of immune active molecules and cells within a host, they comprise a much more diverse array of biological structures and processes that collectively protect an organism from infection. Medication, the prophylactic (pre-infection) or therapeutic (post-infection) use of substances found in the environment to combat infection, is a type of behavioral immune mechanism (1). Medication requires recognition of infection, or infection risk, by the host leading to use of a substance directed against the identified parasite (2, 3). Endoparasitoid wasps are a serious threat to flies in nature (4), and we recently showed that infected D. melanogaster larvae preferentially consume toxic levels of alcohol because the benefit of alcohol-mediated wasp death outweighs the cost to flies of alcohol consumption, an example of therapeutic self-medication (5). Here, we tested whether adult fruit flies choose to lay their eggs in food containing toxic levels of alcohol when wasps are present in the environment as a means of prophylactically medicating their offspring against infection.
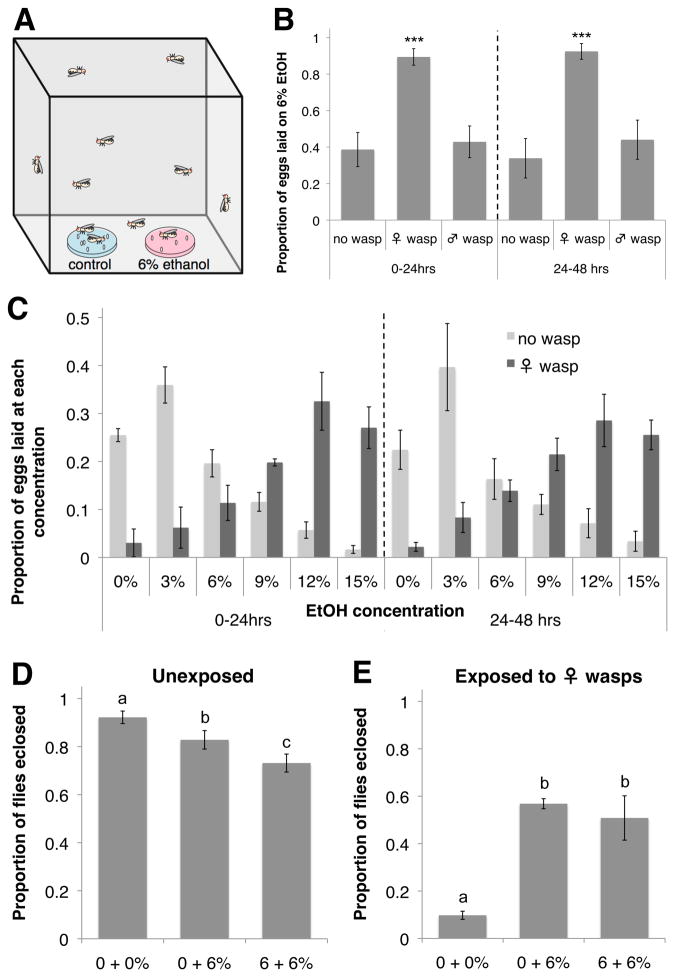
We tested oviposition preferences of adult female D. melanogaster by placing 300 flies in population cages containing two food dishes, one of which contained 6% ethanol by volume (Fig. 1A). Flies were housed with or without 50 female wasps, and fly eggs were counted from separate sets of dishes 24 and 48 hrs later. Control flies preferred to oviposit on dishes containing no ethanol, but in the presence of female Leptopilina heterotoma, a common wasp parasite of D. melanogaster larvae in nature (6), flies laid a significantly greater proportion of eggs on ethanol dishes at both time points (Fig. 1B, Table S1). The flies displayed no such alcohol preference in the presence of male wasps. To determine the extent of fly preference for alcohol-laden oviposition sites in the presence of female wasps, flies were given a choice of various concentrations of ethanol. Control flies preferred to oviposit on dishes containing 3% ethanol (Fig. 1C, Table S2), consistent with the known benefits to fly larvae of low-level alcohol consumption and costs of higher-level consumption (5, 7–9). In the presence of wasps, however, flies overwhelmingly preferred to oviposit on dishes containing ethanol concentrations corresponding to the highest levels found in nature (12 and 15%) (Fig. 1C, Table S2) (10).
Fig. 1.
D. melanogaster medicates offspring with alcohol after exposure to wasps. (A) Standard oviposition preference setup. (B) Proportion eggs laid on 6% ethanol dishes for three wasp treatments, at two time points. ***P < 0.001. (C) Proportion eggs laid on dishes with increasing ethanol (EtOH) concentrations, depending on wasp presence. P < 0.001 for distribution comparisons at both time points. (D) Proportion wasp-exposed fly offspring that eclose when laid in cages with different combinations of oviposition dishes. (E) Proportion unexposed offspring that eclose. For (D to E), letters indicate significance groups at P < 0.01. For (B to E), error bars represent 95% confidence intervals (n = 4).
To determine whether the fly oviposition switch is adaptive, we measured offspring eclosion success in various oviposition setups. In the absence of wasps, the offspring of flies in cages with only 0% ethanol dishes had significantly higher eclosion success than offspring from flies given 6% alcohol food, demonstrating there is normally a fitness detriment to ovipositing in food with such high alcohol levels (Fig. 1D). When female wasps were present, however, offspring of flies given an opportunity to oviposit on alcohol-laden food had significantly higher eclosion success than offspring of flies given no such opportunity (Fig. 1E). This prophylaxis likely arises from both decreased offspring infection and increased offspring success at curing infections (5). Such an induced fly behavioral immune response may serve as alternative to the presumably energetically costly cellular encapsulation response flies mount against wasp eggs.
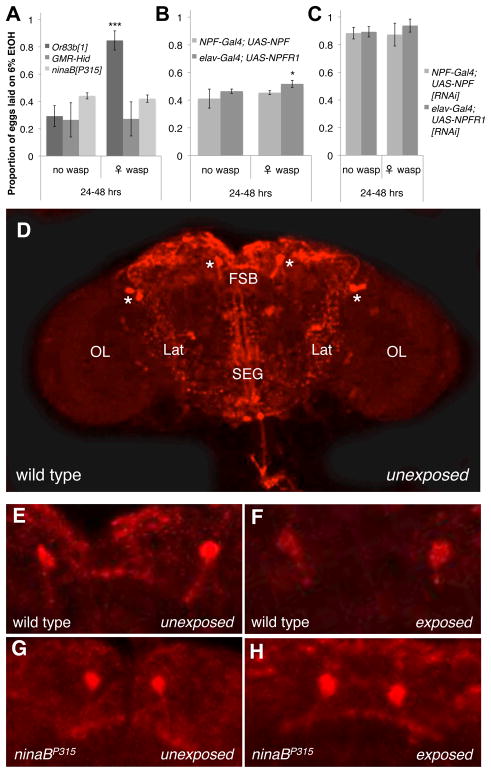
Mutant strains were used to determine whether flies require olfactory or visual cues to sense wasps. Or83b mutants fail to respond to most olfactory stimuli (11), but retained an oviposition preference for ethanol food in the presence of wasps (Fig. 2A, Fig. S1A, and Table S3), suggesting this general olfactory receptor is not required for wasp detection or alcohol sensing (12). GMR-Hid flies express an apoptotic activator in the developing retina leading to dramatically reduced eyes (13), and ninaBP315 mutants fail to synthesize rhodopsin, eliminating vision while leaving the basic morphology of the eye intact (14). Neither vision mutant showed an oviposition preference for ethanol food in the presence of wasps (Fig. 2A, Fig. S1A, and Table S3), indicating flies rely on sight to sense wasps in their environment and initiate the oviposition preference switch.
Fig. 2.
Sight and NPF signaling control fly ability to sense and respond to wasps. Proportion eggs laid on ethanol dishes by (A) smell and sight mutants, (B) NPF and NPFR1 over-expression mutants, and (C) NPF and NPFR1 knockdown mutants in the presence and absence of wasps. For (A to C), y-axis is the same; error bars represent 95% confidence intervals (*P < 0.05, ***P < 0.001, n = 4). (D) NPF immunostain of an unexposed fly brain. * = NPF-expressing neurons, FSB = fan-shaped body, Lat = lateral regions, SEG = subesophageal ganglion, OL = optic lobes. (E to H) NPF immunostained fan-shaped bodies from control and sight mutant flies unexposed or exposed to wasps.
Reduced expression of neuropeptide F (NPF) and its receptor (NPFR1) in fly brains increases ethanol tolerance and preference similar to NPY in mammals (15, 16). Using the yeast Gal4-UAS transcription factor-promoter system, we found NPF-Gal4-driven ectopic expression of NPF eliminated the wasp-induced ethanol oviposition preference (Fig. 2B, Fig. S1B, D to G, and Table S3). Flies with elav-Gal4-driven pan-neuronal ectopic expression of NPFR1 showed a weak but significant increase in alcohol preference in the presence of wasps, but there was a dramatic reduction in oviposition preference for alcohol food compared to wild-type flies (Fig. 1B). RNAi-mediated knockdown of NPF and NPFR1 levels in the brain, using NPF-Gal4 and elav-Gal4 drivers, respectively, led to increased ethanol oviposition preference regardless of wasp presence (Fig. 2C, Fig. S1C to E, H to I, and Table S3).
These results suggest visual perception of wasps by flies might cause decreased NPF levels in fly brains. We immunostained fly brains with NPF antiserum following wasp exposure (Fig. 2D) and found a marginally significant decrease in whole brain fluorescence levels of wild-type flies exposed to wasps for 24 hours but no such change in sight-impaired ninaBP315 flies (Fig. S1J). NPF-expressing neurons send projections to the fan-shaped body, subesophageal ganglion, and lateral regions of the lower central brain (Fig. 2D) (16, 17). There was a significant loss of NPF immunofluorescence in the fan-shaped body of wild-type flies exposed to wasps while the other NPF-positive regions remained unchanged (Fig. 2E to F and Fig. S1K to O). Despite constitutive differences between wild type and ninaBP315 flies, there was no difference in the staining of the fan-shaped body or other brain regions between unexposed and wasp-exposed ninaBP315 flies (Fig. 2G to H and Fig. S1K to O). The fan-shaped body is part of the central complex of the fly brain that regulates both visual pattern recognition and ethanol-stimulated locomotion (18, 19).
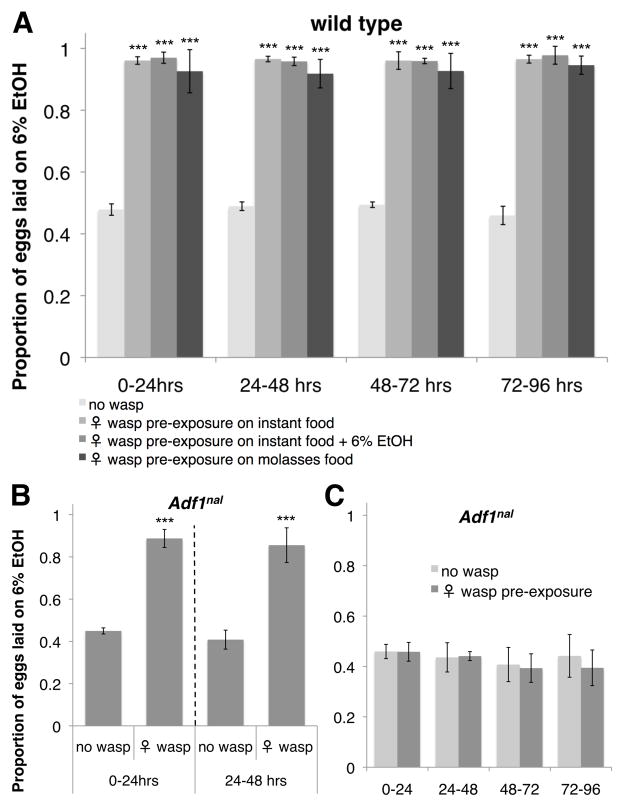
To determine how long fly oviposition preferences are altered after sensing wasps (20), we “pre-exposed” flies to wasps before assaying oviposition preferences in cages devoid of wasps. Flies pre-exposed to wasps showed a strong preference for the ethanol oviposition dishes across a 4-day choice assay (Fig. 3A, Table S4). The oviposition switch occurred even when flies were pre-exposed to wasps in food bottles containing alcohol or completely different media, demonstrating flies do not learn to associate wasp presence with food type (Fig. 3A: P > 0.120 for preference comparisons between pre-exposure food types at each time point, Table S4). Thus, the oviposition preference switch flies maintain after seeing wasps is a natural example of non-associative memory (21).
Fig. 3.
Flies form long-term memories of seeing wasps. Proportion eggs laid on ethanol dishes (A) following wasp pre-exposure on different food types, (B) in the presence of wasps by the long-term memory mutant Adf1nal, and (C) after wasp pre-exposure (on molasses food) in Adf1nal mutants. For A–C, all y-axes are the same, error bars represent 95% confidence intervals (*P < 0.05, ***P < 0.001, n = 4).
The D. melanogaster transcription factor Adf1 is required for long-term memory formation and for regulating Alcohol dehydrogenase expression (21, 22). Adf1nal, a mutant that has normal early memory but lacks long-term memory, switched its oviposition preference to alcohol food in the presence of wasps like wild-type flies (Fig. 3B, Table S4). When Adf1nal mutants were pre-exposed to wasps and then put into cages without wasps, however, the flies failed to retain the wasp exposure memory, showing no oviposition preference for alcohol food (Fig. 3C, Table S4). This result is not explained by any reduced ethanol tolerance of Adf1nal flies (Fig. S2A–B). Furthermore, both vision and NPF signaling were required for initiating memory formation (Fig. S2C to F, Table S4). These data suggest a single protein (Adf1) may simultaneously be responsible for memory of wasp presence and regulation of a gene that controls tolerance to the alcohol-laden food flies become permanently attracted to, and also outline a simple model of wasp-mediated alcohol seeking in flies (Fig. S3).
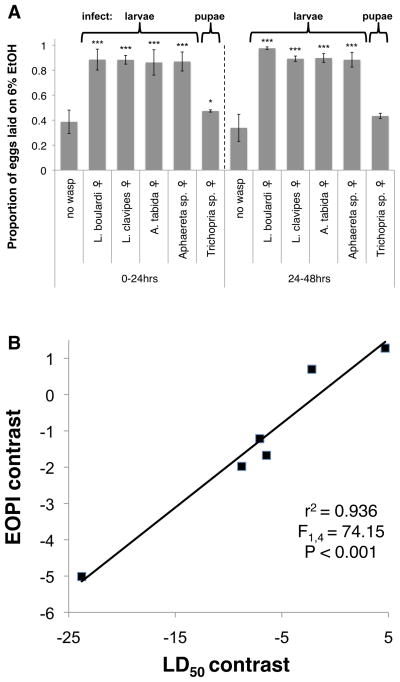
Innate and learned search images are important for numerous organismal interactions (23, 24). To further delimit innate wasp search images D. melanogaster maintains, we assayed fly oviposition behavior during exposure to two more Figitid wasps, L. boulardi and L. clavipes, two Braconids, Aphaereta sp. and Asobara tabida, and a Diapriid pupal endoparasitoid, Trichopria sp. (25). Flies preferred ethanol-laden oviposition dishes in the presence of females of each endoparasitoid species that infects fly larvae, but the preference was weaker upon exposure to the wasp that infects fly pupae, reaching only marginal significance at 0–24 hrs (Fig. 4A, Table S1). This might make adaptive sense given Drosophila larvae often move off their food source before pupating. Thus, D. melanogaster has evolved search images specific enough to distinguish female from male L. heterotoma and a pupal endoparasitoid from larval endoparasitoids, but broad enough to recognize two families of larval endoparasitoids (Fig. S4 for wasp images).
Fig. 4.
Breadth of fly search images and evolution of medication behavior. (A) Proportion eggs laid on ethanol dishes in response to different wasp species. Error bars represent 95% confidence intervals (*P < 0.05, ***P < 0.001, n = 4). (B) Correlation between phylogenetically independent contrasts for ethanol tolerance (LD50) and ethanol oviposition preference index across seven Drosophila species.
To understand the evolution of this behavioral immune mechanism, we tested oviposition preferences in six more Drosophila species, and found that three species (D. simulans, D. hydei, D. virilis) showed a significant increase in oviposition preference for alcohol dishes in the presence of female L. heterotoma wasps, while the other species actively avoided ethanol dishes regardless of wasp presence (Fig. S5A to F, Table S5). Ethanol tolerance LD50 values for each fly species (Fig. S5G to L) were positively correlated with the ethanol oviposition preference index, a measure of how strongly the preference for ovipositing in ethanol food increases in response to wasp exposure (Fig. S5M). This relationship was even stronger when the phylogenetic relationships between the Drosophila species were taken into account using phylogenetically independent contrasts (26) (Fig. 4B). Thus, alcohol tolerance and the alcohol oviposition preference switch have co-evolved across the genus Drosophila.
Materials and Methods
Insects
The D. melanogaster strain Canton S was used as a wild-type strain. Or83b1, ninaBP315, elav-Gal4, UAS-NPFRNAi, and UAS-NPFR1RNAi mutant strains were acquired from the Bloomington Drosophila Stock Center. NPF-Gal4, UAS-NPF, and UAS-NPFR1 strains were provided by Ping Shen (University of Georgia), GMR-Hid was provided by Ken Moberg (Emory University), and Adf1nal was provided by Subhabrata Sanyal (Emory University). D. simulans, D. sechellia, D. kikkawai, D. subobscura, D. virilis, and D. hydei were acquired from the Drosophila Species Stock Center (strains 14021-0251.195, 14021-0248.25, 14028-0561.14, 14011-0131.08, 15010-1051.87, 15085-1641.69, respectively). All flies were maintained on standard cornmeal/yeast/molasses Drosophila medium. Flies aged 3–6 days post-eclosion were used for all experiments.
The Figitid larval endoparasitoid Leptopilina heterotoma (strain Lh14) was used for all experiments unless otherwise noted. Five other endoparasitoid wasp species from three Hymenopteran families were used to test the breadth of D. melanogaster search images: the Figitid larval endoparasitoids L. boulardi (strain Lb17) and L. clavipes (strain LcNet), the Braconid larval endoparasitoids A. tabida (strain AtFr) and Aphaereta sp.1 (strain Aph1Atl), and the Diapriid pupal endoparasitoid Trichopria sp.1 (strain Tri1Fr). Wasp strains Lh14, Lb17, and Tri1Fr were maintained in the lab on D. melanogaster hosts, while strains LcNet, Aph1Atl, and AtFr were maintained on D. virilis. Wasps aged 3–7 days post-eclosion were used for all experiments.
Fly oviposition preference
In the standard oviposition preference assay, 300 female flies were placed in 0.6 m3 population cages and given the choice to oviposit on two food dishes containing either 0 or 6% ethanol by volume. Oviposition dishes were made by mixing 4 g instant Drosophila medium (Formula 4-24, Carolina Biological Supply) with water and high purity ethanol (Pharmco-AAPER #111000190) for a total liquid volume of 16 mL, in 100 mm diameter Petri dishes. For cages containing wasps, 50 female wasps were placed in the cages 2 hours prior to the addition of flies. Oviposition dishes from each cage were replaced 24 hrs after the start of the experiment, and the second set of dishes were removed 48 hrs after the start of the experiment. Fly egg counts from each dish were made at the 0–24 and 24–48 hr time points. All treatments were run at room temperature (23°C) in four replicates. The relative position of the 6% ethanol dish in each cage was switched in each replicate to eliminate any effect of a fly oviposition bias for a particular side of the population cages. Generalized linear models (GLMs) with binomial errors and logit link functions were used to statistically examine the effect of wasp presence on fly oviposition preference, unless the residual deviance was greater than the degrees of freedom, in which case quasi-binomial errors were used.
To test fly oviposition preference across a gradient of food ethanol concentrations (Fig. 1C), modifications were made to the standard oviposition preference assay: Six oviposition dishes were placed equidistant from each other in double-size oviposition cages (0.6 m2 × 1.2 m), in increasing ethanol concentration (0, 3, 6, 9, 12, 15%). 600 female flies were released in the cage, following the release of 100 female wasps 2 hrs prior for assays with wasp exposure. Fly egg counts from each of the six dishes were made as described above, and all treatments were run in four replicates. Chi-square tests were used to compare oviposition distributions between control and wasp-exposure treatments for each independent experimental replicate.
For oviposition experiments testing memory (Fig. 3), three batches of 100 adult female flies were placed into standard Drosophila bottles where they were pre-exposed to 20 adult female wasps (or not) for 24 hrs. These bottles either contained instant Drosophila medium, the same medium supplemented with 6% ethanol, or standard cornmeal/yeast/molasses Drosophila medium. All 300 flies were then pooled and used in a standard oviposition preference assay without wasps. Fly eggs were counted and analyzed as above, except new oviposition dishes were placed in the cages every 24 hrs for 4 days rather than 2 days. All treatments were run in four replicates.
Fly eclosion
Fly eclosion success was measured in the presence or absence of wasps using three oviposition conditions: (i) both oviposition dishes contained no ethanol, (ii) one dish contained no ethanol and the other dish contained 6% ethanol, (iii) or both dishes contained 6% ethanol. 300 female flies were placed in standard oviposition cages for 24 hrs, with or without 50 female wasps. Fly egg counts were then made for each oviposition dish, and these dishes were allowed to sit undisturbed for 24 hrs while fly larvae hatched. The oviposition dishes were then placed into new population cages (0.6 × 0.5 × 0.2 m) for 48 hours with or without 20 female wasps following the adult fly treatment. These wasps were allowed to infect the fly larvae. To prevent oviposition dishes from drying, dishes were replenished with one half of the original liquid volume (with the respective ethanol concentration) every 24 hrs (four times total). Finally, the contents of each dish were moved into standard Drosophila bottles and the number of flies that eclosed from each bottle was measured 20 days later. All treatments were run in four replicates. GLMs with quasi-binomial errors and logit link functions were used to examine the overall effect of oviposition preference treatment on fly eclosion success. From these models, stepdown pairwise comparisons between oviposition choice treatments were assessed using Tukey’s Honestly Significant Difference test.
Brain staining
Immunofluorescence of adult fly brains was performed by fixing adult flies in 4% paraformaldehyde at 4°C at least 16 hours prior to dissection. After fixing, brains were dissected in 1X PBS, permeabilized in PBT (1X PBS + 0.15% Triton X-100) and blocked in PBT with 2% bovine serum albumin and 5% normal goat serum. Brains were then incubated with rabbit NPF antiserum (1:2000) overnight at 4°C. Following washes in PBT, the brains were incubated with TRITC-anti-rabbit secondary antibody (1:200, Jackson Immunoresearch) and mounted in 50% glycerol. Brains were imaged using a Zeiss LSM 510 META confocal microscope. Images were taken as 14 μm sections through the depth of the brain and projections were made using ImageJ software. Average fluorescence intensities across projections were used for quantification while maximum fluorescence intensities across projections were used to generate brain images.
All brain staining treatments were run in three replicates. For whole brain comparisons, we calculated corrected total fluorescence for each brain, which is the image area multiplied by the mean of the average fluorescence intensities across the image, corrected for background fluorescence. This value was averaged over at least three brains per replicate. Exact one-tailed Wilcoxon rank-sum tests were used to examine the effect of wasp exposure on whole-brain NPF immunofluorescence (fig. S1J). For fan-shaped body comparisons, presence/absence of fluorescence was determined by eye, and the proportion of brains showing fan-shaped body fluorescence was calculated from at least three brains per replicate. GLMs with quasi-binomial errors and logit link functions were used to examine the effect of wasp exposure on the proportion of fly brains displaying NPF immunofluorescence in different brain regions (fig. S1K to O).
Relationship between ethanol oviposition preference and ethanol tolerance
To compare the increased propensity of different Drosophila species to oviposit on 6% ethanol food in the presence of wasps with the ethanol tolerance of each fly species, we first devised the ethanol oviposition preference index (EOPI). Using data from standard oviposition preference assays in four replicates, EOPI is calculated as the difference in the mean proportion of fly eggs laid on the 6% ethanol dish between control and wasp-exposed flies at the 24–48 hrs time point. Ethanol tolerance of each fly species was measured by placing batches of ten adult female flies in 35 mm diameter Petri dishes containing instant Drosophila medium supplemented with 0, 4, 6, 8, or 10% ethanol by volume. Counts for living flies were made 1.5, 3, 6, 12, and 24 hrs later. All ethanol tolerance treatments were run in five replicates, and LD50, the alcohol concentration inferred to cause 50% mortality after 24 hours, was calculated from replicate means for each fly species (fig. S5G to L). Simple linear regression was used to examine the relationship between the raw values of EOPI and ethanol LD50 across the seven Drosophila species (fig. S3M).
To determine whether EOPI and ethanol LD50 have co-evolved, fly phylogeny was taken into account using phylogenetically independent contrasts. COI sequences for each fly species were downloaded from Genbank (with D. pseudoobscura used in place of D. subobscura), and Phylip version 3.69 was then used to generate the following unrooted neighbor-joining tree: ((((((sechellia:0.00768,simulans:0.00691):0.01075,melanogaster:0.03308):0.02771,kikkawai:0.0 7770):0.01711,subobscura:0.06897):0.00355,virilis:0.09215):0,hydei:0.11267). Based on this tree, non-directional phylogenetically independent contrasts for EOPI and ethanol LD50 were calculated in Phylip, and simple linear regression was used to examine the relationship between the EOPI and ethanol LD50 contrasts.
Statistics
All statistical analyses were performed in R version 2.13.2 unless otherwise indicated.
Supplementary Material
Acknowledgments
We thank Ping Shen for NPF antiserum, Ping Shen, Ken Moberg, and Subhabrata Sanyal for Drosophila strains, and Jacques van Alphen and Bregje Wertheim for wasp strains. This work was supported by NIH grant AI081879 to T.A.S. and the Integrated Cellular Imaging Microscopy Core of the Emory Neuroscience NINDS Core Facilities grant, P30NS055077. Data are deposited in the Dryad Repository: http://dx.doi.org/10.5061/dryad.j5g7m.
Footnotes
References and Notes
- 1.de Roode JC, Lefevre T. Insects. 2012;3:789. doi: 10.3390/insects3030789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lefevre T, Oliver L, Hunter MD, de Roode JC. Ecol Lett. 2010;13:1485. doi: 10.1111/j.1461-0248.2010.01537.x. [DOI] [PubMed] [Google Scholar]
- 3.Singer MS, Mace KC, Bernays EA. PLoS One. 2009;4:e4796. doi: 10.1371/journal.pone.0004796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fleury F, et al. Genetica. 2004;120:181. doi: 10.1023/b:gene.0000017640.78087.9e. [DOI] [PubMed] [Google Scholar]
- 5.Milan NF, Kacsoh BZ, Schlenke TA. Curr Biol. 2012;22:488. doi: 10.1016/j.cub.2012.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fleury F, Gibert P, Ris N, Allemand R. Adv Parasitol. 2009;70:3. doi: 10.1016/S0065-308X(09)70001-6. [DOI] [PubMed] [Google Scholar]
- 7.Chawla SS, Perron JM, Radoucothomas C. Can Entomol. 1981;113:315. [Google Scholar]
- 8.McKechnie SW, Geer BW. Insect Biochem. 1984;14:231. [Google Scholar]
- 9.Parsons PA, Stanley SM, Spence GE. Austral J Zool. 1979;27:747. [Google Scholar]
- 10.Gibson JB, May TW, Wilks AV. Oecologia. 1981;51:191. doi: 10.1007/BF00540600. [DOI] [PubMed] [Google Scholar]
- 11.Vosshall LB, Amrein H, Morozov PS, Rzhetsky A, Axel R. Cell. 1999;96:725. doi: 10.1016/s0092-8674(00)80582-6. [DOI] [PubMed] [Google Scholar]
- 12.Kong EC, et al. PLoS One. 2010;5:e9954. doi: 10.1371/journal.pone.0009954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goyal L, McCall K, Agapite J, Hartwieg E, Steller H. EMBO J. 2000;19:589. doi: 10.1093/emboj/19.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Lintig J, Dreher A, Kiefer C, Wernet MF, Vogt K. Proc Natl Acad Sci USA. 2001;98:1130. doi: 10.1073/pnas.031576398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thiele TE, Marsh DJ, Ste Marie L, Bernstein IL, Palmiter RD. Nature. 1998;396:366. doi: 10.1038/24614. [DOI] [PubMed] [Google Scholar]
- 16.Wen TQ, Parrish CA, Xu D, Wu Q, Shen P. Proc Natl Acad Sci USA. 2005;102:2141. doi: 10.1073/pnas.0406814102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown MR, et al. Peptides. 1999;20:1035. doi: 10.1016/s0196-9781(99)00097-2. [DOI] [PubMed] [Google Scholar]
- 18.Liu G, et al. Nature. 2006;439:551. doi: 10.1038/nature04381. [DOI] [PubMed] [Google Scholar]
- 19.Strauss R. Curr Opin Neurobiol. 2002;12:633. doi: 10.1016/s0959-4388(02)00385-9. [DOI] [PubMed] [Google Scholar]
- 20.Lefèvre T, De Roode JC, Kacsoh BZ, Schlenke TA. Biol Lett. 2012;8:230. doi: 10.1098/rsbl.2011.0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Margulies C, Tully T, Dubnau J. Curr Biol. 2005;15:R700. doi: 10.1016/j.cub.2005.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.England BP, Heberlein U, Tjian R. J Biol Chem. 1990;265:5086. [PubMed] [Google Scholar]
- 23.Menzel R. In: Experimental Behavioral Ecology and Sociobiology. Holldobler B, Lindauer M, editors. Sinauer Associates; Sunderland, MA: 1985. pp. 55–74. [Google Scholar]
- 24.Pietrewicz AT, Kamil AC. Science. 1979;204:1332. doi: 10.1126/science.204.4399.1332. [DOI] [PubMed] [Google Scholar]
- 25.Kacsoh BZ, Schlenke TA. PLoS One. 2012;7:e34721. doi: 10.1371/journal.pone.0034721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harvey PH, Purvis A. Nature. 1991;351:619. doi: 10.1038/351619a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.