Abstract
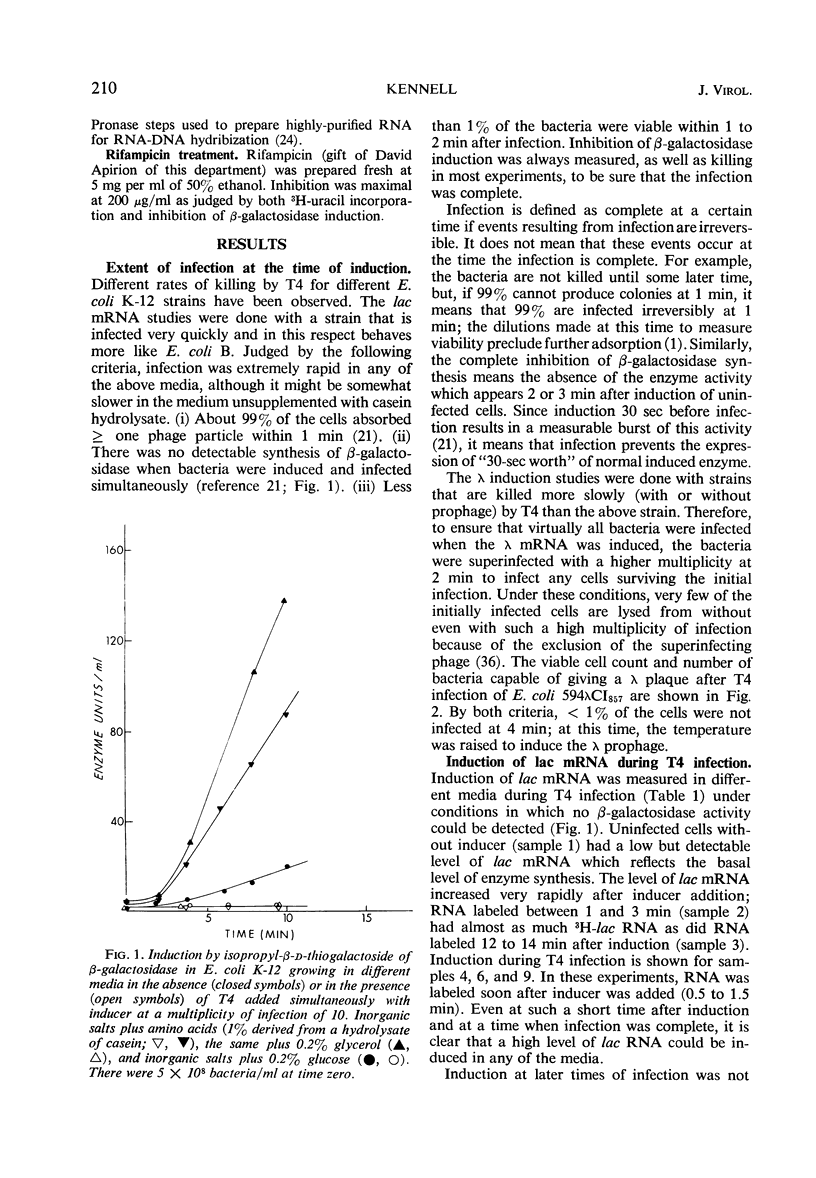
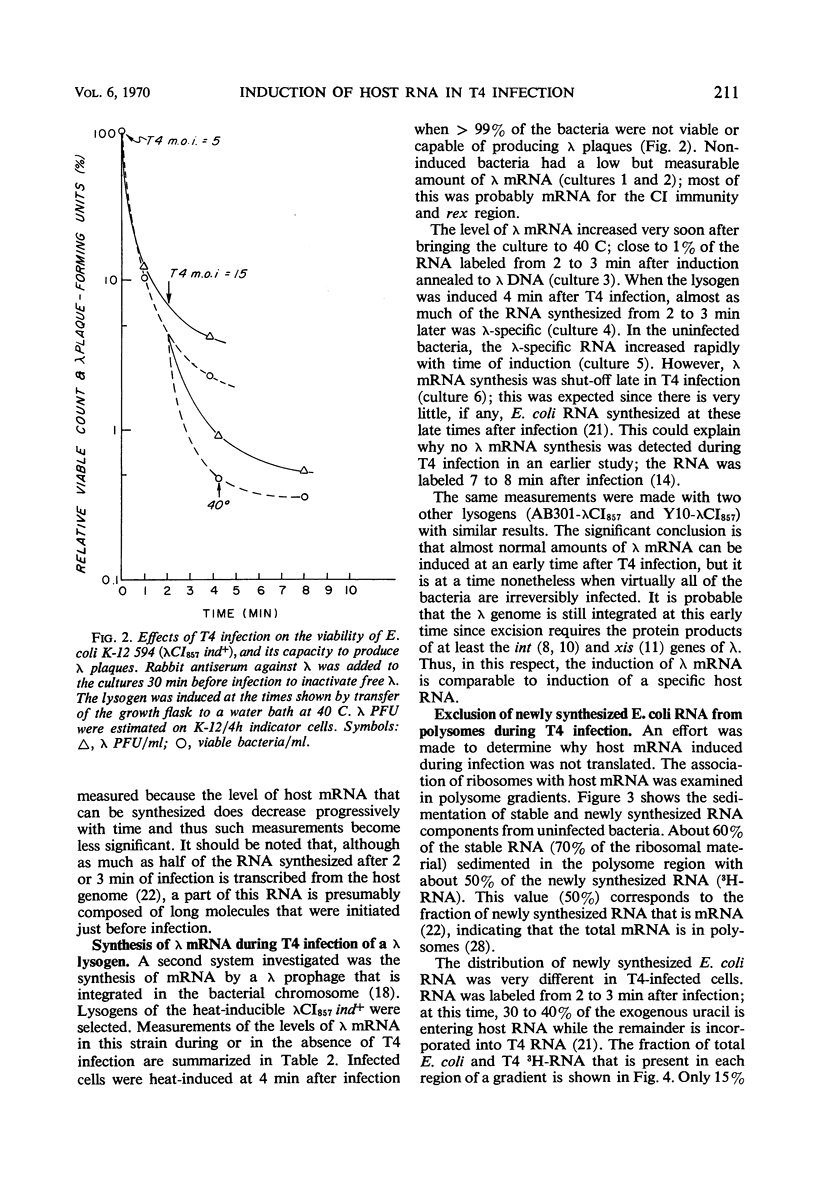
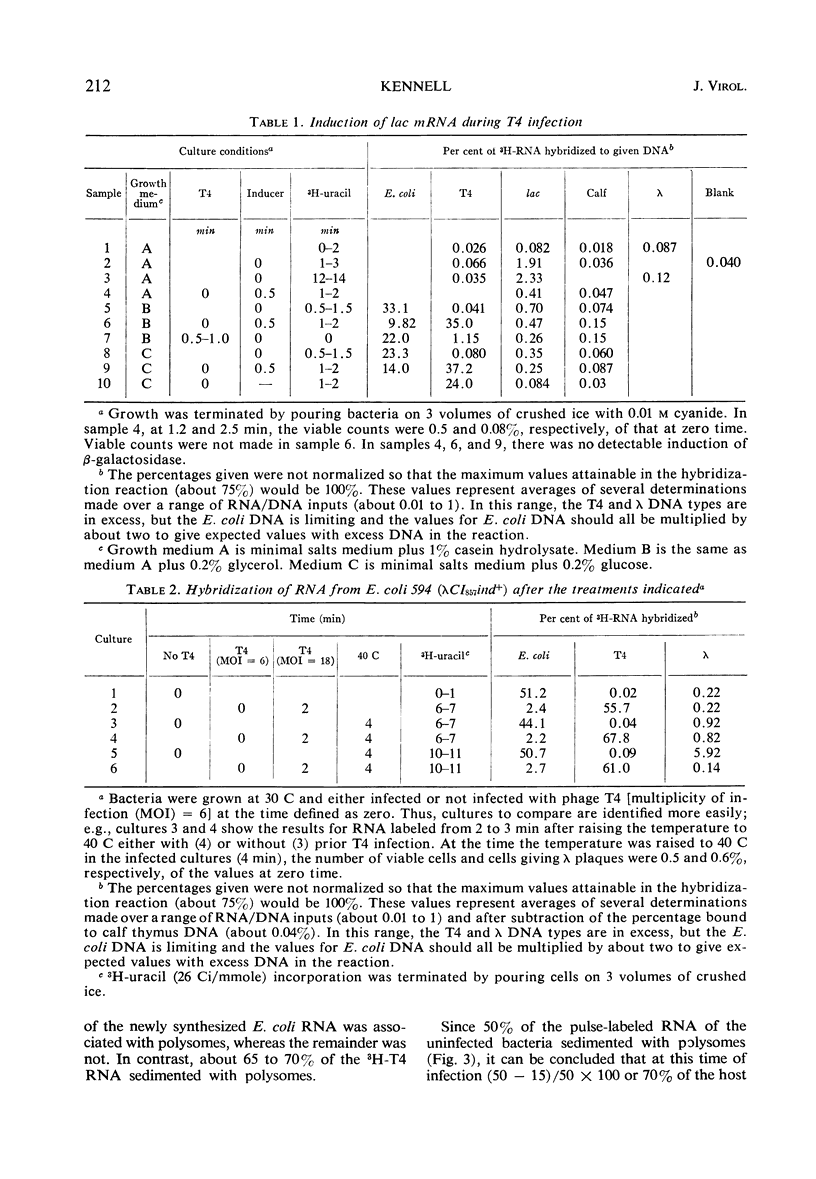
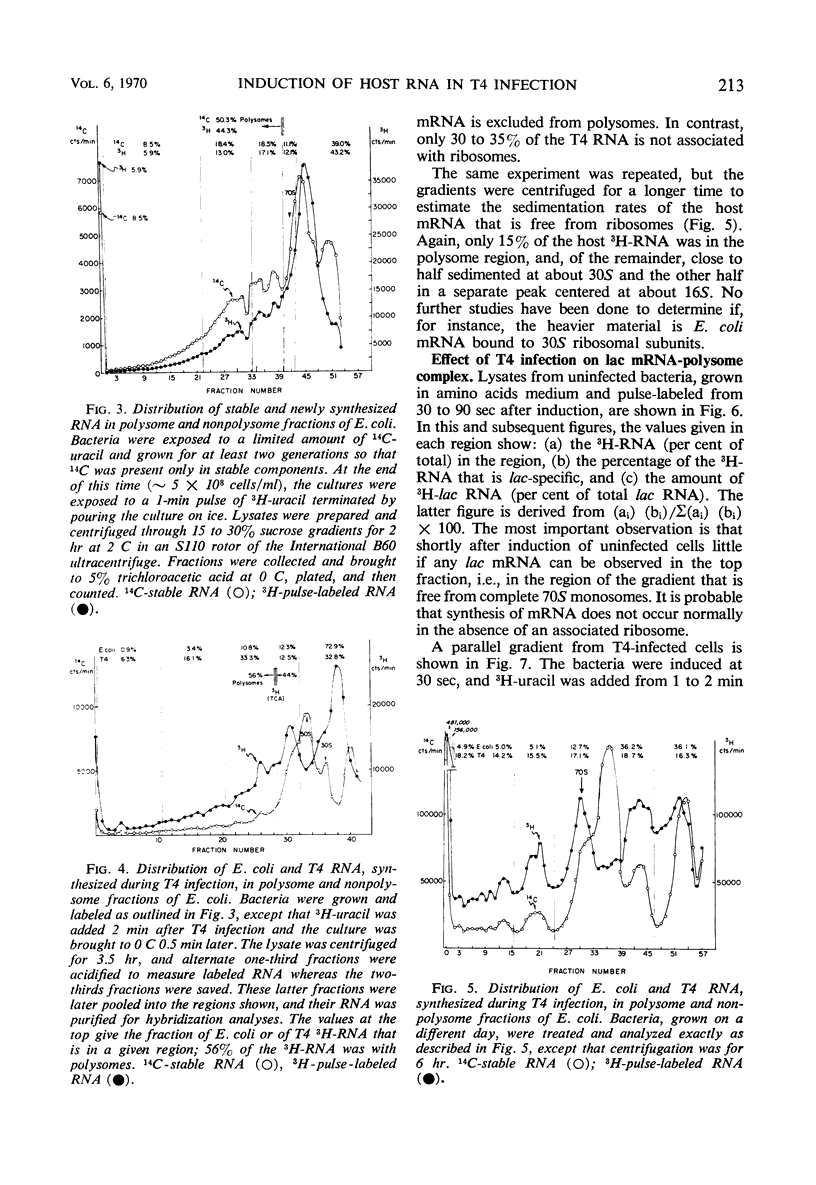
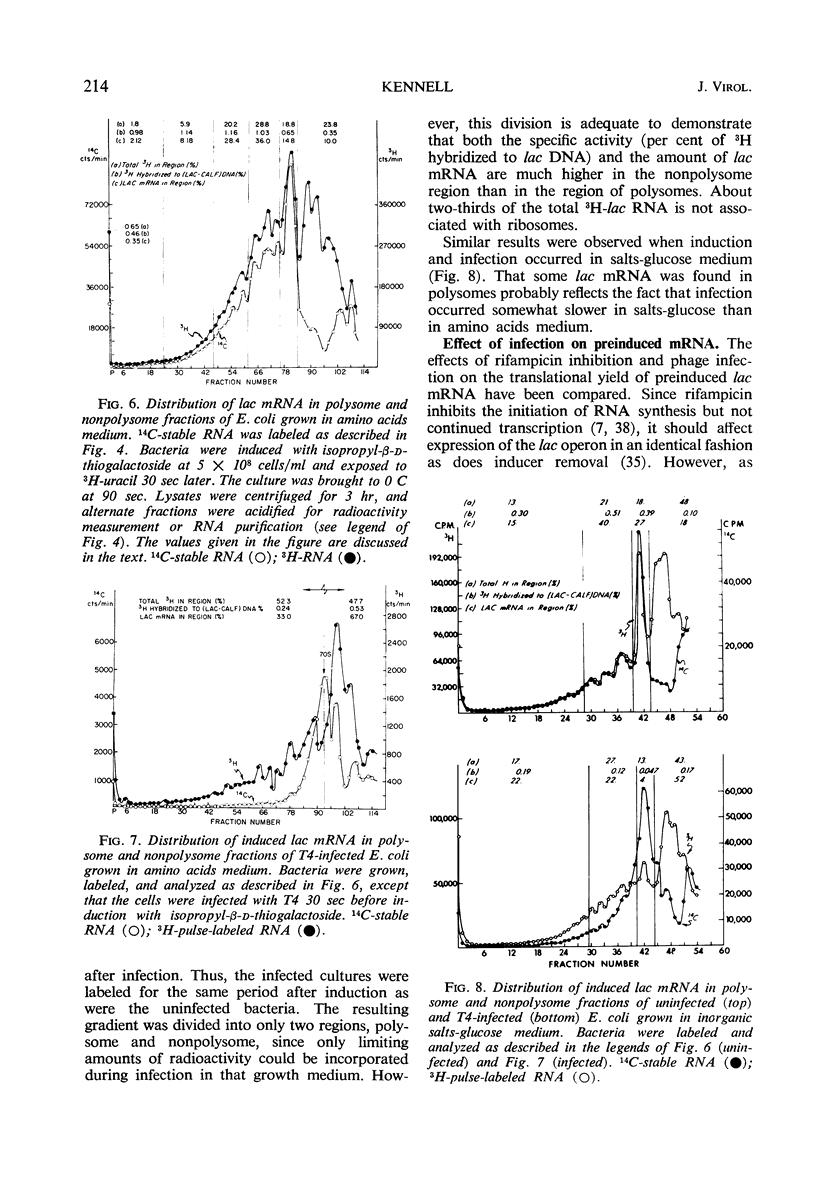
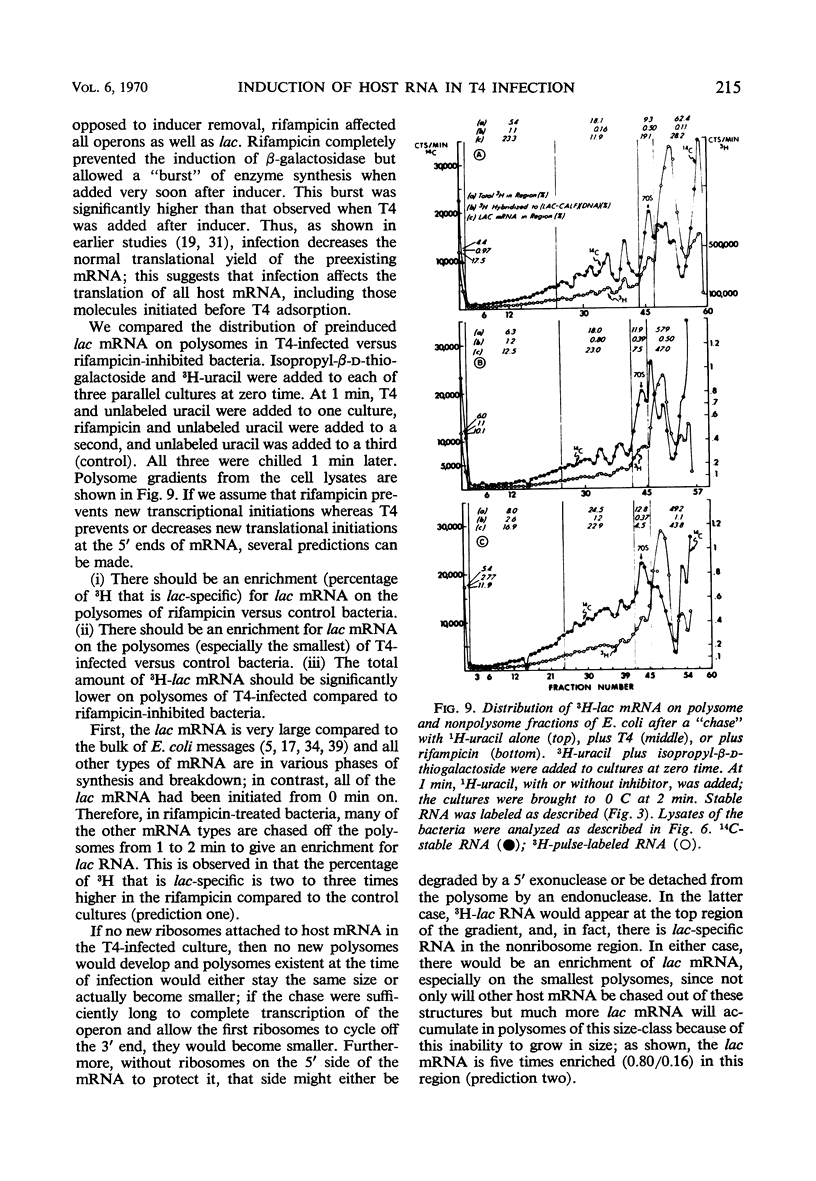
Two gene clusters on the Escherichia coli chromosome were induced at early times after T4 infection when >99% of the cells were infected: the lactose (lac) operon and prophage λ. Their messenger ribonucleic acid (mRNA) was detected by hybridization to φ80 dlac deoxyribonucleic acid (DNA) and λDNA, respectively. Synthesis of host mRNA could be initiated during the first few minutes after T4 infection, although no β-galactosidase activity could be detected. Hybridization analyses of selected fractions from sucrose gradients revealed that most of this lac mRNA induced at very early times of T4 infection was not associated with ribosomes. In contrast, virtually all lac mRNA in uninfected bacteria was associated with polysomes. This exclusion affected all host mRNA; about 70% of E. coli3H-mRNA, labeled from 2 to 3 min after T4 infection, was excluded from polysomes. Infection even reduced the yield of β-galactosidase from lac mRNA induced before infection. Gradients from rifampicin-inhibited cells showed the normal growth of lac mRNA polysomes; in contrast, T4 infection prevented growth of the preinduced lac polysomes. It is concluded that T4 infection interferes within seconds with the reassociation of ribosomes to host mRNA.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENZER S. Induced synthesis of enzymes in bacteria analyzed at the cellular level. Biochim Biophys Acta. 1953 Jul;11(3):383–395. doi: 10.1016/0006-3002(53)90057-2. [DOI] [PubMed] [Google Scholar]
- Baker R. F., Yanofsky C. The periodicity of RNA polymerase initiations: a new regulatory feature of transcription. Proc Natl Acad Sci U S A. 1968 May;60(1):313–320. doi: 10.1073/pnas.60.1.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilezikian J. P., Kaempfer R. O., Magasanik B. Mechanism of tryptophanase induction in Escherichia coli. J Mol Biol. 1967 Aug 14;27(3):495–506. doi: 10.1016/0022-2836(67)90054-x. [DOI] [PubMed] [Google Scholar]
- Brown J. L., Koorajian S., Katze J., Zabin I. Beta-galactosidase. Amino-and carboxyl-terminal studies. J Biol Chem. 1966 Jun 25;241(12):2826–2831. [PubMed] [Google Scholar]
- Gingery R., Echols H. Mutants of bacteriophage lambda unable to integrate into the host chromosome. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1507–1514. doi: 10.1073/pnas.58.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman M. E., Yarmolinsky M. B. Integration-negative mutants of bacteriophage lambda. J Mol Biol. 1968 Feb 14;31(3):487–505. doi: 10.1016/0022-2836(68)90423-3. [DOI] [PubMed] [Google Scholar]
- Guarneros G., Echols H. New mutants of bacteriophage lambda with a specific defect in excision from the host chromosome. J Mol Biol. 1970 Feb 14;47(3):565–574. doi: 10.1016/0022-2836(70)90323-2. [DOI] [PubMed] [Google Scholar]
- HERSHEY A. D., DIXON J., CHASE M. Nucleic acid economy in bacteria infected with bacteriophage T2. I. Purine and pyrimidine composition. J Gen Physiol. 1953 Jul;36(6):777–789. doi: 10.1085/jgp.36.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattman S., Hofschneider P. H. Influence of T4 on the formation of RNA phage-specific polyribosomes and polymerase. J Mol Biol. 1968 Aug 14;35(3):513–522. doi: 10.1016/s0022-2836(68)80011-7. [DOI] [PubMed] [Google Scholar]
- Hattman S., Hofschneider P. H. Interference of bacteriophage T4 in the reproduction of RNA-phage M12. J Mol Biol. 1967 Oct 14;29(1):173–190. doi: 10.1016/0022-2836(67)90189-1. [DOI] [PubMed] [Google Scholar]
- Hayward W. S., Green M. H. Inhibition of Escherichia coli and bacteriophage lambda messenger RNA synthesis by T4. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1675–1678. doi: 10.1073/pnas.54.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu W. T., Weiss S. B. Selective translation of T4 template RNA by ribosomes from T4-infected Escherichia coli. Proc Natl Acad Sci U S A. 1969 Sep;64(1):345–351. doi: 10.1073/pnas.64.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. H., Kennedy E. P. Characterization of the membrane protein component of the lactose transport system of Escherichia coli. J Biol Chem. 1969 Nov 10;244(21):5981–5987. [PubMed] [Google Scholar]
- Joyner A., Isaacs L. N., Echols H., Sly W. S. DNA replication and messenger RNA production after induction of wild-type lambda bacteriophage and lambda mutants. J Mol Biol. 1966 Aug;19(1):174–186. doi: 10.1016/s0022-2836(66)80059-1. [DOI] [PubMed] [Google Scholar]
- Kaempfer R. O., Magasanik B. Effect of infection with T-even phage on the inducible synthesis of beta-glactosidase in Escherichia coli. J Mol Biol. 1967 Aug 14;27(3):453–468. doi: 10.1016/0022-2836(67)90051-4. [DOI] [PubMed] [Google Scholar]
- Kano-Sueoka T., Sueoka N. Characterization of a modified leucyl-tRNA of Escherichia coli after bacteriophage T2 infection. J Mol Biol. 1968 Nov 14;37(3):475–491. doi: 10.1016/0022-2836(68)90116-2. [DOI] [PubMed] [Google Scholar]
- Kennel D. Titration of the gene sites on DNA by DNA-RNA hybridization. II. The Escherichia coli chromosome. J Mol Biol. 1968 May 28;34(1):85–103. doi: 10.1016/0022-2836(68)90236-2. [DOI] [PubMed] [Google Scholar]
- Kennell D. Inhibition of host protein synthesis during infection of Escherichia coli by bacteriophage T4. I. Continued synthesis of host ribonucleic acid. J Virol. 1968 Nov;2(11):1262–1271. doi: 10.1128/jvi.2.11.1262-1271.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennell D., Kotoulas A. Magnesium starvation of Aerobacter aerogenes. II. Rates of nucleic acid synthesis and methods for their measurement. J Bacteriol. 1967 Jan;93(1):345–356. doi: 10.1128/jb.93.1.345-356.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennell D., Kotoulas A. Titration of the gene sites on DNA by DNA-RNA hybridization. I. Problem of measurement. J Mol Biol. 1968 May 28;34(1):71–84. doi: 10.1016/0022-2836(68)90235-0. [DOI] [PubMed] [Google Scholar]
- LEVIN A. P., BURTON K. Inhibition of enzyme formation following infection of Escherichia coli with phage T2r. J Gen Microbiol. 1961 Jun;25:307–314. doi: 10.1099/00221287-25-2-307. [DOI] [PubMed] [Google Scholar]
- Landy A., Spiegelman S. Exhaustive hybridization and its application to an analysis of the ribonucleic acid synthesized in T4-infected cells. Biochemistry. 1968 Feb;7(2):585–591. doi: 10.1021/bi00842a011. [DOI] [PubMed] [Google Scholar]
- Mangiarotti G., Apirion D., Schlessinger D., Silengo L. Biosynthetic precursors of 30S and 50S ribosomal particles in Escherichia coli. Biochemistry. 1968 Jan;7(1):456–472. doi: 10.1021/bi00841a058. [DOI] [PubMed] [Google Scholar]
- Manor H., Goodman D., Stent G. S. RNA chain growth rates in Escherichia coli. J Mol Biol. 1969 Jan 14;39(1):1–29. doi: 10.1016/0022-2836(69)90329-5. [DOI] [PubMed] [Google Scholar]
- SHER I. H., MALLETTE M. F. The adaptive nature of the formation of lysine decarboxylase in Escherichia coli B. Arch Biochem Biophys. 1954 Oct;52(2):331–339. doi: 10.1016/0003-9861(54)90131-9. [DOI] [PubMed] [Google Scholar]
- STEERS E., Jr, CRAVEN G. R., ANFINSEN C. B., BETHUNE J. L. EVIDENCE FOR NONIDENTICAL CHAINS IN THE BETA-GALACTOSIDASE OF ESCHERICHIA COLI K12. J Biol Chem. 1965 Jun;240:2478–2484. [PubMed] [Google Scholar]
- Silver S., Levine E., Spielman P. M. Cation fluxes and permeability changes accompanying bacteriophage infection of Escherichia coli. J Virol. 1968 Aug;2(8):763–771. doi: 10.1128/jvi.2.8.763-771.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler B., Magasanik B. Molecular basis of transient repression of beta-galactosidase in Escherichia coli. J Bacteriol. 1969 Feb;97(2):550–556. doi: 10.1128/jb.97.2.550-556.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VISCONTI N. Resistance to lysis from without in bacteria infected with T2 bacteriophage. J Bacteriol. 1953 Sep;66(3):247–253. doi: 10.1128/jb.66.3.247-253.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOLKIN E., ASTRACHAN L. Phosphorus incorporation in Escherichia coli ribo-nucleic acid after infection with bacteriophage T2. Virology. 1956 Apr;2(2):149–161. doi: 10.1016/0042-6822(56)90016-2. [DOI] [PubMed] [Google Scholar]
- Wehrli W., Knüsel F., Schmid K., Staehelin M. Interaction of rifamycin with bacterial RNA polymerase. Proc Natl Acad Sci U S A. 1968 Oct;61(2):667–673. doi: 10.1073/pnas.61.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann R. A., Levinthal C. Messenger RNA and RNA transcription time. J Mol Biol. 1967 Dec 14;30(2):349–370. [PubMed] [Google Scholar]
- di Mauro E., Synder L., Marino P., Lamberti A., Coppo A., Tocchini-Valentini G. P. Rifampicin sensitivity of the components of DNA-dependent RNA polymerase. Nature. 1969 May 10;222(5193):533–537. doi: 10.1038/222533a0. [DOI] [PubMed] [Google Scholar]


