Abstract
AIM: To analyze phospholipid profiles in intrahepatic bile from patients with primary sclerosing cholangitis (PSC) and secondary sclerosing cholangitis (SSC).
METHODS: Intrahepatic bile specimens collected via endoscopic retrograde cholangiography from 41 patients were analyzed. Fourteen of these patients were diagnosed with PSC, 10 with SSC, 11 with choledocholithiasis or no identifiable biliary disease, and 6 with cholangiocellular carcinoma (CCC). Bile acid, cholesterol, protein, and bilirubin contents as well as pancreas lipase activity in bile were determined by biochemical methods. Phosphatidylcholine (PC) and lysophosphatidylcholine (LPC) species were quantified using nano-electrospray ionization tandem mass spectrometry.
RESULTS: Bile from all the examined patient groups showed a remarkably similar PC and LPC species composition, with only minor statistical differences. Total biliary PC concentrations were highest in controls (8030 ± 1843 μmol/L) and lowest in patients with CCC (1969 ± 981 μmol/L) (P = 0.005, controls vs SSC and CCC, respectively, P < 0.05). LPC contents in bile were overall low (4.2% ± 1.8%). Biliary LPC/PC ratios and ratios of biliary PC to bilirubin, PC to cholesterol, PC to protein, and PC to bile acids showed no intergroup differences.
CONCLUSION: PC and LPC profiles being similar in patients with or without sclerosing cholangitis, these phospholipids are likely not of major pathogenetic importance in this disease group.
Keywords: Primary sclerosing cholangitis, Secondary sclerosing cholangitis, Cholangiocellular carcinoma, Phosphatidylcholine, Lysophosphatidylcholine, Bile, Mass spectrometry
Core tip: Based on the idea that unfavorable alterations of biliary phospholipids might play a role in the pathogenesis of sclerosing cholangitis, phosphatidylcholine (PC) and lysophosphatidylcholine (LPC) species profiles were analyzed in endoscopically-acquired intrahepatic bile using nano-electrospray ionization tandem mass spectrometry. The examination of specimens from 14 patients with primary sclerosing cholangitis, 10 patients with secondary sclerosing cholangitis, 11 patients with choledocholithiasis/no biliary disease and 6 patients with cholangiocellular carcinoma revealed strikingly similar PC and LPC species patterns, implicating at the most a minor role of biliary phospholipid changes in sclerosing cholangitis.
INTRODUCTION
Primary sclerosing cholangitis (PSC) is a chronic cholestatic liver disease with fibroobliterative sclerosis of intra- and/or extrahepatic bile ducts, eventually leading to biliary cirrhosis[1,2]. The etiopathogenesis of the disease is not yet completely understood[3].
Secondary sclerosing cholangitis (SSC) also belongs to the group of chronic sclerosing cholangitis. SSC is thought to develop as a consequence of known injuries or secondary to pathological processes of the biliary tree[4]. The mechanisms leading to cholangiopathy in critically ill patients are mostly unknown; however, the available clinical data indicate that ischemic injury to the intrahepatic biliary tree may be one of the earliest events responsible for the development of this severe form of sclerosing cholangitis. Therapeutic options for most forms of SSC are limited, and patients with SSC who do not undergo transplantation have significantly reduced survival compared to those with PSC. Sclerosing cholangitis in critically ill patients, in particular, is associated with rapid disease progression and poor outcome[4,5]. PSC and SSC can be treated successfully only by liver transplantation.
Genetic or chemical modifications of bile composition have been found to induce sclerosing cholangitis and liver fibrosis in a number of animal models, which gave rise to the “toxic bile” concept[6]. Bile contains various biochemical components whose alterations could lead to an imbalance between its protective and harmful effects, thus leading to chronic inflammation and, finally, to the destruction of small and large bile ducts. These alterations could be primary or secondary to inflammatory processes of different origins.
Phospholipids are an essential ingredient of bile. They represent one of its major lipid components besides cholesterol and bile salts. Among bile phospholipids, there are mostly mixed diacylphosphatidylcholines. They have a hydrophilic, zwitter-ionic phosphocholine head group and two hydrophobic fatty acid side chains[7]. Phospholipids are considered to potently emulsify hydrophobic molecules, such as certain bile acids, and thereby attenuate their toxicity[8]. If it can be shown that they are unfavorably altered in concentration or species composition, then the “toxic bile” concept could be considered relevant in the pathogenesis of sclerosing cholangitis, especially the one of PSC.
To date, no reports have been published on the comparison of the biliary phospholipid composition in PSC patients, SSC patients, and controls. The present study was aimed at determining their potential differences, especially concerning the phosphatidylcholine (PC) species composition in bile, which could help obtain further insight into the pathogenesis of sclerosing cholangitis and might be useful as a diagnostic tool for easier differentiation between biliary diseases of various origins. The hypothesis that alterations in the phospholipid composition of bile are involved in the pathogenesis of sclerosing cholangitis is supported by the fact that mice with targeted disruption of the Mdr2 (Abcb4) gene, which encodes canalicular phospholipid flippase, spontaneously develop cholangitis and typical onion-skin-type periductal fibrosis, which mirrors some of the key features of human PSC[1,9,10]. However, the composition of bile in PSC patients without elevated serum bilirubin has been shown to be normal[11]. Furthermore, the role of MDR3 variants in the pathogenesis of PSC in humans is not yet clear[12]. Our group showed a reduced PC/bile acid ratio in bile from a patient suffering from inborn chronic cholestatic liver disease with fibrosis. He had a homozygous missense mutation of Abcb4 encoding MDR3[13]. These findings still suggest that changes in biliary lipid composition and total concentrations could play an important role in the pathogenesis of PSC and maybe SSC, which seem to differ in etiological factors and pathogenesis, but have a similar phenotype, although the latter, being recently identified, has been scarcely described. Also to be mentioned in this context are previous publications dealing with potential protective effects of phospholipids, especially glycerophospholipids, in other liver diseases[14]. For example, it was shown that alcohol-induced hepatic fibrosis could be alleviated by polyunsaturated lecithin[14,15].
In the light of the above-mentioned data and theories, we focused on a systematic electrospray mass spectrometric analysis of bile phospholipids in two types of sclerosing cholangitis and compared them to the data from specimens of patients with choledocholithiasis or malignant biliary disease. If the bile phospholipid composition was involved in the pathogenesis of sclerosing cholangitis, one would expect differences in phospholipid concentrations and/or species patterns between bile from patients with or without sclerosing cholangitis. The evaluation of the ratios of PC concentrations to bile acid as well as lysophosphatidylcholine (LPC) concentrations are particularly interesting in that respect, since certain bile acids and LPC are thought to have cytotoxic properties[16] which might be alleviated by PC.
MATERIALS AND METHODS
Hepatic bile specimens were collected from the following four groups of patients: controls, PSC patients, SSC patients, and patients with cholangiocellular carcinoma (CCC). Clinical data originated from a data base predominantly set up for the collection of samples from PSC patients. As the sample collection is also used for other studies, only specimens with sufficient amounts of material left could be used. A maximum number of 14 patients per group was predefined. Fourteen PSC patients were randomly chosen from the sample bank by a technical assistant who was not involved in phospholipid measurements. For the other groups, less than 14 samples in every group were available. Clinical data were extracted from the database, which had been set up prospectively for a wide array of research projects. The clinical characteristics of the included patient groups are presented separately in Table 1. All procedures in this study were compliant with the Declaration of Helsinki and approved by the local ethics committee. All patients had provided written informed consent before their specimens and data were included in the database. Bile samples were collected during endoscopic retrograde cholangiography (ERC) at the Department of Endoscopy at the University Hospital Heidelberg between 2007 and 2012. For all groups, the serum albumin level, as a parameter of liver synthesis function, was within the normal range.
Table 1.
Clinical characteristics of the four included patient groups
| Controls (n = 11) | PSC (n = 14) | SSC (n = 10) | CCC (n = 6) with PSC (n = 2) | P value | |
| Gender (M/F) | 9/2 | 10/4 | 10/0 | 3/3 | |
| Age at ERC (yr) | 52.8 ± 6.6 | 41.1 ± 2.3 | 52.1 ± 4.0 | 64.5 ± 5.2 | 0.02; PSCa |
| Serum albumin level (g/L) | 41.5 ± 1.5 | 39.2 ± 1.9 | 37.6 ± 3.1 | 34.5 ± 1.4 | 0.13 |
| (ND in 3) | (ND in 5) | ||||
| Serum AP level (U/L) | 221.3 ± 99.9 | 271.5 ± 37.1 | 1004 ± 304 | 357 ± 62.4 | 0.003; controlsd |
| (ND in 1) | (ND in 3) | ||||
| Serum bilirubin level (mg/dL) | 1.6 ± 0.5 | 2.7 ± 0.8 | 5.3 ± 2.3 | 4.9 ± 1.9 | 0.39 |
| (ND in 1) | (ND in 2) | ||||
| Sterile bile or scarce bacterial growth/moderate or abundant bacterial growth | 6/5 | 9/5 | 5/5 | 4/1 (ND in 1) | |
| Intake of UDCA (yes/no) | 0/11 | 13/1 | 6/2 | 3/3 | |
| (2 unknown) | |||||
| Dominant bile duct stenosis (yes/no) | 0/11 | 4/10 | 1/9 | 6/0 | |
| Choledocholithiasis and/or sludge (yes/no) | 9/2 | 0/14 | 2/8 | 0/6 | |
| Diagnosis of inflammatory bowel disease (yes/no) | 0/11 | 10/4 | 0/10 | 2/4 |
M: Male; F: Female; PSC: Primary sclerosing cholangitis; AP: Alkaline phosphatase; ERC: Endoscopic retrograde cholangiography; UDCA: Ursodeoxycholic acid; ND: Not done.
P < 0.05 vs Cholangiocellular carcinoma (CCC);
P < 0.01 vs Secondary sclerosing cholangitis (SSC).
Control group
The control group (11 patients in all; two women and nine men; aged 24 to 81 years) comprised nine patients with choledocholithiasis without signs of relevant cholangitis when ERC was performed. The remaining two patients had undergone ERC for unexplained elevation of serum alkaline phosphatase (AP) levels; one of them had recurrent right side abdominal pain. ERC findings in both of these patients were completely normal.
Patients with PSC
PSC was diagnosed on the basis of typical ERC findings. Of the 14 patients with PSC (four women, ten men; aged 25 to 55 years), four had no inflammatory bowel disease, nine had ulcerative colitis, and one suffered from Crohn’s disease. The mean disease duration of PSC at sample acquisition was 8.9 ± 1.4 years. Four of the patients underwent endoscopic dilation for dominant stenosis of a major bile duct during the ERC. All the PSC patients received ursodeoxycholic acid (UDCA) at doses between 1000 and 1500 mg per day, except one patient who had to discontinue the drug due to adverse effects. More detailed information is provided in Table 1.
Patients with SSC
Patients who had cholestatic liver disease with the ERC morphology of SSC without evidence of pre-existing hepatobiliary disease and who had previously required long-term treatment in an intensive care unit were included. Polytrauma and sepsis were the main reasons for long-term intensive care treatment among the patients. Ten patients (all male; aged 35 to 70 years) were included. The mean disease duration since first diagnosis of SSC was less than one year. None of these patients suffered from inflammatory bowel disease. Two of the patients did not take UDCA; six were on UDCA at daily doses between 500 and 1500 mg, and for two patients, the history of medication was unknown. Further clinical data are indicated in Table 1.
Patients with CCC
All patients in this group were diagnosed with CCC on the basis of morphological and histological findings. They all had relevant stenosis of the common bile duct and had visited the hospital for a change in or the insertion of a bile duct endoprosthesis. Of the eight patients who were initially to be included, two had to be excluded since their bile was colorless and phospholipids were below the limit of detection. This could be explained by massive cholestasis in these two patients. Two of the six patients who were finally included had PSC-related CCC, and four had CCC unrelated to PSC. All the patients with PSC-related CCC and one of the other patients were on UDCA at daily doses between 750 and 1500 mg (Table 1).
Collection and storage of bile specimens
For endoscopic collection of bile specimens, the papilla of Vater was selectively cannulated. Bile samples were obtained by suction and, if possible, before injection of contrast medium and any therapeutic procedure. In patients with whom bile collection was not possible before injection of contrast medium into the bile duct, a volume equivalent to that of the contrast medium was first extracted by suction into a syringe to be discarded before the syringe for the actual bile specimen was attached. This was performed in order to minimize effects of dilution. All specimens were snap-frozen in liquid nitrogen and stored at -80 °C before further use. Bile specimens from 41 patients were included in this study (see above).
Determination of levels of pancreas lipase activity, protein, cholesterol, bile acids, and bilirubin in bile
As an indicator of the amount of refluxing pancreatic juice in the bile specimens, pancreas lipase activity was determined photometrically by using the chromogenic lipase substrate DGGMR (1,2-O-dilauryl-rac-glycero-glutaric acid ester)[17]. Total protein concentrations in the bile were determined using the 2-D-Quant kit (Amersham Biosciences, Amersham, United Kingdom). Bile cholesterol levels were determined by the CHOD-PAP enzymatic photometric test (“Cholesterol FS*”, Diagnostic Systems International GmbH, Holzheim, Germany)[18]. Total bile salt concentrations were measured spectrophotometrically by using 3α-hydroxysteroid dehydrogenase[19]. Biliary bilirubin concentration was determined using the Jendrassik-Grof method[20].
Bacterial cultures
Aliquots of fresh bile specimens were sent to our Department of Microbiology directly after acquisition for aerobic and anaerobic bacterial cultures. Bacterial growth was semiquantitatively described as non-existent, scarce, moderate, or abundant.
Lipid extraction from bile specimens
Extraction of lipids from bile specimens was performed according to Folch[21]. One-microliter aliquots of bile were diluted in 75 μL distilled water each. Four non-physiological PC and two non-physiological LPC standards were added before extraction. Both these phospholipid classes constitute more than 95% of biliary phospholipids[7], and no additional phospholipid standards were used. Crude lipid extracts were completely dried. For mass spectrometry, each sample was redissolved in 50 μL of methanol/chloroform 2:1 (v/v). Table 2 provides detailed information about the phospholipid standards used. It includes molecular weights (Da), numbers of carbon atoms and double bonds of fatty acids, names of the molecules as well as their structures and the companies where they were purchased.
Table 2.
Phospholipids used as internal standards to determine phospholipid concentrations in bile
| Fatty acid(s) | Molecular structure name | Provider | |
| LPC standards | |||
| 482 Da | 15:00 | 1-pentadecanoyl-2-hydroxy-sn-glycero-3-PC | Avanti (Alabaster, AL, United States) |
| 552 Da | 20:00 | 1-arachidoyl-2-hydroxy-sn-glycero-3-PC | Avanti (Alabaster, AL, United States) |
| PC standards | |||
| 622 Da | 12:0/12:0 | 1, 2-didodecanoyl-sn-glycero-3-PC | Sigma (Deisenhofen, Germany) |
| 678 Da | 14:0/14:0 | 1, 2-tetradecanoyl-sn-glycero-3-PC | Sigma (Deisenhofen, Germany) |
| 846 Da | 20:0/20:0 | 1, 2-dieicosanoyl-sn-glycero-3-PC | Sigma (Deisenhofen, Germany) |
| 902 Da | 22:0/22:0 | 1, 2-didocosanoyl-sn-glycero-3-PC | Sigma (Deisenhofen, Germany) |
PC: Phosphatidylcholine.
Nano-electrospray ionization tandem mass spectrometry
Mass spectrometry (MS) analyses were performed using a triple quadrupole instrument (Finnegan MAT, San Jose, CA, model TSQ 7000) with a nano-electrospray source operating at a typical flow rate of 20-50 nL/min. The electrospray capillary was positioned at a distance of 0.5-1 mm from the orifice of the heated transfer capillary (140 °C). Argon was used as the collision gas (2 mTorr). Lipid extracts were infused into the heated capillary. The mass spectrometric resolution was set to the approximate nominal mass resolution for the scan range of an m/z of 400-1000. All specimens were analyzed in the precursor ion-scan mode for an m/z of 184. At least 100 consecutive scans of four seconds each were averaged for every measurement. After comparison of all spectra, the most abundant physiological PC and LPC species were identified. For the quantification of physiological phospholipid species, regression curves were determined from the non-physiological standards as per the method described by Brügger et al[22]. A typical spectrum from a PSC patient is presented in Figure 1. Total amounts of PC and LPC were calculated by addition of all single species.
Figure 1.
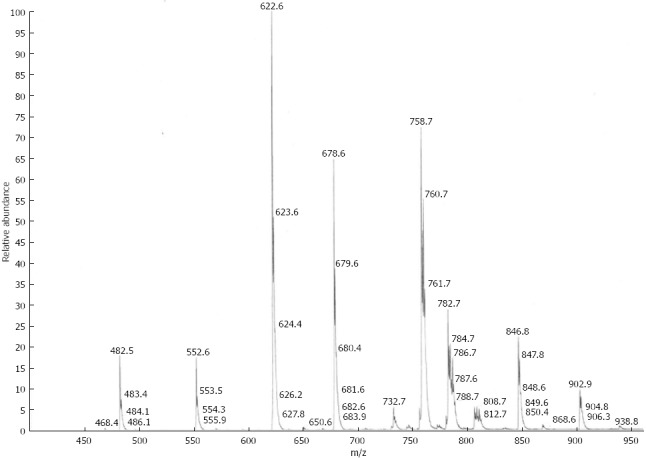
Electrospray mass spectrum of the total lipid extract from human bile of a patient with primary sclerosing cholangitis. For explanation of standards view Table 2.
Statistical analysis
The results have been expressed in terms of mean and standard error of the mean (SE) values. Due to small sample sizes, no assumptions of normality were made, and non-parametric tests (Kruskal-Wallis test and Dunn’s post-test) were used to compare disease groups. P values of < 0.05 were considered to be statistically significant. All statistical analyses were performed using GraphPad Prism 3.0 (GraphPad Inc., CA).
RESULTS
Clinical data
Statistical differences in the clinical data of the four patient groups were noted for serum AP levels at the time of ERC and for patient age (Table 1). Serum bilirubin concentrations, however, did not differ significantly between the groups.
Biliary phospholipid analysis
The amounts of total phosphatidylcholine (PC) in relation to hepatic bile volume were compared between the four groups of patients (Table 3). They ranged between 47 and 22570 μmol/L (median 2977 μmol/L). The highest biliary PC concentrations were noted in the controls (8030 ± 1843 μmol/L), the lowest ones in SSC patients (2501 ± 790 μmol/L) and CCC patients (1969 ± 981 μmol/L) (overall P = 0.05, controls vs CCC and controls vs SSC, P < 0.05, respectively). In PSC patients, we found intermediate concentrations (6205 ± 1465 μmol/L).
Table 3.
Results of biochemical bile analyses of 41 human bile samples from four patient groups
| Controls (n = 11) | PSC (n = 14) | SSC (n = 10) | CCC (n = 6) with PSC (n = 2) | P value | |
| Bile bilirubin (mg/dL) | 41.6 ± 11.1 | 11.3 ± 1.8 | 6.0 ± 1.7 | 14.0 ± 6.4 | 0.006; controlsb |
| 1 ND | 2 ND, 1 BLD | 1 BLD | |||
| Bile protein (g/dL) | 3.5 ± 0.9 | 3.1 ± 0.5 | 12.5 ± 9.5 | 3.7 ± 1.0 | 0.89 |
| 1 ND | 3 ND | 1 BLD | |||
| Bile cholesterol (mmol/L) | 1.10 ± 0.32 | 0.53 ± 0.18 | 0.25 ± 0.07 | 0.31 ± 0.13 | 0.10 |
| 2 BLD | 4 BLD | 4 BLD | |||
| Bile total bile acids (mmol/L) | 21.3 ± 3.1 | 17.4 ± 6.8 | 7.8 ± 3.1 | 5.9 ± 1.5 | 0.003; controlsb, controlsc |
| Bile total PC per volume (μmol/L) | 8030 ± 1843 | 6205 ± 1465 | 2501 ± 790 | 1969 ± 981 | 0.005; controlsa, controlsc |
| Bile total LPC per volume (μmol/L) | 256 ± 90 | 200 ± 97 | 91.3 ± 50.0 | 12.2 ± 5.4 | Over-all 0.02; Dunn’s post-test: NS |
| LPC/PC (molar ratio) | 0.04 ± 0.01 | 0.09 ± 0.05 | 0.03 ± 0.011 | 0.008 ± 0.001 | 0.54 |
| PC/bilirubin (molar ratio) | 0.13 ± 0.07 | 0.35 ± 0.08 | 0.30 ± 0.07 | 0.24 ± 0.09 | 0.09 |
| PC/protein [μmol/L/(g/dL)] | 5131 ± 1918 | 3597 ± 1350 | 2782 ± 2241 | 725 ± 229 | 0.09 |
| PC/cholesterol (molar ratio) | 8.9 ± 2.6 | 14.0 ± 3.8 | 8.4 ± 1.4 | 6.2 ± 2.3 | 0.46 |
| PC/bile acids (molar ratio) | 0.40 ± 0.07 | 0.45 ± 0.06 | 0.34 ± 0.07 | 0.31 ± 0.09 | 0.45 |
One value was excluded (outlier); for calculation of SEM, values below detection level were set at 0; ND: Not done (not sufficient material left for determination); BLD: Below limit of detection; NS: Not significant; PSC: Primary sclerosing cholangitis; SSC: Secondary sclerosing cholangitis; CCC: Cholangiocellular carcinoma.
P < 0.05,
P < 0.01 vs SSC;
P < 0.05 vs CCC.
Biliary PC species profiles
PC species in significant amounts were identified at molecular weights of 732, 734, 756, 758, 760, 782, 784, 786, 788, 804, 806, 808, and 810 Da. This corresponds to molecules with the following ratios of fatty acid (FA) carbon numbers to numbers of double bonds: 32:1, 32:0, 34:3, 34:2, 34:1, 36:4, 36:3, 36:2, 36:1, 38:7, 38:6, 38:5, and 38:4. Bile samples from the four patient groups showed a remarkably similar PC molecular species composition. Together constituting more than 50% of total PC, PC 34:1 and 34:2 represented in all cases the two most abundant PC species. DPPC (PC 34:0, 16:0-16:0) which is the most abundant PC species in pulmonary surfactant, represented only a very small percentage of biliary PC, ranging between 1.1% in controls and 3.6% in SSC patients (Table 4). Only minor intergroup differences were noted in biliary PC species patterns. These can be viewed in detail in Table 4 (for PC and LPC species, numbers of carbon atoms and double bonds of fatty acid side chains as well as molecular weights are indicated). Not even minor differences were found between PC species profiles in patients with PSC vs patients with SSC.
Table 4.
Molar percentages of phosphatidylcholine and lysophosphatidylcholine species in bile from four patient groups
| Molecular weight (Da) | Controls (n = 11) | PSC (n = 14) | SSC (n = 10) | CCC ± PSC (n = 6) | P value | |
| PC molecular species | ||||||
| 32:1 | 732 | 2.8 ± 0.5 | 3.3 ± 0.4 | 9.2 ± 3.9 | 4.2 ± 0.7 | 0.036, controls vs SSCa |
| 32:0 | 734 | 1.1 ± 0.1 | 1.4 ± 0.2 | 3.6 ± 1.2 | 1.5 ± 0.2 | 0.029, controls vs SSCa |
| 34:3 | 756 | 2.2 ± 0.3 | 2.7 ± 0.2 | 2.7 ± 0.4 | 2.0 ± 0.4 | 0.19 |
| 34:2 | 758 | 33.7 ± 1.1 | 28.5 ± 0.7 | 25.8 ± 3.1 | 29.8 ± 0.2 | 0.017, controls vs PSCc |
| 34:1 | 760 | 22.8 ± 0.9 | 22.1 ± 0.7 | 22.2 ± 0.9 | 24.1 ± 1.3 | 0.55 |
| 36:4 | 782 | 10.5 ± 0.6 | 10.3 ± 0.7 | 10.1 ± 1.8 | 8.6 ± 0.5 | 0.24 |
| 36:3 | 784 | 8.8 ± 0.3 | 10.0 ± 0.4 | 8.4 ± 0.8 | 7.1 ± 0.7 | 0.013, CCC vs PSCc |
| 36:2 | 786 | 7.3 ± 0.4 | 9.0 ± 0.6 | 8.1 ± 0.9 | 9.3 ± 0.9 | 0.13 |
| 36:1 | 788 | 3.3 ± 0.3 | 3.5 ± 0.3 | 3.7 ± 0.4 | 3.2 ± 0.4 | 0.65 |
| 38:7 | 804 | 0.8 ± 0.2 | 0.9 ± 0.3 | 0.4 ± 0.3 | 0.2 ± 0.1 | 0.10 |
| 38:6 | 806 | 2.6 ± 0.2 | 3.3 ± 0.3 | 2.5 ± 0.5 | 6.3 ± 2.5 | 0.05 |
| 38:5 | 808 | 2.1 ± 0.1 | 2.7 ± 0.3 | 1.6 ± 0.4 | 2.0 ± 0.3 | 0.09 |
| 38:4 | 810 | 1.9 ± 0.2 | 2.3 ± 0.3 | 1.8 ± 0.4 | 1.9 ± 0.3 | 0.51 |
| LPC molecular species | ||||||
| 16:0 | 496 | 59.2 ± 7.0 | 56.0 ± 5.8 | 85.0 ± 5.2 | 58.9 ± 7.0 | 0.05, controls vs SSCa, PSC vs SSCb |
| 18:2 | 520 | 21.2 ± 6.0 | 12.6 ± 4.0 | 0.9 ± 0.9 | 30.0 ± 9.0 | 0.02, stones vs SSCa, CCC vs SSCb |
| 18:1 | 522 | 9.7 ± 2.3 | 16.0 ± 2.9 | 7.4 ± 2.6 | 4.0 ± 4.0 | 0.08 |
| 18:0 | 524 | 9.9 ± 5.4 | 15.5 ± 2.3 | 6.8 ± 2.2 | 7.2 ± 7.2 | 0.06 |
P < 0.05,
P < 0.01 vs secondary sclerosing cholangitis (SSC) group;
P < 0.05 vs primary sclerosing cholangitis (PSC). CCC: Cholangiocellular carcinoma; PC: Phosphatidylcholine; LPC: Lysophosphatidylcholine.
LPC concentrations and LPC/PC ratios
Total LPC contents per bile volume ranged from 12.2 ± 5.4 μmol/L in CCC patients to 256 ± 90 μmol/L in the control group (overall, P = 0.02; Dunn’s post-test: no differences between single groups).
LPC species found in human hepatic bile had molecular weights of 496, 520, 522, and 524 Da, corresponding to 16:0, 18:3, 18:2, and 18:1, respectively. The distribution of different LPC species in the groups is shown in Table 4. Bile specimens obtained from patients with SSC contained relatively more LPC 496 (16:0) than those from patients with PSC and controls and relatively less LPC 520 (18:3) than those from patients with CCC and from controls.
Since the cytotoxic effect of LPC on the bile duct mucosa is likely to be alleviated by the presence of PC[23], it was interesting to examine the LPC/PC ratios in bile. Except for two bile specimens obtained from PSC patients and one bile specimen from a SSC patient (LPC/PC ratios: 0.25, 0.64 and 10.5), the biliary LPC/PC ratios were remarkably low in the other patients, LPC accounting for 4.2% ± 1.8% of total PC (mean ± SE). LPC/PC ratios were not different between the four groups.
In order to determine the extent of potential reflux of pancreatic juice in the bile specimens, pancreatic lipase activity was quantified in the samples. In 31 of 41 specimens examined, pancreas lipase activity could be determined. Its average in these specimens was 266 ± 95.3 U/L. Interestingly, no significant correlation was found between pancreas lipase activity in bile and LPC/PC ratios (P = 0.66, r = 0.08), suggesting that bile LPC in the examined patients originated more likely from a different source.
Ratios of phospholipids to other bile components
As shown in Table 3, PC or LPC/bilirubin, PC or LPC/protein, PC or LPC/cholesterol as well as PC or LPC/total bile salt ratios in hepatic bile did not differ between controls and patients with PSC, SSC, or CCC.
Bacterial cultures and biliary phospholipids
At certain concentrations, bacteria in hepatic bile are known to lead to the degradation of protective biliary PC and subsequent increase in the amounts of potentially cytotoxic LPC[24]. Thereby, they might cause chronic irritation of the bile duct mucosa and subsequently fibrosis. The 41 patients included in this study were divided into two groups according to the amount of bacterial growth in their bile. Group 1 comprised patients with no or only scarce amounts of bacteria and/or Candida organisms, while group 2 comprised patients with at least moderate amounts of bacteria in their bile specimens. According to this classification, 24 of 40 patients (no microbiological results available in one of the CCC patients) belonged to group 1. Among the bacterial species identified were mainly Enterococcus faecalis and Enterococcus faecium as well as Escherichia coli. Other species identified were Enterobacter cloacae, Klebsiella pneumoniae, Pseudomonas aeruginosa, Stenotrophomonas maltophilia, and Raoultella planticola. Interestingly, biliary LPC/PC ratios did not differ significantly between the two groups.
DISCUSSION
The main hypothesis of the present study was that, according to the “toxic bile” concept, alterations in biliary phospholipid concentrations and percentual distribution of species might play a role in the process of chronic inflammation and subsequent fibrosis in sclerosing cholangitis, such as PSC and SSC. Based on this hypothesis, we expected to find a biliary phospholipid imbalance with a lack of presumably cytoprotective PC and an abundance of presumably cytotoxic LPC in bile specimens from patients with sclerosing cholangitis.
To our knowledge, no data on MS of PC and LPC species patterns in human hepatic bile from patients with PSC and SSC have been published thus far. Most previous studies on biliary phospholipids have been performed using samples of gall bladder bile acquired during surgery in patients with gall stones[25-27].
Compared to conventional methods of phospholipid analysis (e.g., thin layer chromatography, derivatization, HPLC, and gas chromatography), electrospray-ionization (ESI)-tandem mass spectrometry (MS/MS) has several advantages since it has a high basic sensitivity for the detection of phospholipids (analyte concentrations between 0.1 and 50 pmol/μL), and since the specificity of MS/MS scan modes enables direct analysis of crude lipid extracts[28]. Thus, as little as 1 μL of bile from every patient was sufficient for phospholipid analyses in the present study.
Further, for the analysis of bile in respect to potential secondary alterations of bile ducts, intrahepatic bile is more reliable than gall bladder bile. However, obtaining bile specimens during ERC has some drawbacks. Mostly, it is not justifiable from an ethical position to obtain specimens from completely healthy controls via ERC, since the procedure can cause complications, such as post-ERC pancreatitis, which is - of course - no different for patients in surgery. This is why we selected mainly choledocholithiasis patients without signs of chronic inflammation as the controls in this study. However, lithogenic bile is known to show an imbalance between cholesterol, bile salts, and phospholipids. The primary pathophysiological defect in cholesterol gallstone disease is hypersecretion of hepatic cholesterol into bile with less frequent hyposecretion of bile salts and/or phospholipids[7]. For this reason, all the above-mentioned bile compounds were assessed in the present study.
The amount of total PC in relation to bile volume differed significantly between the controls and patients with SSC or CCC, with the latter two groups displaying lower concentrations (overall P = 0.005, controls vs SSC patients or CCC patients P < 0.05, respectively). The interpretation of this result is challenging since it cannot be ruled out that a few of the bile samples might have been diluted by contrast medium. However, the cases in which bile cannot be aspirated through the ERC-catheter before intervention are usually rare according to our experience, and we took care to first aspirate the contrast medium in such a case, and to discard it before obtaining the bile sample in a second syringe. Unfortunately, it was not indicated in the databank in which cases specimens were obtained without prior injection of contrast medium. Reduced biliary PC concentrations might be caused by lack or malfunction of biliary phospholipid transporters. Another reason for which the evaluation of total PC and LPC per bile volume might be hampered is that a high degree of cholestasis (with high serum levels of AP and bilirubin) can go along with low concentrations of bilirubin and the other analytes in bile. Bilirubin levels in serum from patients with SSC or CCC exceeded those of the other groups, albeit not significantly. AP levels in serum from patients with SSC were significantly higher than those in serum from controls. This is why we feel that - given the small sample sizes in the present study - the fact that we found lower levels of PC and LPC per bile volume should not be overrated. Yet we think that if our results can be confirmed in further studies with larger sample numbers and matched samples, a diagnostic tool might thereby be established. For such a future study, it would be helpful to take a note of the mode of bile acquisition in every included patient.
Importantly, the difference observed between the groups concerning total biliary PC per bile volume could not be reproduced when the groups were compared for the biliary PC/bile salt ratio, the reason for which might be dilution, as indicated above. The biliary PC/bile salt ratio was assumed to be of special pathophysiological importance since it may be indicative of a potential imbalance between factors protecting cholangiocytes and those harming cholangiocytes. Thus, before starting the study, we had hypothesized that PC-to-bile salt ratios in bile would be reduced in patients with sclerosing cholangitis vs controls. Our hypothesis was also due to recently published data showing that nonanastomotic strictures after liver transplantation were present more often in patients with low biliary phospholipids/bile salt ratios than in patients with high biliary phospholipids/bile salt ratios[29]. When planning another study on this subject, it would be important to include more patients, and to not only focus on total bile salts as a reference parameter, but to also quantitate different bile salts with variable effects on cholangiocytes. Such an approach may also help to attenuate the intake of UDCA by many patients as a confounder.
Further, no major differences were noted in the biliary LPC/PC ratios and, most interestingly, in the LPC and PC species patterns in bile between the groups. As the distribution of biliary PC and LPC species was not relevantly changed in sclerosing cholangitis, we suggest that it plays no major role in the pathogenesis of the disease; based on our study, PC and LPC species patterns cannot be used as diagnostic tools to differentiate between PSC and SSC.
What is the role of LPC in bile Remarkably, this study confirms that in normal bile, LPC is just a minor constituent. Analysis of the data for all 41 patients of the study together showed that total LPC accounted for less than 5% of total PC, after the exclusion of three patients with exceedingly high ratios, who were treated as outliers. In line with previous studies by other authors, our results revealed only traces of LPC in hepatic bile from “controls”[30]. LPC can be derived from PC by hydrolysis within the bile ducts. Nakano et al[24] found that most bacterial strains isolated from bile possess both phospholipase A1 and A2 activity. Shimada et al[27] showed in their study that patients with an anomalous pancreaticobiliary ductal junction have considerable amounts of LPC in their intrahepatic bile, which could not be correlated with concentrations of bacteria in bile, but with phospholipase A2 activity from refluxing pancreatic juice.
Although the four groups did not differ with respect to the biliary LPC/PC ratios in the present study, a few bile specimens displayed high biliary LPC/PC ratios. Retrospective analysis of these patients showed high lipase concentrations and/or abundant bacterial growth in some, but not in all, cases; some patients also showed abundant growth of bacteria in bile and/or high lipase concentrations with very low LPC/PC ratios. Again, the small numbers of subgroups might disguise potentially important differences here. Furthermore, most bile specimens with abundant bacterial growth contained predominantly gram-positive cocci. The latter have been previously shown to produce just minor amounts of phospholipases[24]. Considering all the data, it is still reasonable to suggest that recurrent bacterial cholangitis caused by certain bacterial strains in patients with a prior diagnosis of sclerosing cholangitis might lead to the aggravation of disease activity via increased LPC/PC ratios in bile, even though this could not be proven in the present study.
Data have recently been published on the lack of PC in the bile from patients with CCC[31]. Although total biliary PC contents per volume in the specimens from CCC patients in the present study were the lowest compared to the other groups, ratios of PC contents to any other biliary compound did not differ between groups. Since the number of CCC patients was below ten in both the above-mentioned study and ours, further studies with larger sample numbers are necessary to determine whether patients with CCC indeed have lower biliary PC contents. This would be of major importance as there is still no diagnostic tool available for early diagnosis of CCC, especially in patients with known PSC.
PC and LPC species patterns in human bile were quite similar to those found in intestinal mucus in a previous study[32]. Unlike the case with pulmonary surfactant, biliary PC molecules with fatty acids containing at least one double bond clearly overweigh those with saturated fatty acid side chains. The fact that PC and LPC species patterns were remarkably similar between the groups supports the idea that biliary PC and LPC form a highly preserved system that cannot easily be changed by external influences. Previous reports indicating that bile of various animal species displayed a very similar phospholipid molecular species composition are also supportive of this notion[33].
In conclusion, we showed that electrospray MS/MS is a very convenient method for bile phospholipid analysis in very low volume samples. Surprisingly, there were no major differences concerning the biliary PC and LPC species profiles between patients with PSC, SSC, CCC and controls. Yet our data can serve as an incentive and reference for further studies using the same methods for larger groups of patients or other disease conditions. Also, the lower total PC and LPC concentrations found in bile from patients with SSC and CCC compared to controls might be of diagnostic importance, if the results were certified in further studies with larger sample numbers and after exclusion of dilutional effects.
COMMENTS
Background
Primary sclerosing cholangitis (PSC) and secondary sclerosing cholangitis (SSC) are progressive diseases where chronic inflammation leads to scarring and strictures of the bile ducts. The process eventually results in liver cirrhosis and makes liver transplantation necessary. The two diseases have very similar features, but their pathogeneses seem to be different even though they are not yet fully known. In SSC, the afflictions of the bile ducts originate from a trigger in the patient’s history, like e.g., hypoxemia. SSC also progresses more rapidly than PSC. Biliary phospholipid transporter defects have been shown to lead to bile duct fibrosis in mice. Phosphatidylcholine (PC) is thought to have protective properties in bile. These facts led to the hypothesis that a deficiency of PC or an imbalance of its single species, or a relative abundance of potentially toxic lysophosphatidylcholine (LPC) could play a role in the pathogenesis of SSC and more significantly PSC. Such a finding could lead to the development of novel therapeutic options.
Research frontiers
Due to its unfavorable disease course, lack of thorough understanding of the underlying pathophysiology, and absence of effective medical therapy, sclerosing cholangitis has become a subject of increasing scientific interest. In contrast to PSC, SSC has not been described at all until recently, so research in this field is breaking new ground. Research has been published on the relation between changes in biliary phospholipids and bile duct strictures after liver transplantation, but none on biliary PC and LPC species in PSC as well as SSC. The “toxic bile concept” - the theory which states that imbalances in the composition of bile could lead to chronic bile duct inflammation and destruction - remains relevant, as a therapy would likely arise from the identification and regulation of these potential imbalances. Such a therapy would be effective regardless of whether these imbalances were primary or secondary factors of disease activity.
Innovations and breakthroughs
Even though the data presented here have no direct implications for diagnosis or therapy, they can serve as motivation for future studies in the field using the very convenient and resource-economic method of nano-electrospray ionization mass spectrometry for bile phospholipid analysis. In this study, data on PC and LPC profiles in intrahepatic human bile in SSC are published for the first time.
Applications
The method of nano-electrospray ionization mass spectrometry of bile phospholipids can be used for the examination of larger patient groups. Biliary phospholipid composition in cases of cholangiocellular carcinoma and after liver transplantation might be of special interest. As little as 1 μL of bile is needed for analysis of all PC and LPC species.
Terminology
PC is not only an essential component of biological membranes, but also of a wide array of functionally important body fluids like pulmonary surfactant, gastric and intestinal mucus, synovial fluid, peritoneal fluid and bile. It is a glycerophospholipid featuring a choline head group and two fatty acid side chains. The special biophysical qualities of these zwitter-ionic molecules depend on the composition of these fatty acid side chains. For example, PC in pulmonary surfactant has mostly saturated fatty acids, while in intestinal mucus - having to meet completely different functional challenges - PC almost always contains at least one unsaturated fatty acid side chain. This is why the exact composition of PC species of a fluid like bile is so important. LPC is produced from PC by partial hydrolysis, resulting in removal of one fatty acid side chain.
Peer review
This manuscript is quite interesting, with a good methodology. There are not many patients, but due to ERC and rare etiologies, these could be enough. The authors took into account their limitations and made a clear discussion. This small study about the pathophysiology of sclerosing cholangitis is well designed and presented.
Footnotes
Supported by A Grant from Deutsche Forschungsgemeinschaft (DFG)
P- Reviewers Kaya M, Tischendorf JJW, Trapero-Marugan M S- Editor Wen LL L- Editor A E- Editor Zhang DN
References
- 1.Pollheimer MJ, Halilbasic E, Fickert P, Trauner M. Pathogenesis of primary sclerosing cholangitis. Best Pract Res Clin Gastroenterol. 2011;25:727–739. doi: 10.1016/j.bpg.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gotthardt D, Stiehl A. Endoscopic retrograde cholangiopancreatography in diagnosis and treatment of primary sclerosing cholangitis. Clin Liver Dis. 2010;14:349–358. doi: 10.1016/j.cld.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Mendes F, Lindor KD. Primary sclerosing cholangitis: overview and update. Nat Rev Gastroenterol Hepatol. 2010;7:611–619. doi: 10.1038/nrgastro.2010.155. [DOI] [PubMed] [Google Scholar]
- 4.Ruemmele P, Hofstaedter F, Gelbmann CM. Secondary sclerosing cholangitis. Nat Rev Gastroenterol Hepatol. 2009;6:287–295. doi: 10.1038/nrgastro.2009.46. [DOI] [PubMed] [Google Scholar]
- 5.Gelbmann CM, Rümmele P, Wimmer M, Hofstädter F, Göhlmann B, Endlicher E, Kullmann F, Langgartner J, Schölmerich J. Ischemic-like cholangiopathy with secondary sclerosing cholangitis in critically ill patients. Am J Gastroenterol. 2007;102:1221–1229. doi: 10.1111/j.1572-0241.2007.01118.x. [DOI] [PubMed] [Google Scholar]
- 6.Krones E, Graziadei I, Trauner M, Fickert P. Evolving concepts in primary sclerosing cholangitis. Liver Int. 2012;32:352–369. doi: 10.1111/j.1478-3231.2011.02607.x. [DOI] [PubMed] [Google Scholar]
- 7.Wang DQ, Cohen DE, Carey MC. Biliary lipids and cholesterol gallstone disease. J Lipid Res. 2009;50 Suppl:S406–S411. doi: 10.1194/jlr.R800075-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ehlken H, Kondylis V, Heinrichsdorff J, Ochoa-Callejero L, Roskams T, Pasparakis M. Hepatocyte IKK2 protects Mdr2-/- mice from chronic liver failure. PLoS One. 2011;6:e25942. doi: 10.1371/journal.pone.0025942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smit JJ, Schinkel AH, Oude Elferink RP, Groen AK, Wagenaar E, van Deemter L, Mol CA, Ottenhoff R, van der Lugt NM, van Roon MA. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell. 1993;75:451–462. doi: 10.1016/0092-8674(93)90380-9. [DOI] [PubMed] [Google Scholar]
- 10.Fickert P, Fuchsbichler A, Wagner M, Zollner G, Kaser A, Tilg H, Krause R, Lammert F, Langner C, Zatloukal K, et al. Regurgitation of bile acids from leaky bile ducts causes sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology. 2004;127:261–274. doi: 10.1053/j.gastro.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 11.de Vree JML. Dissertation: Defects in hepatobiliary lipid transport: genetics and therapy of progressive familial cholestasis type 3. Amsterdam: University of Amsterdam; 1999. p. 32. Available from: http://dare.uva.nl/en/record/73737. [Google Scholar]
- 12.Trauner M, Fickert P, Wagner M. MDR3 (ABCB4) defects: a paradigm for the genetics of adult cholestatic syndromes. Semin Liver Dis. 2007;27:77–98. doi: 10.1055/s-2006-960172. [DOI] [PubMed] [Google Scholar]
- 13.Gotthardt D, Runz H, Keitel V, Fischer C, Flechtenmacher C, Wirtenberger M, Weiss KH, Imparato S, Braun A, Hemminki K, et al. A mutation in the canalicular phospholipid transporter gene, ABCB4, is associated with cholestasis, ductopenia, and cirrhosis in adults. Hepatology. 2008;48:1157–1166. doi: 10.1002/hep.22485. [DOI] [PubMed] [Google Scholar]
- 14.Küllenberg D, Taylor LA, Schneider M, Massing U. Health effects of dietary phospholipids. Lipids Health Dis. 2012;11:3. doi: 10.1186/1476-511X-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lieber CS, DeCarli LM, Mak KM, Kim CI, Leo MA. Attenuation of alcohol-induced hepatic fibrosis by polyunsaturated lecithin. Hepatology. 1990;12:1390–1398. doi: 10.1002/hep.1840120621. [DOI] [PubMed] [Google Scholar]
- 16.Weltzien HU. Cytolytic and membrane-perturbing properties of lysophosphatidylcholine. Biochim Biophys Acta. 1979;559:259–287. doi: 10.1016/0304-4157(79)90004-2. [DOI] [PubMed] [Google Scholar]
- 17.Panteghini M, Bonora R, Pagani F. Measurement of pancreatic lipase activity in serum by a kinetic colorimetric assay using a new chromogenic substrate. Ann Clin Biochem. 2001;38:365–370. doi: 10.1258/0004563011900876. [DOI] [PubMed] [Google Scholar]
- 18.Deeg R, Ziegenhorn J. Kinetic enzymic method for automated determination of total cholesterol in serum. Clin Chem. 1983;29:1798–1802. [PubMed] [Google Scholar]
- 19.Turley SD, Dietschy JM. Re-evaluation of the 3 alpha-hydroxysteroid dehydrogenase assay for total bile acids in bile. J Lipid Res. 1978;19:924–928. [PubMed] [Google Scholar]
- 20.Jendrassik L, Grof P. Vereinfachte photometrische Methode zur Bestimmung des Blutbilirubins. Biochem Z. 1938;297:81–89. [Google Scholar]
- 21.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 22.Brügger B, Erben G, Sandhoff R, Wieland FT, Lehmann WD. Quantitative analysis of biological membrane lipids at the low picomole level by nano-electrospray ionization tandem mass spectrometry. Proc Natl Acad Sci USA. 1997;94:2339–2344. doi: 10.1073/pnas.94.6.2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poncelet P, Thompson AG. Action of bile phospholipids on the pancreas. Am J Surg. 1972;123:196–202. doi: 10.1016/0002-9610(72)90333-9. [DOI] [PubMed] [Google Scholar]
- 24.Nakano T, Yanagisawa J, Nakayama F. Phospholipase activity in human bile. Hepatology. 1988;8:1560–1564. doi: 10.1002/hep.1840080615. [DOI] [PubMed] [Google Scholar]
- 25.Jüngst D, Lang T, Huber P, Lange V, Paumgartner G. Effect of phospholipids and bile acids on cholesterol nucleation time and vesicular/micellar cholesterol in gallbladder bile of patients with cholesterol stones. J Lipid Res. 1993;34:1457–1464. [PubMed] [Google Scholar]
- 26.Pereira SP, Bain IM, Kumar D, Dowling RH. Bile composition in inflammatory bowel disease: ileal disease and colectomy, but not colitis, induce lithogenic bile. Aliment Pharmacol Ther. 2003;17:923–933. doi: 10.1046/j.1365-2036.2003.01529.x. [DOI] [PubMed] [Google Scholar]
- 27.Shimada K, Yanagisawa J, Nakayama F. Increased lysophosphatidylcholine and pancreatic enzyme content in bile of patients with anomalous pancreaticobiliary ductal junction. Hepatology. 1991;13:438–444. [PubMed] [Google Scholar]
- 28.Lehmann WD, Koester M, Erben G, Keppler D. Characterization and quantification of rat bile phosphatidylcholine by electrospray-tandem mass spectrometry. Anal Biochem. 1997;246:102–110. doi: 10.1006/abio.1996.9941. [DOI] [PubMed] [Google Scholar]
- 29.Buis CI, Geuken E, Visser DS, Kuipers F, Haagsma EB, Verkade HJ, Porte RJ. Altered bile composition after liver transplantation is associated with the development of nonanastomotic biliary strictures. J Hepatol. 2009;50:69–79. doi: 10.1016/j.jhep.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 30.Gottfries A, Nilsson S, Samuelsson B, Scherstén T. Phospholipids in human hepatic bile, gall bladder bile, and plasma in cases with acute cholecystitis. Scand J Clin Lab Invest. 1968;21:168–176. doi: 10.3109/00365516809084279. [DOI] [PubMed] [Google Scholar]
- 31.Hashim Abdalla MS, Taylor-Robinson SD, Sharif AW, Williams HR, Crossey MM, Badra GA, Thillainayagam AV, Bansi DS, Thomas HC, Waked IA, et al. Differences in phosphatidylcholine and bile acids in bile from Egyptian and UK patients with and without cholangiocarcinoma. HPB (Oxford) 2011;13:385–390. doi: 10.1111/j.1477-2574.2011.00296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braun A, Treede I, Gotthardt D, Tietje A, Zahn A, Ruhwald R, Schoenfeld U, Welsch T, Kienle P, Erben G, et al. Alterations of phospholipid concentration and species composition of the intestinal mucus barrier in ulcerative colitis: a clue to pathogenesis. Inflamm Bowel Dis. 2009;15:1705–1720. doi: 10.1002/ibd.20993. [DOI] [PubMed] [Google Scholar]
- 33.Alvaro D, Cantafora A, Attili AF, Ginanni Corradini S, De Luca C, Minervini G, Di Biase A, Angelico M. Relationships between bile salts hydrophilicity and phospholipid composition in bile of various animal species. Comp Biochem Physiol B. 1986;83:551–554. doi: 10.1016/0305-0491(86)90295-6. [DOI] [PubMed] [Google Scholar]


