Abstract
Accumbal deep brain stimulation (DBS) is a promising therapeutic modality for the treatment of addiction. Here, we demonstrate that DBS in the nucleus accumbens shell, but not the core, attenuates cocaine priming-induced reinstatement of drug seeking, an animal model of relapse, in male Sprague Dawley rats. Next, we compared DBS of the shell with pharmacological inactivation. Results indicated that inactivation using reagents that influenced (lidocaine) or spared (GABA receptor agonists) fibers of passage blocked cocaine reinstatement when administered into the core but not the shell. It seems unlikely, therefore, that intrashell DBS influences cocaine reinstatement by inactivating this nucleus or the fibers coursing through it. To examine potential circuit-wide changes, c-Fos immunohistochemistry was used to examine neuronal activation following DBS of the nucleus accumbens shell. Intrashell DBS increased c-Fos induction at the site of stimulation as well as in the infralimbic cortex, but had no effect on the dorsal striatum, prelimbic cortex, or ventral pallidum. Recent evidence indicates that accumbens DBS antidromically stimulates axon terminals, which ultimately activates GABAergic interneurons in cortical areas that send afferents to the shell. To test this hypothesis, GABA receptor agonists (baclofen/muscimol) were microinjected into the anterior cingulate, and prelimbic or infralimbic cortices before cocaine reinstatement. Pharmacological inactivation of all three medial prefrontal cortical subregions attenuated the reinstatement of cocaine seeking. These results are consistent with DBS of the accumbens shell attenuating cocaine reinstatement via local activation and/or activation of GABAergic interneurons in the medial prefrontal cortex via antidromic stimulation of cortico-accumbal afferents.
Introduction
Despite decades of research, there are currently no effective pharmacologic treatments for cocaine addiction or the prevention of cocaine relapse. Emerging preclinical evidence indicates that deep brain stimulation (DBS) of the nucleus accumbens, a limbic structure that plays an important role in the reinforcing effects of drugs of abuse, may be a therapeutic option in the treatment of addiction. Thus, DBS of the nucleus accumbens prevented morphine-conditioned place preference (Liu et al., 2008), attenuated cocaine priming-induced reinstatement of drug seeking (Vassoler et al., 2008), and decreased alcohol preference and/or intake in rats (Knapp et al., 2009; Henderson et al., 2010). There are two main subregions of the nucleus accumbens, the core and shell, which can be differentiated both functionally and anatomically. As one example, the infralimbic and ventral prelimbic cortices preferentially innervate the shell, whereas the anterior cingulate and dorsal prelimbic cortices project mainly to the core (Heimer et al., 1997; Zahm, 2000). Although DBS of the medial accumbens shell attenuated cocaine priming-induced reinstatement of drug seeking (Vassoler et al., 2008), the influence of core DBS has not yet been examined.
Surprisingly, the mechanism of action of DBS remains unclear. There are several general hypotheses regarding the therapeutic effects of DBS, which typically involves constant high-frequency (130–160 Hz) stimulation. For instance, some evidence indicates that DBS increases neuronal activity within the stimulated nucleus (McIntyre et al., 2004; Montgomery and Gale, 2008). In contrast, other results suggest that DBS produces inhibition either through depolarization blockade or activation of inhibitory neurons (Boraud et al., 1996; Benazzouz and Hallett, 2000; Kiss et al., 2002). DBS also was shown to preferentially stimulate axon terminals and axons of passage relative to cell bodies (Nowak and Bullier, 1998), which results in broader, circuit-wide influences (Windels et al., 2000; Vitek, 2002; McCracken and Grace, 2007; Gradinaru et al., 2009). These mechanisms are not necessarily mutually exclusive since activation of GABAergic cell bodies in the nucleus accumbens produces local inhibition through recurrent collaterals (Taverna et al., 2004). Moreover, DBS may inhibit the accumbens through the activation of GABAergic interneuron axon terminals.
Recent evidence indicated that DBS of the nucleus accumbens has complex effects on afferent brain regions. Thus, accumbens DBS of urethane-anesthetized rats increased spontaneous gamma power at the site of stimulation as well as in the orbitofrontal cortex (OFC) and medial prefrontal cortex (mPFC; McCracken and Grace, 2009). These results suggest that the therapeutic effects of DBS may be due to enhanced rhythmicity and synchronous inhibition at the site of stimulation as well as in brain regions sending afferents to the nucleus accumbens (McCracken and Grace, 2009). Accumbens DBS reduced spontaneous activity of OFC-accumbal glutamatergic neurons but activated OFC interneurons, which is consistent with accumbens DBS producing recurrent inhibition in the OFC following antidromic stimulation of OFC-accumbal neurons (McCracken and Grace, 2007).
In the current study, we used pharmacological inactivation of specific nuclei coupled with c-Fos immunoreactivity to examine potential mechanisms underlying the effects of accumbens shell DBS on the reinstatement of cocaine-seeking behavior. Our results are consonant with accumbens shell DBS attenuating cocaine reinstatement by antidromically activating inhibitory interneurons in the infralimbic cortex.
Materials and Methods
Animals and housing.
Male Sprague Dawley rats (Rattus norvegicus) weighing 250–300 g were obtained from Taconic Laboratories. Animals were individually housed with food and water available ad libitum. A 12 h light/dark cycle was used with the lights on at 7:00 A.M. All experimental procedures were performed during the light cycle.
Materials.
All experiments used Med Associates instrumentation enclosed within ventilated, sound-attenuating chambers. The apparatus was equipped with response levers, stimulus lights, food pellet dispensers, and injection pumps for injecting drugs intravenously.
Surgery.
Before surgery, the rats were anesthetized with 80 mg/kg ketamine and 12 mg/kg xylazine (intraperitoneally injected). An indwelling Silastic catheter was placed into the right jugular vein (side opposite the heart) and sutured into place. The catheter was then threaded subcutaneously over the shoulder blade and was routed to a mesh backmount platform (CamCaths) that was sutured below the skin dorsal to the shoulder blades. Catheters were flushed daily with 0.3 ml of an antibiotic (Timentin, 0.93 mg/ml) dissolved in heparinized saline. The catheters were sealed with plastic obturators when not in use.
Following catheter implantation, the rats were mounted in a stereotaxic apparatus (Kopf Instruments), and bipolar stainless steel electrodes (Plastics One) or stainless steel guide cannulae (14 mm for accumbens or 10 mm for mPFC, 24 gauge; Small Parts Inc.) were implanted into the nucleus accumbens shell, nucleus accumbens core, infralimbic prefrontal cortex, prelimbic prefrontal cortex, or anterior cingulate prefrontal cortex according to the following coordinates, relative to bregma (Paxinos and Watson, 1997): electrodes: nucleus accumbens shell: +1.0 mm anteroposterior (A/P), ±3.0 mm mediolateral (M/L), −7.3 mm dorsoventral (D/V), 17° angle; nucleus accumbens core: +1.0 mm A/P, ±3.0 mm M/L, −7.07 mm D/V, 8.13° angle; guide cannulae: nucleus accumbens shell: +1.0 mm A/P, ±1.0 mm M/L, −5.0 mm D/V; nucleus accumbens core: +1.0 mm A/P, ±2.5 mm M/L, −5.0 mm D/V; infralimbic prefrontal cortex: +2.5 mm A/P, ±0.5 mm M/L, −3.0 mm D/V; prelimbic prefrontal cortex: +2.5 mm A/P, ±0.5 mm M/L, −2.0 mm D/V; anterior cingulate prefrontal cortex: +2.5 mm A/P, ±0.5 mm M/L, −1.0 mm D/V. Electrodes and cannulae were cemented in place by affixing dental acrylic to three stainless steel screws fastened to the skull. Stainless steel obturators (14 mm, 33 gauge) were inserted into the guide cannulae, where they remained until the microinjections were performed.
Cocaine self-administration, extinction, and reinstatement.
Following a 7 d period for recovery from surgery, the rats were placed in operant chambers and were allowed to press a lever for intravenous cocaine infusions (0.25 mg of cocaine, 56 μl of saline, infusion over 5 s). Rats initially were trained using a fixed ratio 1 (FR1) schedule of reinforcement. When the animals achieved a stable response with the FR1 schedule (i.e., <15% variation in response rates over 3 consecutive days), they were switched to an FR5 schedule. A 20 s timeout period during which responses have no scheduled consequences followed each cocaine infusion. The rats were limited to a maximum of 30 cocaine infusions per daily 2 h self-administration session.
After 21 d of total cocaine self-administration, the animals underwent an extinction phase during which cocaine was replaced with saline. Daily 2 h extinction sessions were conducted until responding was <15% of the response rate maintained by cocaine self-administration. Following the extinction phase, the ability of an acute priming injection of cocaine (10 mg/kg, i.p.) to reinstate drug-seeking behavior was assessed. For the reinstatement sessions, the FR5 schedule was used. Satisfaction of the response requirements for each component resulted in a saline infusion rather than a cocaine infusion. Each reinstatement session was followed by extinction sessions until responding was <15% of the response rate maintained by cocaine self-administration.
Using this experimental design, the rats underwent a series of extinction and reinstatement sessions that lasted ∼16 d. During this period, extinction of the ability of cocaine to induce reinstatement is a concern. However, we have previously shown that reinstatement of cocaine seeking persists for at least 20 d after the initial extinction of cocaine self-administration (Park et al., 2002; Anderson et al., 2003). Moreover, since the drug treatments were counterbalanced across reinstatement days, in the current experiments we were able to assess the magnitude of reinstatement across sessions. Thus, reinstatement of cocaine seeking was assessed at the beginning, middle, and end of the reinstatement phase. All subjects demonstrated stable drug seeking throughout the reinstatement phase of these experiments.
Deep brain stimulation.
In most DBS experiments (both clinical and preclinical), many parameters are fixed and uniform across studies. We used alternating current with biphasic symmetrical pulses (60 μs pulse width and a 160 Hz frequency), parameters that are consistent with previous work in this field (Chang et al., 2003; Mayberg et al., 2005). Stimulation intensities, in contrast, are often varied within and between studies, usually in the range of 50–200 μA (Benazzouz and Hallett, 2000; Chang et al., 2003; Mayberg et al., 2005). We previously reported that 150 μA of current is an effective stimulation intensity in our reinstatement paradigm (Vassoler et al., 2008). Immediately before the start of a reinstatement session, 0 or 150 μA current was delivered continuously to the bipolar electrodes. The stimulation continued until the end of the reinstatement session. Throughout the 0 μA condition, the stimulation tethers were attached in the exact same manner as the 150 μA condition. The 0 and 150 μA currents were administered in a counterbalanced fashion across the multiple reinstatement test days.
Microinjections.
Before a reinstatement test session, obturators were removed from the guide cannulae, and 33 gauge stainless steel microinjectors (Small Parts Inc.) were inserted. These microinjectors were cut to a length that extended 2 mm below the ventral end of the guide cannulae and into the brain region of interest. Bilateral infusions were performed simultaneously over a 2 min time period in a total volume of 0.5 μl per hemisphere. The microinjectors were left in place for 1 min to allow the solution to diffuse away from the tips of the microinjectors before they were removed. A systemic priming injection of cocaine (10 mg/kg, i.p.) was administered 10 min following microinjections, and animals were then placed into the operant chambers and the reinstatement session began immediately. Each animal served as its own control and received two microinjections per brain region (i.e., one drug and one vehicle injection per region). To control for potential order effects of drug and vehicle administrations, all drug and vehicle treatments were counterbalanced across reinstatement sessions.
Drugs.
Cocaine was obtained from the National Institute on Drug Abuse and dissolved in bacteriostatic 0.9% saline solution. Baclofen (0.3 nmol), muscimol (0.03 nmol), saclofen (50 and 100 ng), bicuculline (50 and 100 ng), and lidocaine (100 μg/side; Sigma-Aldrich) were dissolved in sterile 0.9% saline. Drug doses were based on similar previous studies (Peters et al., 2008; Kantak et al., 2009; Koya et al., 2009).
Verification of electrode and cannulae placements.
Following the completion of all experiments, the animals were given an overdose of pentobarbital (100 mg/kg) and perfused intracardially with 0.9% saline followed by 10% formalin. The brain was removed, and coronal sections (100 μm) were taken at the level of the nucleus accumbens or prefrontal cortex with a vibratome (Amphenol Technical Products International). An investigator who was unaware of the animals' behavioral responses determined electrode and cannulae placements, as well as potential electrode-induced neuronal damage. Animals with electrode or cannulae placements outside of the areas of interest, or with excessive mechanical damage, were excluded from subsequent data analysis.
c-Fos Immunohistochemistry.
For the c-Fos staining experiments, rats were implanted with electrodes in the nucleus accumbens shell as described above. Following 7 d of recovery, animals began a habituation process in which they were attached to the stimulation tethers during daily 1 h sessions for a total of 7 d. On the eighth day, animals received either 30 min of stimulation (160 Hz, 150 μA) followed by 30 min of no stimulation or 1 h of no stimulation (attached to the tethers in the same manner as those animals that received stimulation). c-Fos expression is known to peak ∼60 min following a stimulus. Therefore, we stimulated animals for 30 min and looked for c-Fos expression 30 min later to capture peak activation resulting from stimulation. All animals were perfused 70 min after the onset of stimulation. Animals were deeply anesthetized with sodium pentobarbital (100 mg/kg) and perfused with 200 ml of ice-cold PBS followed by 200 ml of ice-cold 4% paraformaldehyde (PFA). The brains were subsequently removed and stored in 4% PFA for 24 h, at which point the brains were switched to a 30% sucrose solution in PBS for 72 h. Coronal sections (30 μm) were taken using a vibratome in 1% Na azide in PBS and then processed for immunohistochemistry.
c-Fos immunoreactivity was detected using a rabbit polyclonal antibody (1:1000; SC-52, Santa Cruz Biotechnology). Slices were mounted on electrostatic slides (both conditions, stimulated and not stimulated, were mounted on the same slide) and allowed to dry. They were then washed with PBS and blocked for 1 h in PBS with 0.1% Triton X-100 and 3% normal donkey serum. Following the blocking step, slides were incubated overnight in primary antibody, 0.1% Triton X-100, and 3% normal donkey serum at 4°C. The following day, the slides were washed in PBS and incubated for 2 h at room temperature in a fluorescent secondary antibody (CY-3 anti-rabbit, 1:500) and 3% normal donkey serum. The slides were washed for a final time, dehydrated in increasing EtOH concentrations, and coverslipped using DPX mountant. Staining was visualized with a fluorescence microscope.
Statistics.
All reinstatement experiments were analyzed with mixed-factors ANOVAs with repeated measures over reinstatement days. Pairwise analyses were made with Bonferroni post-tests (p < 0.05).
Results
DBS of the nucleus accumbens shell, but not the core, attenuated cocaine priming-induced reinstatement of drug seeking
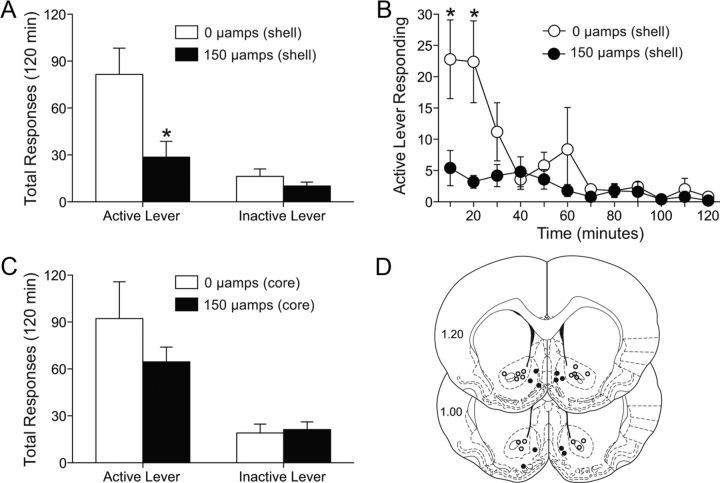
Following cocaine self-administration and extinction, deep brain stimulation of the nucleus accumbens shell (0 or 150 μA) was administered throughout a 2 h cocaine-primed reinstatement session. Total active and inactive lever responding from the reinstatement session during which DBS was delivered to the accumbens shell are presented in Figure 1A. These data were analyzed with a two-way mixed-factors ANOVA (repeated measures over lever press), which revealed significant main effects of treatment (F(1,8) = 10.21, p < 0.0127) and lever response (F(1,8) = 14.64, p < 0.005), as well as a marginally significant interaction between these factors (F(1,8) = 4.589, p < 0.0646). Subsequent pairwise analyses (Bonferroni's correction, p < 0.01) showed that the total active lever responses were significantly different between 0 and 150 μA treatments. The time course of the active lever responding is shown in Figure 1B. These data were analyzed with a mixed-factors ANOVA (repeated measures over time), the results of which revealed significant main effects of treatment (F(1,8) = 8.613, p < 0.0189) and time (F(11,88) = 6.004, p < 0.0001), as well as a significant interaction between these factors (F(11,88) = 3.306, p < 0.0008). Subsequent pairwise analyses showed that the active lever responses were significantly different between the 0 and 150 μA treatments over the first 20 min of the reinstatement session (Bonferroni's correction, p < 0.001). There were five subjects per treatment. Previous results indicated that this effect is reinforce specific in that DBS of the accumbens shell had no influence on the reinstatement of sucrose seeking (Vassoler et al., 2008).
Figure 1.
Deep brain stimulation of the nucleus accumbens shell, but not core, attenuates cocaine priming-induced reinstatement. A, C, Mean (±SEM) active and inactive lever responses from reinstatement sessions with 0 or 150 μA of stimulation aimed at the shell (A) or core (C). DBS began immediately following administration of 10 mg/kg (i.p.) cocaine and continued throughout the 2 h reinstatement session. B, Time course of active lever responding from 0 or 150 μA stimulation of the accumbens shell. D, Electrode placements from both the shell (closed circles) and core (open circles). The values are in millimeters, relative to bregma. *p < 0.05 0 μamps compared with 150 μamps. There were five to eight animals per group.
In contrast, DBS of the accumbens core (Fig. 1C) had no effect on the reinstatement of cocaine seeking. Total lever presses were analyzed with a two-way repeated-measures ANOVA (repeated measures over lever press), which revealed a significant main effect of lever response (F(1,14) = 16.99, p < 0.001), but no main effect of treatment and no significant interaction. There were eight subjects per treatment. The electrode placements are shown in Figure 1D. The open circles represent core placements, which were all clustered around the anterior commissure. The shell placements are depicted by closed circles, and were in the medial portion of the shell and on the border between the shell and the olfactory tubercles.
Pharmacological inactivation of the accumbens core, but not the shell, impairs the reinstatement of cocaine seeking
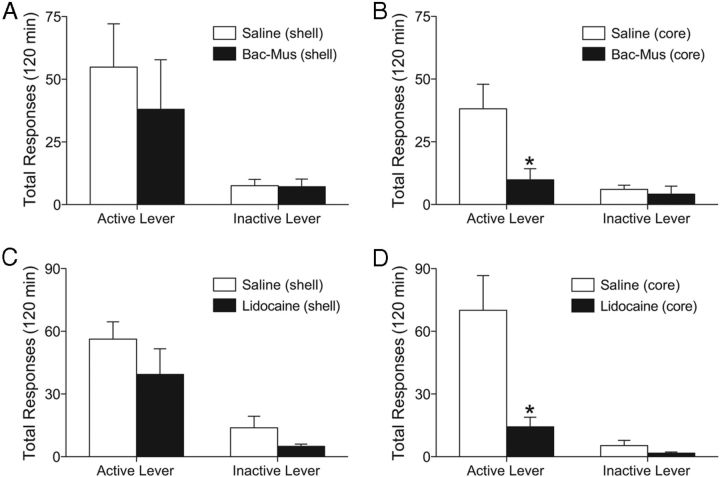
Some evidence indicates that DBS produces therapeutic effects by inhibiting cell bodies in the region of stimulation via depolarization inactivation (Beurrier et al., 2001; Anderson et al., 2004). To test this hypothesis, we inactivated the core or shell of the nucleus accumbens by microinjecting a cocktail of a GABAA agonist (muscimol, 0.03 mmol per side) and a GABAB agonist (baclofen, 0.3 mmol per side) into these structures before a priming injection of cocaine. Total active and inactive lever responding during the reinstatement test session following administration of baclofen/muscimol into the shell or core are presented in Figure 2, A and B, respectively. The shell data were analyzed with a two-way repeated-measures ANOVA (repeated measures over lever response), which showed a significant main effect of lever response (F(1,12) = 9.767, p < 0.0088), but no effect of treatment and no significant interaction (n = 7). Analysis of the core data revealed significant main effects of lever response (F(1,12) = 11.04, p < 0.0061) and treatment (F(1,12) = 7.014, p < 0.0212), as well as a significant interaction between these factors (F(1,12) = 5.38, p < 0.0388). Subsequent pairwise analyses (Bonferroni's correction, p < 0.05) indicated that total active lever responses were significantly different between treatments (n = 7).
Figure 2.
Baclofen-muscimol or lidocaine microinjected into the core but not the shell of the nucleus accumbens attenuated cocaine priming-induced reinstatement. A–D, Mean (±SEM) active and inactive lever responses from reinstatement sessions with saline or baclofen and muscimol microinjected into the nucleus accumbens shell (A) or core (B) and lidocaine microinjected into the shell (C) or core (D). Cocaine (10 mg/kg, i.p.) was administered 10 min following the baclofen and muscimol microinjection or immediately following the lidocaine injection. *p < 0.05 compared to saline microinjections. There were 7–12 animals per group.
Lidocaine administration into the accumbens core, but not the shell, attenuates the reinstatement of cocaine seeking
Baclofen/muscimol administration inhibits neuronal activity but has no effect on fibers of passage, which could be influenced by DBS. To assess the role of fibers of passage in the behavioral effects of DBS, a sodium channel blocker (lidocaine, 100 μg/side) was microinjected into the shell or core of the nucleus accumbens immediately before cocaine priming-induced reinstatement, as the effect of lidocaine is very rapid (Kantak et al., 2002). Total active and inactive lever responses from the reinstatement session are presented in Figure 2, C (shell) and D (core). The shell data were analyzed with a two-way mixed-factors ANOVA (repeated measures over lever response), which revealed a significant main effect of lever response (F(1,12) = 31.15, p < 0.001), but no main effect of lidocaine treatment and no significant interaction (Fig. 3A). Analysis of the core data showed a significant main effect of lever response (F(1,19) = 25.91, p < 0.0001) and lidocaine treatment (F(1,19) = 14.71, p < 0.0011), as well as a significant interaction between these factors (F(1,19) = 11.79, p < 0.0028). Subsequent pairwise analyses (Bonferroni post-tests, p < 0.001) indicated that the total active lever responses were significantly different between the lidocaine and vehicle treatments. There were 9–12 subjects per group.
Figure 3.
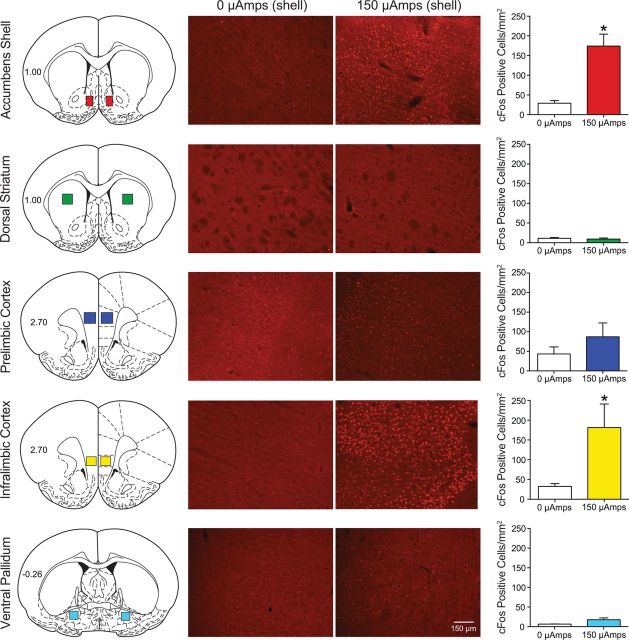
DBS of the nucleus accumbens shell induces c-Fos expression in the nucleus accumbens shell and the infralimbic prefrontal cortex. All animals received bilateral nucleus accumbens shell DBS (0 or 150 μA) for 30 min. Animals were perfused 70 min from the start of stimulation, and c-Fos immunoreactivity was measured to assess neuronal activation following accumbens DBS. Figure 3 shows a representative cartoon depicting the area of c-Fos staining quantification from the five brain regions where c-Fos was counted (nucleus accumbens shell, dorsal striatum, prelimbic cortex, infralimbic cortex, and ventral pallidum), a representative image from both the 0 and 150 μA condition, and the quantification (mean ± SEM c-Fos-positive cells per square millimeter). *p < 0.05 0 μamps compared with 150 μamps. There were five animals per group.
GABA antagonists administered into the accumbens core have no effect on the reinstatement of cocaine seeking
Since intracore microinjection of GABA agonists attenuated reinstatement, we postulated that GABA antagonists might promote reinstatement, which would suggest that a decrease in accumbens GABAergic transmission promotes cocaine reinstatement. Therefore, we administered a cocktail of a GABAA antagonist (bicuculline, 50 or 100 ng/side) and a GABAB antagonist (saclofen, 50 or 100 ng/side) into the nucleus accumbens core in the absence of an intraperitoneal cocaine priming injection. Our results indicated that intracore saclofen/bicuculline injection failed to promote the reinstatement of cocaine seeking (mean ± SEM active lever responses: bicuculline-saclofen, 3.833 ± 1.195, n = 6).
DBS of the shell produced local activation and antidromic stimulation of the infralimbic prefrontal cortex
The results summarized above are not consistent with DBS attenuating the reinstatement of cocaine seeking by either suppressing local (within the nucleus accumbens) neuronal activity or inhibiting fibers of passage in the accumbens shell. It has been shown that DBS activates afferent axons (Gradinaru et al., 2009). Specifically, DBS of the accumbens shell produces antidromic stimulation of afferent structures like the mPFC (McCracken and Grace, 2007, 2009). To examine the activating effects of DBS, we used immunohistochemistry to determine the levels of the immediate early gene c-fos following DBS. Animals were implanted with bilateral electrodes aimed at the nucleus accumbens shell and were administered either 0 or 150 μA of stimulation for 30 min (n = 5). c-Fos immunoreactivity was subsequently examined in a number of relevant brain regions. These data were analyzed with a mixed-factors ANOVA (repeated measures over brain region), which revealed significant main effects of DBS (F(1,8) = 7.748, p < 0.0238) and brain region (F(4,32) = 11.45, p < 0.0001), as well as a significant interaction between these factors (F(4,32) = 6.957, p < 0.0004). Subsequent pairwise analyses indicated significant differences between 0 or 150 μA stimulation in the accumbens shell and infralimbic cortex (Bonferroni's correction, p < 0.05). Thus, DBS of the nucleus accumbens shell induced c-Fos expression both at the site of stimulation (locally) and in the infralimbic subregion of the mPFC, but did not induce c-Fos expression in the prelimbic cortex, dorsal striatum, ventral pallidum (Fig. 3), hippocampus, or dorsal raphe nucleus (data not shown). We also examined the influence of the same DBS stimulation of the nucleus accumbens core. Results indicated that core DBS produced significant c-Fos immunoreactivity in the core (mean ± SEM: 0 μA = 5.66 ± 3.18; 150 μA = 103.5 ± 10.12; t(5) = 7.976, p < 0.005) but had no significant effect in the infralimbic cortex, prelimbic cortex, dorsolateral striatum, or ventral pallidum. These results indicate that DBS of the shell and core produce very similar effects in the stimulated region but drastically different circuit-wide influences, which likely accounts for the divergent effects of core and shell DBS on the reinstatement of cocaine seeking.
Baclofen and muscimol microinjected into the mPFC attenuated priming-induced reinstatement of cocaine seeking
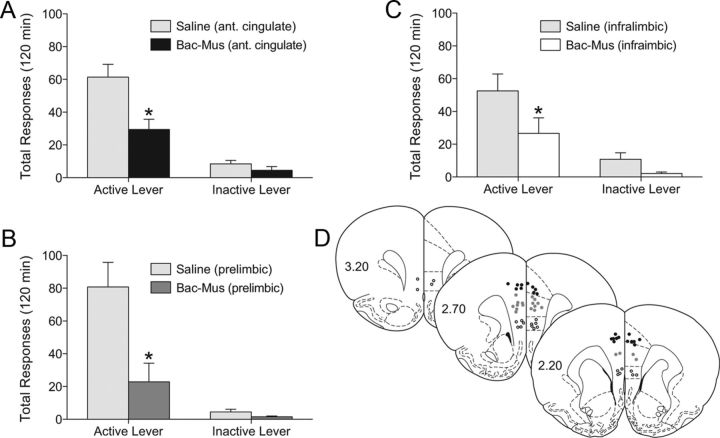
Our c-Fos data agree with recent electrophysiological findings indicating that DBS of the shell produces antidromic stimulation that ultimately activates inhibitory interneurons in afferent cortical structures (McCracken and Grace, 2007, 2009). To examine this hypothesis, we again used microinjections of baclofen and muscimol to inhibit the three subregions of the mPFC (i.e., anterior cingulate, and prelimbic and infralimbic cortices). Our results, shown in Figure 4, revealed that baclofen and muscimol administration into each of these cortical subregions attenuated the reinstatement of cocaine seeking. Each dataset was analyzed with a mixed-factors ANOVA (repeated measures over lever response). The results of the cingulate cortex analyses showed a significant main effect of treatment (F(1,18) = 9.169; p < 0.0072) and lever response (F(1,18) = 62.91; p < 0.0001), as well as a significant treatment × lever response interaction (F(1,18) = 8.161; p < 0.0105). Bonferroni post-tests revealed a significant active lever responding difference between treatments (p < 0.001). There were 11 saline values and 9 baclofen-muscimol data points. In the prelimbic cortex, there was a significant main effect of treatment (F(1,17) = 9.962; p < 0.0058) and lever response (F(1,17) = 29.58; p < 0.0001), and a significant interaction between these factors (F(1,17) = 9.345; p < 0.0071). Pairwise comparisons showed a significant difference in active lever responding between the baclofen-muscimol and saline treatments (Bonferroni's correction, p < 0.001). There were 9–10 animals per treatment. In the infralimbic cortex, there was a marginally significant main effect of treatment (F(1,20) = 4.298; p < 0.0513), a significant main effect of lever response (F(1,20) = 29.37; p < 0.0001), and no significant interaction between these factors (n = 11). Nonetheless, planned comparisons revealed a significant difference between treatments in terms of active lever responding (Bonferroni's correction, p < 0.05).
Figure 4.
Baclofen and muscimol microinjected into the infralimbic, prelimbic, and cingulate cortex attenuates cocaine priming-induced reinstatement. A–C, Mean (±SEM) active and inactive lever responding from a reinstatement session in which baclofen and muscimol or saline was microinjected 10 min before a 10 mg/kg cocaine priming injection into the cingulate cortex (A), the prelimbic cortex (B), or the infralimbic cortex (C). D, Cannulae placements from infrlimbic (open circles), prelimbic (gray circles), and cingulate (black circles). The values are in mm relative to bregma. *p < 0.05 Baclofen and muscimol compared to saline microinjections. There were 9–11 animals per group.
Discussion
The present results showed that DBS of the shell, but not the core, of the nucleus accumbens attenuated cocaine priming-induced reinstatement of drug seeking. This effect does not appear to be due to inactivation of the target nuclei or fibers of passage since GABA agonists or a sodium channel blocker microinjected into the accumbens shell did not mimic the effects of DBS. Our results also revealed that intrashell DBS increased c-Fos immunoreactivity at both the site of stimulation (locally) as well as in the infralimbic prefrontal cortex, which indicates that DBS is producing antidromic activation of afferent structures. Consistent with the hypothesis that accumbens DBS activates inhibitory interneurons in afferent structures antidromically (McCracken and Grace, 2007, 2009), GABA agonists microinjected into the subregions of the prefrontal cortex attenuated the reinstatement of cocaine seeking.
Pharmacological inactivation of accumbens subregions has differential effects on the reinstatement of cocaine seeking
We previously showed that DBS of the shell, but not the dorsal striatum, attenuated the reinstatement of cocaine seeking (Vassoler et al., 2008). The current findings replicate the effect of DBS in the shell and show further anatomical specificity in that DBS of the other major accumbens subregion, the core, had no effect on cocaine reinstatement. In contrast, administration of a cocktail of GABA agonists or lidocaine into the core, but not the shell, reduced cocaine reinstatement. These results suggest that the effect of shell DBS on cocaine reinstatement is not due to inactivation of presynaptic or postsynaptic neuronal transmission induced by GABA receptor agonists or lidocaine. Moreover, since lidocaine inhibits neuronal activity in axons of passage, this effect also cannot account for the influence of shell DBS on the reinstatement of cocaine seeking.
These baclofen/muscimol and lidocaine data are similar to the results of comparable experiments and suggest that the core, but not the shell, is the critical subregion involved in modulating priming-induced reinstatement of cocaine seeking (McFarland and Kalivas, 2001). However, when specific neurotransmitter systems are targeted, different effects have been observed. For example, administration of dopamine receptor agonists into the shell, but not the core, reinstate cocaine seeking (Schmidt and Pierce, 2006; Schmidt et al., 2006). Administration of D1-like or D2-like dopamine receptor antagonists into the shell, and not the core, attenuated cocaine reinstatement (Anderson et al., 2003, 2006). Together, these findings indicate that caution should be used when drawing definitive conclusions regarding the role of brain region in a specific behavior based on a single pharmacological manipulation.
Mechanism of DBS
Although some results indicate that DBS inhibits neuronal activity via depolarization blockade and/or activation of inhibitory neurons (Boraud et al., 1996; Benazzouz and Hallett, 2000; Kiss et al., 2002), the present results suggest that local inhibitory effects are unlikely to underlie the influence of accumbens DBS on cocaine reinstatement. Other credible findings indicate that DBS produces local neuronal activation (McIntyre et al., 2004; Montgomery and Gale, 2008). Consistent with these findings, our results showed that shell DBS increased c-Fos immunoreactivity in this nucleus. However, previous work demonstrated that increasing neuronal activation in the nucleus accumbens promoted cocaine seeking (Cornish et al., 1999; Ping et al., 2008). Therefore, it seems unlikely that DBS-induced activation of the nucleus accumbens alone would attenuate the reinstatement of cocaine seeking.
A growing body of evidence indicates that the effects of DBS are much more complex than local excitation or inhibition. Indeed, it is becoming clear that DBS produces circuit-wide influences (Windels et al., 2000; Vitek, 2002; McCracken and Grace, 2007; Gradinaru et al., 2009). Specifically, electrophysiological results suggest that accumbens DBS inhibits spontaneous activity of cortico-accumbal glutamatergic neurons while also stimulating cortical interneurons, apparently via recurrent inhibition following antidromic stimulation (McCracken and Grace, 2007). The current results support this hypothesis in that shell DBS produced pronounced activation of the infralimbic cortex as measured by c-Fos immunoreactivity. Furthermore, pharmacological inactivation of the infralimbic cortex with GABA agonists attenuated cocaine priming-induced reinstatement of drug seeking. These c-Fos and behavioral data, collectively, are consistent with shell DBS influencing cocaine reinstatement by antidromically activating inhibitory interneurons in the prefrontal cortex, thereby normalizing addiction-related aberrant activity in the cortico-accumbal system.
The role of cortico-accumbal projections in the reinstatement of cocaine seeking
There is general agreement that activation of the prefrontal cortico-accumbal glutamatergic pathway plays a critical role in the reinstatement of cocaine seeking (Kalivas et al., 2005; Schmidt and Pierce, 2010). There are, however, conflicting views on the specific roles of the infralimbic cortex–shell and prelimbic cortex–core projections on cocaine seeking. Recent findings indicated that administration of baclofen/muscimol into the infralimbic cortex promoted cocaine seeking, and microinjection of AMPA into this nucleus attenuated the reinstatement of cocaine seeking (Peters et al., 2008). These and similar results suggested that the infralimbic cortex plays an important role in the consolidation of information related to the extinction of cocaine seeking (LaLumiere et al., 2010). These findings are the opposite of what was observed in the present report, which is perplexing since the behavioral paradigms were quite similar. Recent work demonstrated that activation of the glutamatergic pathway from the ventral mPFC to the accumbens shell promotes the reinstatement of heroin seeking (Bossert et al., 2012), which is consistent with the present results as well as results from experiments examining alcohol reinstatement (Willcocks and McNally, 2013).
It also was shown that baclofen/muscimol injected into the shell reinstated cocaine seeking (Peters et al., 2008). This finding is in direct disagreement with the current results as well as previous work indicating that administration of an AMPA receptor antagonist or suppression of AMPA receptor transcription in the accumbens shell attenuated the reinstatement of cocaine seeking (Famous et al., 2008; Ping et al., 2008), and manipulations that reduced AMPA receptor-mediated synaptic strength in the shell blocked cocaine seeking (Anderson et al., 2008; Famous et al., 2008). Moreover, activation of AMPA receptors in the shell promoted cocaine seeking in the absence of a cocaine priming injection (Ping et al., 2008). These conflicting results make it difficult to draw conclusions about the role of the infralimbic cortex–accumbens shell glutamatergic transmission in the reinstatement of cocaine seeking. However, recent findings with the cue-induced reinstatement paradigm suggest that the manner in which the shell processes information received from the infralimbic cortex is complex and critically dependent on the interplay of glutamate and other neurotransmitters, particularly dopamine (LaLumiere et al., 2012).
The results of experiments focusing on the prelimbic cortex–accumbens core glutamatergic pathway in the reinstatement of cocaine seeking are much more cohesive. Thus, previous work showed that inactivation of the prelimbic cortex and the accumbens core attenuated the reinstatement of cocaine seeking (McFarland and Kalivas, 2001; Capriles et al., 2003), which is consonant with the current results. Moreover, optogenetic inhibition of the prelimbic cortex or accumbens core impaired the reinstatement of cocaine seeking (Stefanik et al., 2013). The reinstatement of cocaine seeking also is associated with increased glutamate release in the accumbens core (McFarland et al., 2003) and enhanced surface expression of GluA2-lacking AMPA receptors in the core (Conrad et al., 2008; McCutcheon et al., 2011). Importantly, attenuation of AMPA-mediated transmission in the core attenuated the reinstatement of cocaine seeking (Conrad et al., 2008; Famous et al., 2008).
Summary and Conclusions
Clinical experiments are beginning to validate the safety and efficacy of DBS of the nucleus accumbens as a treatment for multiple forms of drug addiction (Mantione et al., 2010; Kuhn et al., 2011; Zhou et al., 2011). Importantly, clinical studies report that DBS of the nucleus accumbens is neither reinforcing nor aversive (Sturm et al., 2003; Kuhn et al., 2007a,b; Okun et al., 2007; Schlaepfer et al., 2008). We previously showed that shell DBS alone failed to promote drug seeking in rats, which suggests that DBS is unlikely to promote drug craving (Vassoler et al., 2008). We also found that DBS of the nucleus accumbens shell does not attenuate food-seeking behavior (Vassoler et al., 2008). These basic and clinical results indicate that DBS does not disrupt normal behavior, is not aversive, and does not cause a generalized disorganization of cognitive function. Although it is unclear exactly why DBS is reinforcer specific, it is likely that the neuronal circuits subserving cocaine- and food-seeking behaviors are at least partially segregated (Carelli et al., 2000; Horvath and Diano, 2004).
To optimize this therapeutic strategy, it is important to better understand the specific mechanisms whereby shell DBS modulates drug craving and intake. Although there is some disagreement over the specific roles of cortico-accumbal pathways, the preponderance of evidence indicates that increased activity in at least some pathways from the mPFC to the nucleus accumbens plays a critical role in promoting the reinstatement of drug seeking induced by a cocaine priming injection. The present work suggests that DBS of the accumbens shell attenuated the reinstatement of cocaine seeking by normalizing activity in the cortico-accumbal pathway. Specifically, the results reported here support the notion that shell DBS antidromically activates afferent fibers and stimulates GABAergic interneurons in the prefrontal cortex (McCracken and Grace, 2007).
Footnotes
This work was funded by National Institutes of Health Grants R01 DA33641, K02 DA18678 (RCP), T32- MH14654 (FMV), and MH087581 (to O.B. and J.E.).
References
- Anderson SM, Schmidt HD, Pierce RC. Administration of the D2 dopamine receptor antagonist sulpiride into the shell, but not the core, of the nucleus accumbens attenuates cocaine priming-induced reinstatement of drug seeking. Neuropsychopharmacology. 2006;31:1452–1461. doi: 10.1038/sj.npp.1300922. [DOI] [PubMed] [Google Scholar]
- Anderson SM, Bari AA, Pierce RC. Administration of the D1-like dopamine receptor antagonist SCH-23390 into the medial nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug-seeking behavior in rats. Psychopharmacology (Berl) 2003;168:132–138. doi: 10.1007/s00213-002-1298-5. [DOI] [PubMed] [Google Scholar]
- Anderson SM, Famous KR, Sadri-Vakili G, Kumaresan V, Schmidt HD, Bass CE, Terwilliger EF, Cha JH, Pierce RC. CaMKII: a biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nat Neurosci. 2008;11:344–353. doi: 10.1038/nn2054. [DOI] [PubMed] [Google Scholar]
- Anderson T, Hu B, Pittman Q, Kiss ZH. Mechanisms of deep brain stimulation: an intracellular study in rat thalamus. J Physiol. 2004;559:301–313. doi: 10.1113/jphysiol.2004.064998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benazzouz A, Hallett M. Mechanism of action of deep brain stimulation. Neurology. 2000;55(12 Suppl 6):S13–S16. [PubMed] [Google Scholar]
- Beurrier C, Bioulac B, Audin J, Hammond C. High-frequency stimulation produces a transient blockade of voltage-gated currents in subthalamic neurons. J Neurophysiol. 2001;85:1351–1356. doi: 10.1152/jn.2001.85.4.1351. [DOI] [PubMed] [Google Scholar]
- Boraud T, Bezard E, Bioulac B, Gross C. High frequency stimulation of the internal Globus Pallidus (GPi) simultaneously improves parkinsonian symptoms and reduces the firing frequency of GPi neurons in the MPTP-treated monkey. Neurosci Lett. 1996;215:17–20. doi: 10.1016/S0304-3940(96)12943-8. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Stern AL, Theberge FR, Marchant NJ, Wang HL, Morales M, Shaham Y. Role of projections from ventral medial prefrontal cortex to nucleus accumbens shell in context-induced reinstatement of heroin seeking. J Neurosci. 2012;32:4982–4991. doi: 10.1523/JNEUROSCI.0005-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capriles N, Rodaros D, Sorge RE, Stewart J. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2003;168:66–74. doi: 10.1007/s00213-002-1283-z. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Ijames SG, Crumling AJ. Evidence that separate neural circuits in the nucleus accumbens encode cocaine versus “natural” (water and food) reward. J Neurosci. 2000;20:4255–4266. doi: 10.1523/JNEUROSCI.20-11-04255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JY, Shi LH, Luo F, Woodward DJ. High frequency stimulation of the subthalamic nucleus improves treadmill locomotion in unilateral 6-hydroxydopamine lesioned rats. Brain Res. 2003;983:174–184. doi: 10.1016/S0006-8993(03)03053-1. [DOI] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JL, Duffy P, Kalivas PW. A role for nucleus accumbens glutamate transmission in the relapse to cocaine-seeking behavior. Neuroscience. 1999;93:1359–1367. doi: 10.1016/S0306-4522(99)00214-6. [DOI] [PubMed] [Google Scholar]
- Famous KR, Kumaresan V, Sadri-Vakili G, Schmidt HD, Mierke DF, Cha JH, Pierce RC. Phosphorylation-dependent trafficking of GluR2-containing AMPA receptors in the nucleus accumbens plays a critical role in the reinstatement of cocaine seeking. J Neurosci. 2008;28:11061–11070. doi: 10.1523/JNEUROSCI.1221-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science. 2009;324:354–359. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer L, Alheid GF, de Olmos JS, Groenewegen HJ, Haber SN, Harlan RE, Zahm DS. The accumbens: beyond the core-shell dichotomy. J Neuropsychiatry Clin Neurosci. 1997;9:354–381. doi: 10.1176/jnp.9.3.354. [DOI] [PubMed] [Google Scholar]
- Henderson MB, Green AI, Bradford PS, Chau DT, Roberts DW, Leiter JC. Deep brain stimulation of the nucleus accumbens reduces alcohol intake in alcohol-preferring rats. Neurosurg Focus. 2010;29:E12. doi: 10.3171/2010.4.FOCUS10105. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Diano S. The floating blueprint of hypothalamic feeding circuits. Nat Rev Neurosci. 2004;5:662–667. doi: 10.1038/nrn1479. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB. Dissociable effects of lidocaine inactivation of the rostral and caudal basolateral amygdala on the maintenance and reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2002;22:1126–1136. doi: 10.1523/JNEUROSCI.22-03-01126.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantak KM, Mashhoon Y, Silverman DN, Janes AC, Goodrich CM. Role of the orbitofrontal cortex and dorsal striatum in regulating the dose-related effects of self-administered cocaine. Behav Brain Res. 2009;201:128–136. doi: 10.1016/j.bbr.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss ZH, Mooney DM, Renaud L, Hu B. Neuronal response to local electrical stimulation in rat thalamus: physiological implications for mechanisms of deep brain stimulation. Neuroscience. 2002;113:137–143. doi: 10.1016/S0306-4522(02)00122-7. [DOI] [PubMed] [Google Scholar]
- Knapp CM, Tozier L, Pak A, Ciraulo DA, Kornetsky C. Deep brain stimulation of the nucleus accumbens reduces ethanol consumption in rats. Pharmacol Biochem Behav. 2009;92:474–479. doi: 10.1016/j.pbb.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koya E, Uejima JL, Wihbey KA, Bossert JM, Hope BT, Shaham Y. Role of ventral medial prefrontal cortex in incubation of cocaine craving. Neuropharmacology. 2009;56(Suppl 1):177–185. doi: 10.1016/j.neuropharm.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn J, Lenartz D, Mai JK, Huff W, Lee SH, Koulousakis A, Klosterkoetter J, Sturm V. Deep brain stimulation of the nucleus accumbens and the internal capsule in therapeutically refractory Tourette-syndrome. J Neurol. 2007a;254:963–965. doi: 10.1007/s00415-006-0404-8. [DOI] [PubMed] [Google Scholar]
- Kuhn J, Lenartz D, Huff W, Lee S, Koulousakis A, Klosterkoetter J, Sturm V. Remission of alcohol dependency following deep brain stimulation of the nucleus accumbens: valuable therapeutic implications? J Neurol Neurosurg Psychiatry. 2007b;78:1152–1153. doi: 10.1136/jnnp.2006.113092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn J, Gründler TO, Bauer R, Huff W, Fischer AG, Lenartz D, Maarouf M, Bührle C, Klosterkötter J, Ullsperger M, Sturm V. Successful deep brain stimulation of the nucleus accumbens in severe alcohol dependence is associated with changed performance monitoring. Addict Biol. 2011;16:620–623. doi: 10.1111/j.1369-1600.2011.00337.x. [DOI] [PubMed] [Google Scholar]
- LaLumiere RT, Niehoff KE, Kalivas PW. The infralimbic cortex regulates the consolidation of extinction after cocaine self-administration. Learn Mem. 2010;17:168–175. doi: 10.1101/lm.1576810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Smith KC, Kalivas PW. Neural circuit competition in cocaine-seeking: roles of the infralimbic cortex and nucleus accumbens shell. Eur J Neurosci. 2012;35:614–622. doi: 10.1111/j.1460-9568.2012.07991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HY, Jin J, Tang JS, Sun WX, Jia H, Yang XP, Cui JM, Wang CG. Chronic deep brain stimulation in the rat nucleus accumbens and its effect on morphine reinforcement. Addict Biol. 2008;13:40–46. doi: 10.1111/j.1369-1600.2007.00088.x. [DOI] [PubMed] [Google Scholar]
- Mantione M, van de Brink W, Schuurman PR, Denys D. Smoking cessation and weight loss after chronic deep brain stimulation of the nucleus accumbens: therapeutic and research implications: case report. Neurosurgery. 2010;66:E218. doi: 10.1227/01.NEU.0000360570.40339.64. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- McCracken CB, Grace AA. High-frequency deep brain stimulation of the nucleus accumbens region suppresses neuronal activity and selectively modulates afferent drive in rat orbitofrontal cortex in vivo. J Neurosci. 2007;27:12601–12610. doi: 10.1523/JNEUROSCI.3750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken CB, Grace AA. Nucleus accumbens deep brain stimulation produces region-specific alterations in local field potential oscillations and evoked responses in vivo. J Neurosci. 2009;29:5354–5363. doi: 10.1523/JNEUROSCI.0131-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, Wang X, Tseng KY, Wolf ME, Marinelli M. Calcium-permeable AMPA receptors are present in nucleus accumbens synapses after prolonged withdrawal from cocaine self-administration but not experimenter-administered cocaine. J Neurosci. 2011;31:5737–5743. doi: 10.1523/JNEUROSCI.0350-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre CC, Savasta M, Kerkerian-Le Goff L, Vitek JL. Uncovering the mechanism(s) of action of deep brain stimulation: activation, inhibition, or both. Clin Neurophysiol. 2004;115:1239–1248. doi: 10.1016/j.clinph.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Montgomery EB, Jr, Gale JT. Mechanisms of action of deep brain stimulation(DBS) Neurosci Biobehav Rev. 2008;32:388–407. doi: 10.1016/j.neubiorev.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Nowak LG, Bullier J. Axons, but not cell bodies, are activated by electrical stimulation in cortical gray matter. II. Evidence from selective inactivation of cell bodies and axon initial segments. Exp Brain Res. 1998;118:489–500. doi: 10.1007/s002210050305. [DOI] [PubMed] [Google Scholar]
- Okun MS, Mann G, Foote KD, Shapira NA, Bowers D, Springer U, Knight W, Martin P, Goodman WK. Deep brain stimulation in the internal capsule and nucleus accumbens region: responses observed during active and sham programming. J Neurol Neurosurg Psychiatry. 2007;78:310–314. doi: 10.1136/jnnp.2006.095315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park WK, Bari AA, Jey AR, Anderson SM, Spealman RD, Rowlett JK, Pierce RC. Cocaine administered into the medial prefrontal cortex reinstates cocaine-seeking behavior by increasing AMPA receptor-mediated glutamate transmission in the nucleus accumbens. J Neurosci. 2002;22:2916–2925. doi: 10.1523/JNEUROSCI.22-07-02916.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York: Academic; 1997. [DOI] [PubMed] [Google Scholar]
- Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci. 2008;28:6046–6053. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping A, Xi J, Prasad BM, Wang MH, Kruzich PJ. Contributions of nucleus accumbens core and shell GluR1 containing AMPA receptors in AMPA- and cocaine-primed reinstatement of cocaine-seeking behavior. Brain Res. 2008;1215:173–182. doi: 10.1016/j.brainres.2008.03.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, Joe AY, Kreft M, Lenartz D, Sturm V. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008;33:368–377. doi: 10.1038/sj.npp.1301408. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Pierce RC. Cooperative activation of D1-like and D2-like dopamine receptors in the nucleus accumbens shell is required for the reinstatement of cocaine-seeking behavior in the rat. Neuroscience. 2006;142:451–461. doi: 10.1016/j.neuroscience.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Pierce RC. Cocaine-induced neuroadaptations in glutamate transmission: potential therapeutic targets for craving and addiction. Ann N Y Acad Sci. 2010;1187:35–75. doi: 10.1111/j.1749-6632.2009.05144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HD, Anderson SM, Pierce RC. Stimulation of D1-like or D2 dopamine receptors in the shell, but not the core, of the nucleus accumbens reinstates cocaine-seeking behaviour in the rat. Eur J Neurosci. 2006;23:219–228. doi: 10.1111/j.1460-9568.2005.04524.x. [DOI] [PubMed] [Google Scholar]
- Stefanik MT, Moussawi K, Kupchik YM, Smith KC, Miller RL, Huff ML, Deisseroth K, Kalivas PW, LaLumiere RT. Optogenetic inhibition of cocaine seeking in rats. Addict Biol. 2013;18:50–53. doi: 10.1111/j.1369-1600.2012.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm V, Lenartz D, Koulousakis A, Treuer H, Herholz K, Klein JC, Klosterkötter J. The nucleus accumbens: a target for deep brain stimulation in obsessive-compulsive- and anxiety-disorders. J Chem Neuroanat. 2003;26:293–299. doi: 10.1016/j.jchemneu.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Taverna S, van Dongen YC, Groenewegen HJ, Pennartz CM. Direct physiological evidence for synaptic connectivity between medium-sized spiny neurons in rat nucleus accumbens in situ. J Neurophysiol. 2004;91:1111–1121. doi: 10.1152/jn.00892.2003. [DOI] [PubMed] [Google Scholar]
- Vassoler FM, Schmidt HD, Gerard ME, Famous KR, Ciraulo DA, Kornetsky C, Knapp CM, Pierce RC. Deep brain stimulation of the nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug seeking in rats. J Neurosci. 2008;28:8735–8739. doi: 10.1523/JNEUROSCI.5277-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitek JL. Mechanisms of deep brain stimulation: excitation or inhibition. Mov Disord. 2002;17(Suppl 3):S69–S72. doi: 10.1002/mds.10144. [DOI] [PubMed] [Google Scholar]
- Willcocks AL, McNally GP. The role of medial prefrontal cortex in extinction and reinstatement of alcohol-seeking in rats. Eur J Neurosci. 2013;37:259–268. doi: 10.1111/ejn.12031. [DOI] [PubMed] [Google Scholar]
- Windels F, Bruet N, Poupard A, Urbain N, Chouvet G, Feuerstein C, Savasta M. Effects of high frequency stimulation of subthalamic nucleus on extracellular glutamate and GABA in substantia nigra and globus pallidus in the normal rat. Eur J Neurosci. 2000;12:4141–4146. doi: 10.1046/j.1460-9568.2000.00296.x. [DOI] [PubMed] [Google Scholar]
- Zahm DS. An integrative neuroanatomical perspective on some subcortical substrates of adaptive responding with emphasis on the nucleus accumbens. Neurosci Biobehav Rev. 2000;24:85–105. doi: 10.1016/S0149-7634(99)00065-2. [DOI] [PubMed] [Google Scholar]
- Zhou H, Xu J, Jiang J. Deep brain stimulation of nucleus accumbens on heroin-seeking behaviors: a case report. Biol Psychiatry. 2011;69:e41–e42. doi: 10.1016/j.biopsych.2011.02.012. [DOI] [PubMed] [Google Scholar]