Abstract
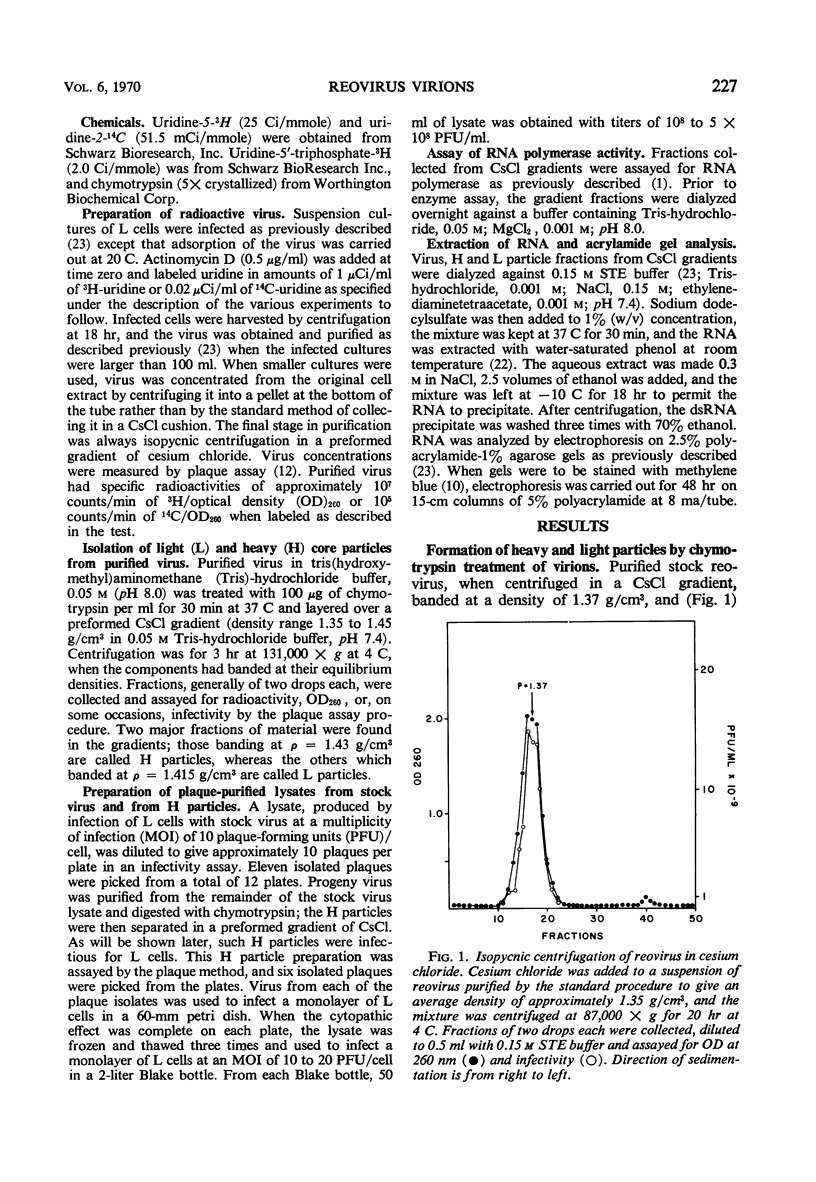
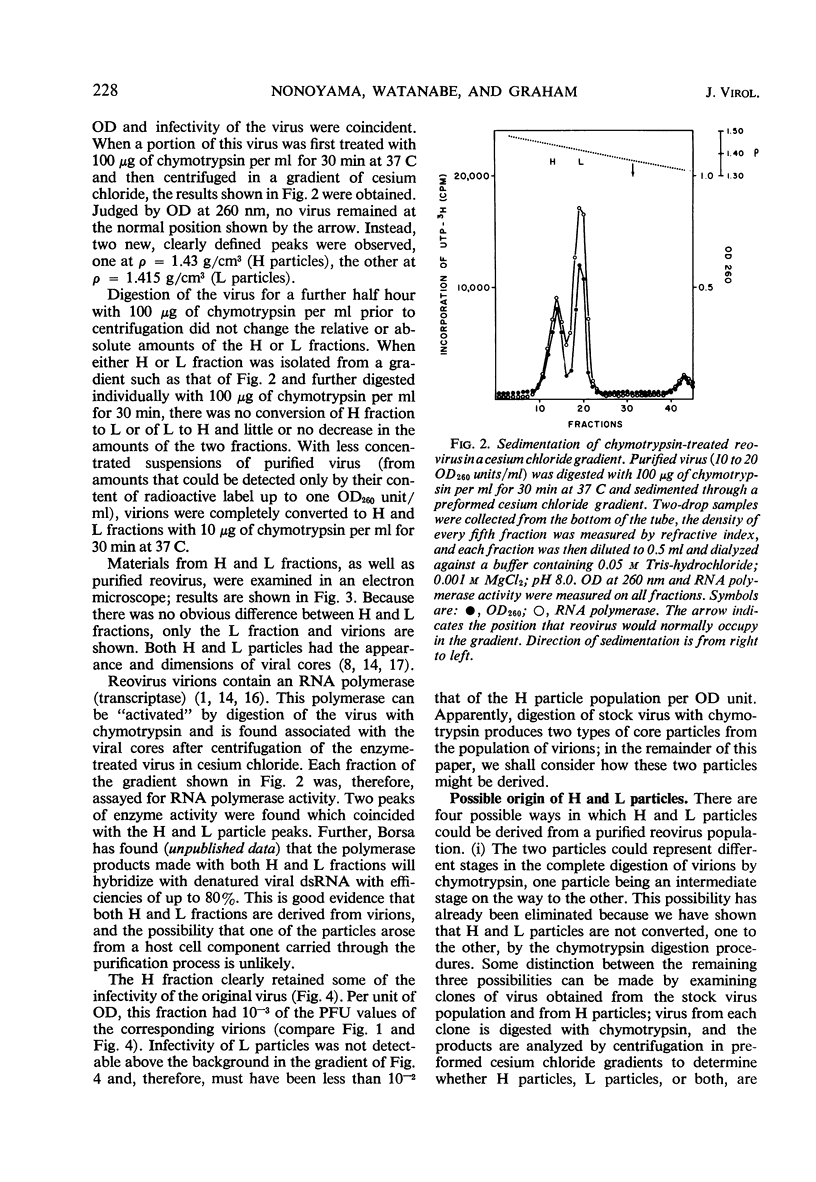
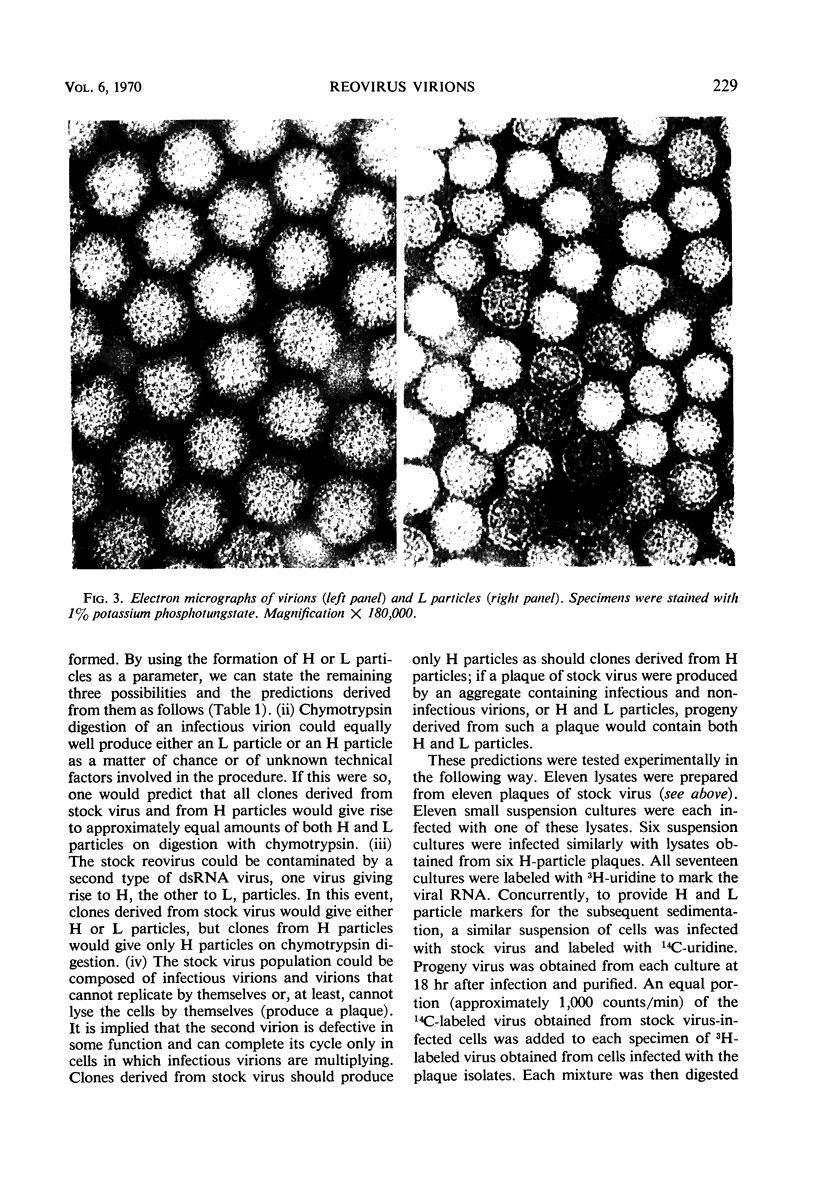
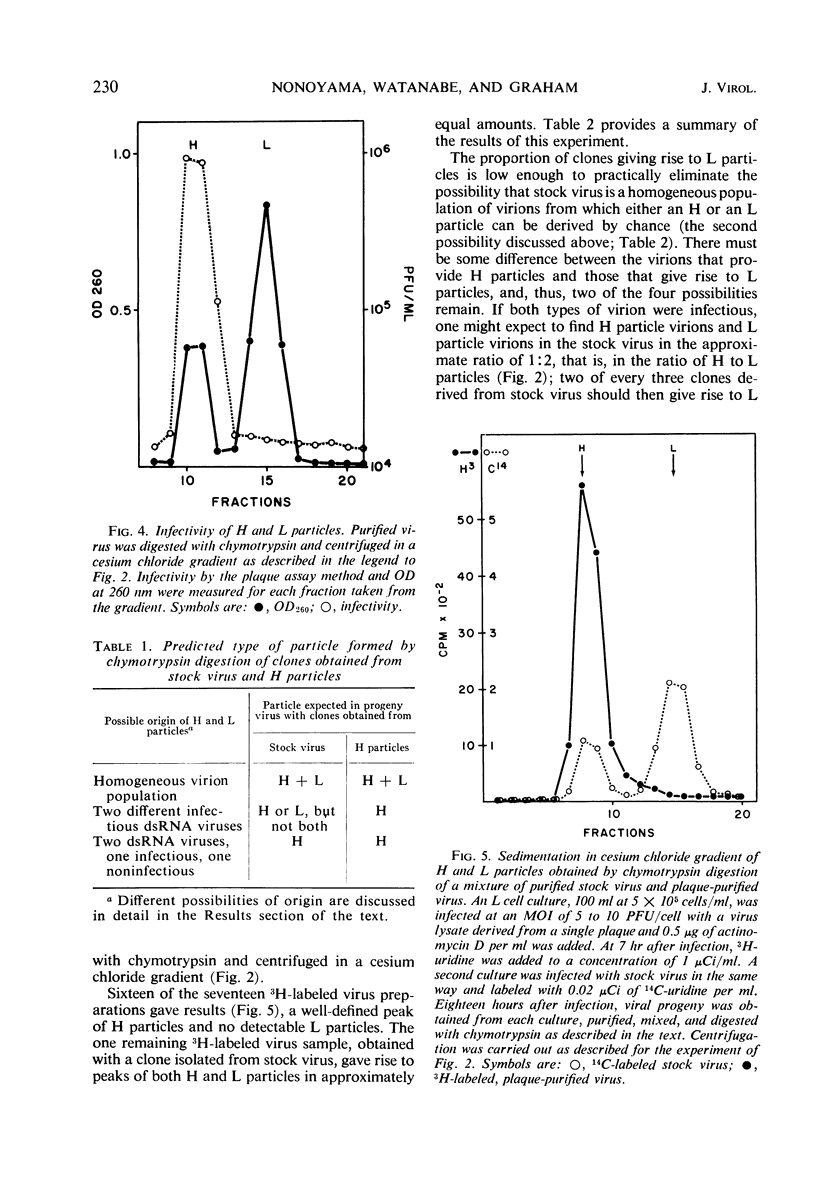
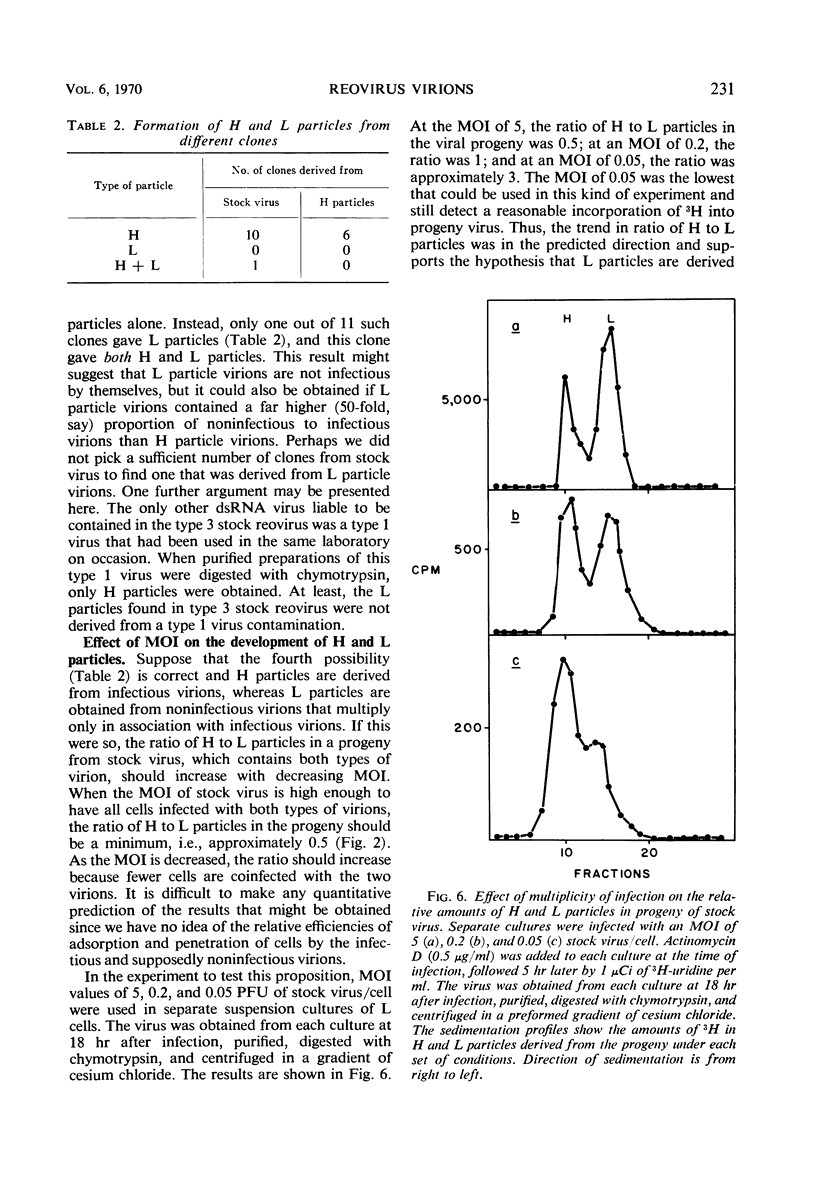
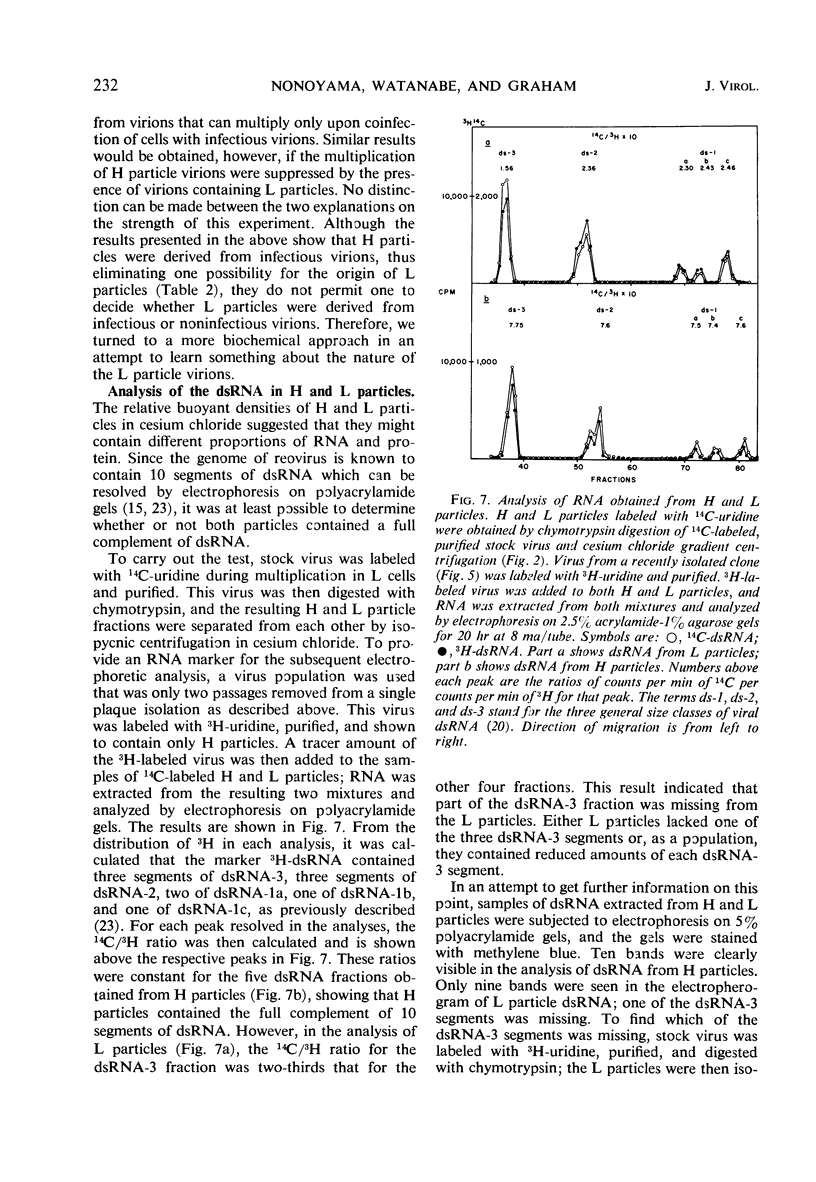
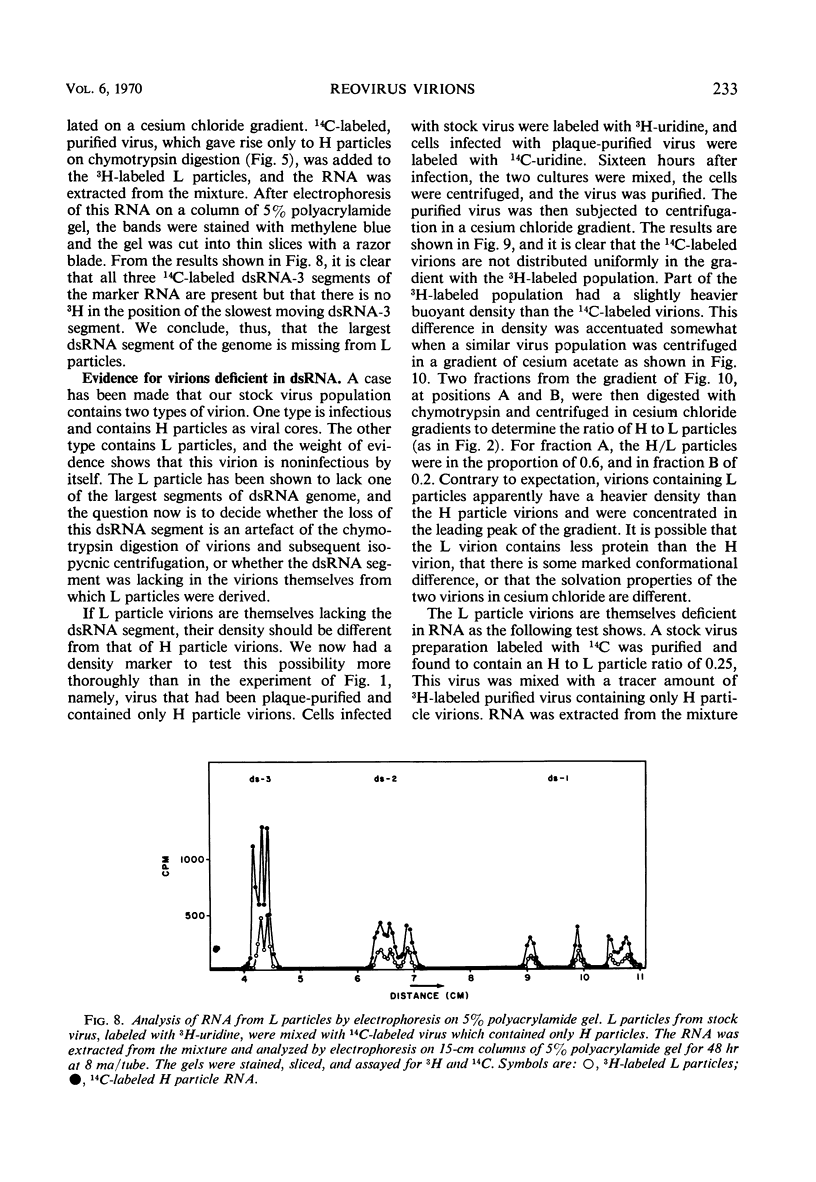
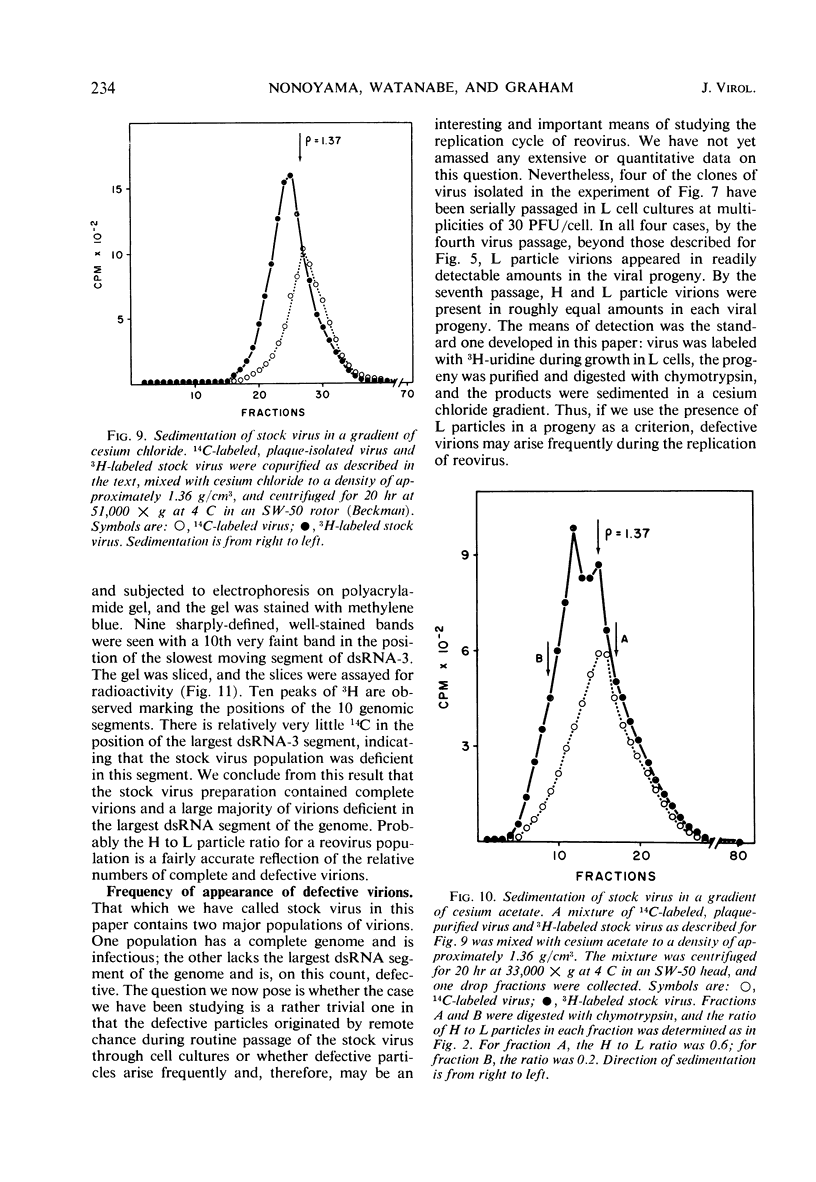
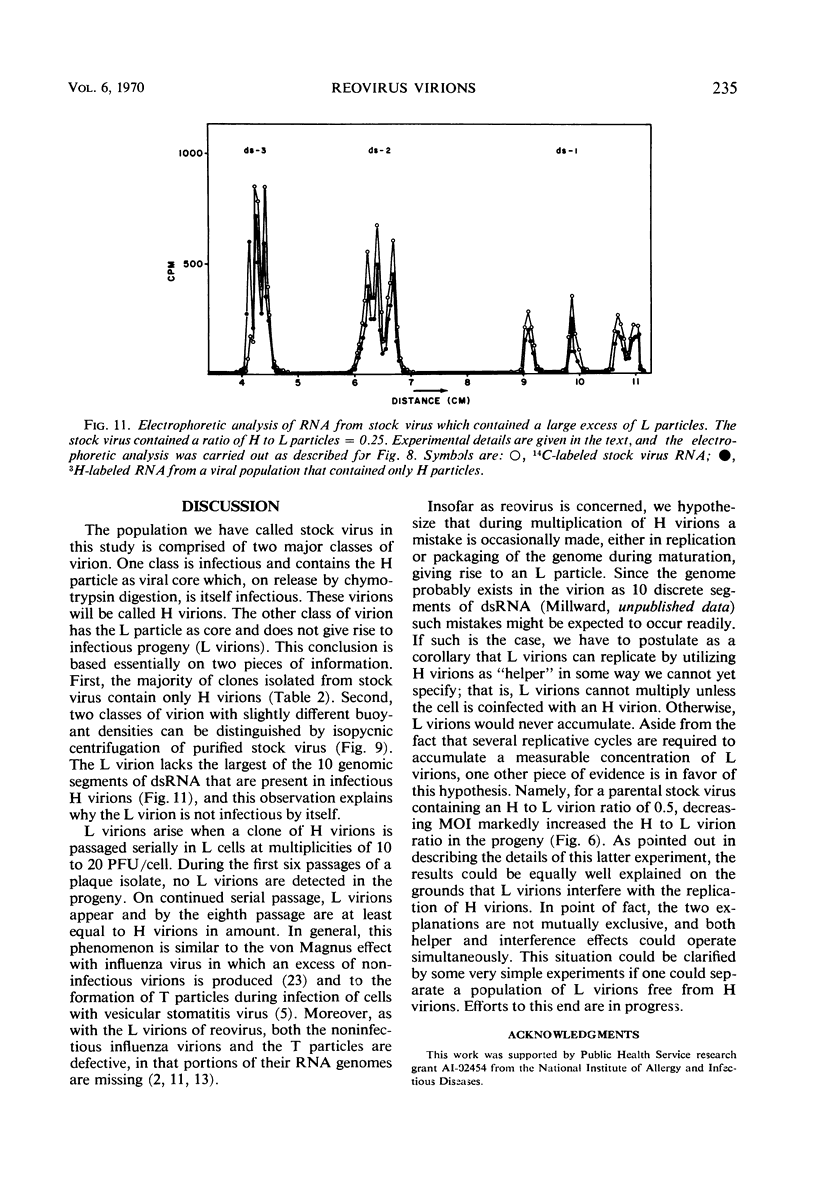
When purified preparations of stock reovirus, type 3, were digested with chymotrypsin, the virions were converted into two different types of particle. These new particles could be separated from each other by isopycnic centrifugation in cesium chloride gradients. One particle banded at a buoyant density of 1.43 g/cm3, the other at a density of 1.415 g/cm3. The former particle is termed the heavy (H) particle, the latter is the light (L) particle. The ratio of H/L particles varied between 0.5 and 0.25 in various purified preparations of virus. In electron micrographs, both H and L particles had the appearance and dimensions of viral cores. H particles were infectious for L cells. When plaques formed by stock virus, or by H particles, were picked and propagated in L cells, the majority of the clones gave rise only to H particles on chymotrypsin digestion. On continued serial passage of the clones, virions containing L particles again appeared in the progeny. The simplest explanation of these results was that stock virus was comprised of two populations of virions. One type of virion which contained H particles was infectious, whereas the other, which contained L particles, was not itself infectious and could replicate only in cells coinfected with an H particle virion. Added weight was given to this hypothesis by two observations. First, a small but definite separation of H and L virions could be achieved by isopycnic centrifugation in a gradient of cesium chloride. Second, L particles and virions containing L particles were both shown to lack the largest of the ten segments of double-stranded ribonucleic acid genome. Thus, L particle virions have defective genomes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borsa J., Graham A. F. Reovirus: RNA polymerase activity in purified virions. Biochem Biophys Res Commun. 1968 Dec 30;33(6):895–901. doi: 10.1016/0006-291x(68)90396-3. [DOI] [PubMed] [Google Scholar]
- Brown F., Martin S. J., Cartwright B., Crick J. The ribonucleic acids of the infective and interfering components of vesicular stomatitis virus. J Gen Virol. 1967 Oct;1(4):479–486. doi: 10.1099/0022-1317-1-4-479. [DOI] [PubMed] [Google Scholar]
- DALES S., GOMATOS P. J., HSU K. C. THE UPTAKE AND DEVELOPMENT OF REOVIRUS IN STRAIN L CELLS FOLLOWED WITH LABELED VIRAL RIBONUCLEIC ACID AND FERRITIN-ANTIBODY CONJUGATES. Virology. 1965 Feb;25:193–211. doi: 10.1016/0042-6822(65)90199-6. [DOI] [PubMed] [Google Scholar]
- GOMATOS P. J., TAMM I., DALES S., FRANKLIN R. M. Reovirus type 3: physical characteristics and interaction with L cells. Virology. 1962 Jul;17:441–454. doi: 10.1016/0042-6822(62)90139-3. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Greenawalt J. W., Wagner R. R. Defective T particles of vesicular stomatitis virus. I. Preparation, morphology, and some biologic properties. Virology. 1966 Oct;30(2):161–172. doi: 10.1016/0042-6822(66)90092-4. [DOI] [PubMed] [Google Scholar]
- LOH P. C., HOHL H. R., SOERGEL M. FINE STRUCTURE OF REOVIRUS TYPE 2. J Bacteriol. 1965 Apr;89:1140–1144. doi: 10.1128/jb.89.4.1140-1144.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAYOR H. D., JAMISON R. M., JORDAN L. E., VANMITCHELL M. REOVIRUSES. II. STRUCTURE AND COMPOSITION OF THE VIRION. J Bacteriol. 1965 Jun;89:1548–1556. doi: 10.1128/jb.89.6.1548-1556.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor H. D., Jordan L. E. Preparation and properties of the internal capsid components of reovirus. J Gen Virol. 1968 Sep;3(2):233–237. doi: 10.1099/0022-1317-3-2-233. [DOI] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Resolution of multiple ribonucleic acid species by polyacrylamide gel electrophoresis. Biochemistry. 1967 Jun;6(6):1818–1827. doi: 10.1021/bi00858a033. [DOI] [PubMed] [Google Scholar]
- Pons M. W., Hirst G. K. Polyacrylamide gel electrophoresis of influenza virus RNA. Virology. 1968 Feb;34(2):385–388. doi: 10.1016/0042-6822(68)90257-2. [DOI] [PubMed] [Google Scholar]
- Prevec L., Watanabe Y., Gauntt C. J., Graham A. F. Transcription of the genomes of type 1 and type 3 reoviruses. J Virol. 1968 Apr;2(4):289–297. doi: 10.1128/jvi.2.4.289-297.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer F. L., Hackett A. J., Soergel M. E. Vesicular stomatitis virus RNA: complementarity between infected cell RNA and RNA's from infectious and autointerfering viral fractions. Biochem Biophys Res Commun. 1968 Jun 10;31(5):685–692. doi: 10.1016/0006-291x(68)90616-5. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J., Sipe J. D., Loh P. Separation of ten reovirus genome segments by polyacrylamide gel electrophoresis. J Virol. 1968 Oct;2(10):986–991. doi: 10.1128/jvi.2.10.986-991.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatkin A. J., Sipe J. D. RNA polymerase activity in purified reoviruses. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1462–1469. doi: 10.1073/pnas.61.4.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel J. J., Joklik W. K. Studies on the in vitro transcription of reovirus RNA catalyzed by reovirus cores. Virology. 1969 Dec;39(4):822–831. doi: 10.1016/0042-6822(69)90019-1. [DOI] [PubMed] [Google Scholar]
- Smith R. E., Zweerink H. J., Joklik W. K. Polypeptide components of virions, top component and cores of reovirus type 3. Virology. 1969 Dec;39(4):791–810. doi: 10.1016/0042-6822(69)90017-8. [DOI] [PubMed] [Google Scholar]
- VASQUEZ C., TOURNIER P. NEW INTERPRETATION OF THE REOVIRUS STRUCTURE. Virology. 1964 Sep;24:128–130. doi: 10.1016/0042-6822(64)90162-x. [DOI] [PubMed] [Google Scholar]
- VASQUEZ C., TOURNIER P. The morphology of reovirus. Virology. 1962 Aug;17:503–510. doi: 10.1016/0042-6822(62)90149-6. [DOI] [PubMed] [Google Scholar]
- VON MAGNUS P. Incomplete forms of influenza virus. Adv Virus Res. 1954;2:59–79. doi: 10.1016/s0065-3527(08)60529-1. [DOI] [PubMed] [Google Scholar]
- Watanabe Y., Graham A. F. Structural units of reovirus ribonucleic acid and their possible functional significance. J Virol. 1967 Aug;1(4):665–677. doi: 10.1128/jvi.1.4.665-677.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Millward S., Graham A. F. Regulation of transcription of the Reovirus genome. J Mol Biol. 1968 Aug 28;36(1):107–123. doi: 10.1016/0022-2836(68)90223-4. [DOI] [PubMed] [Google Scholar]
- Watanabe Y., Prevec L., Graham A. F. Specificity in transcription of the reovirus genome. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1040–1046. doi: 10.1073/pnas.58.3.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]



