Abstract
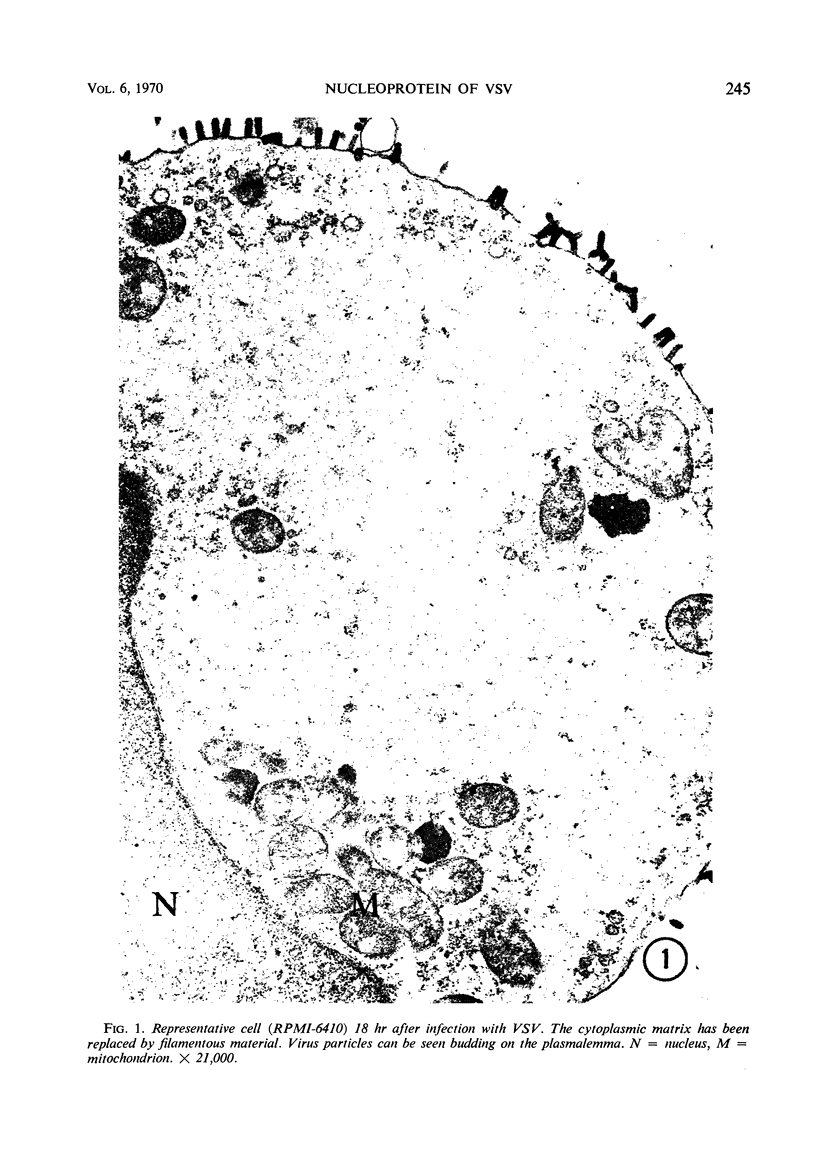
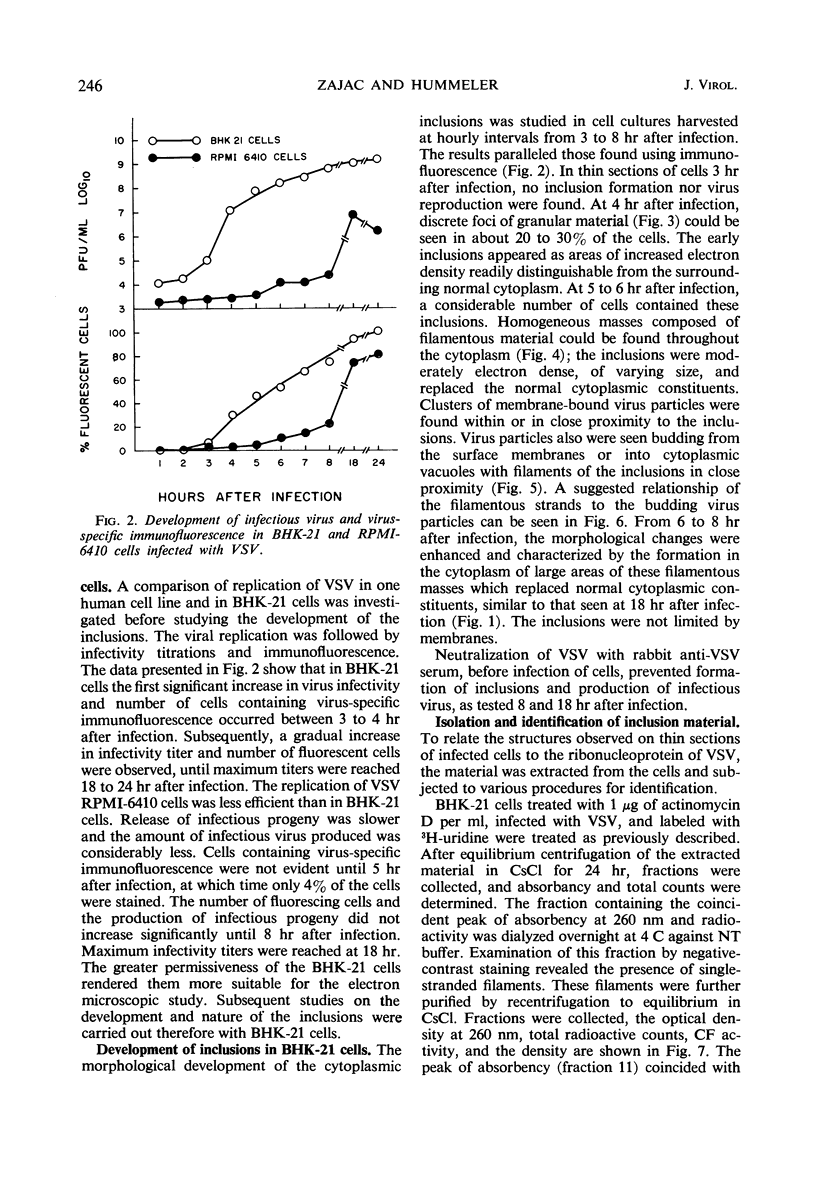
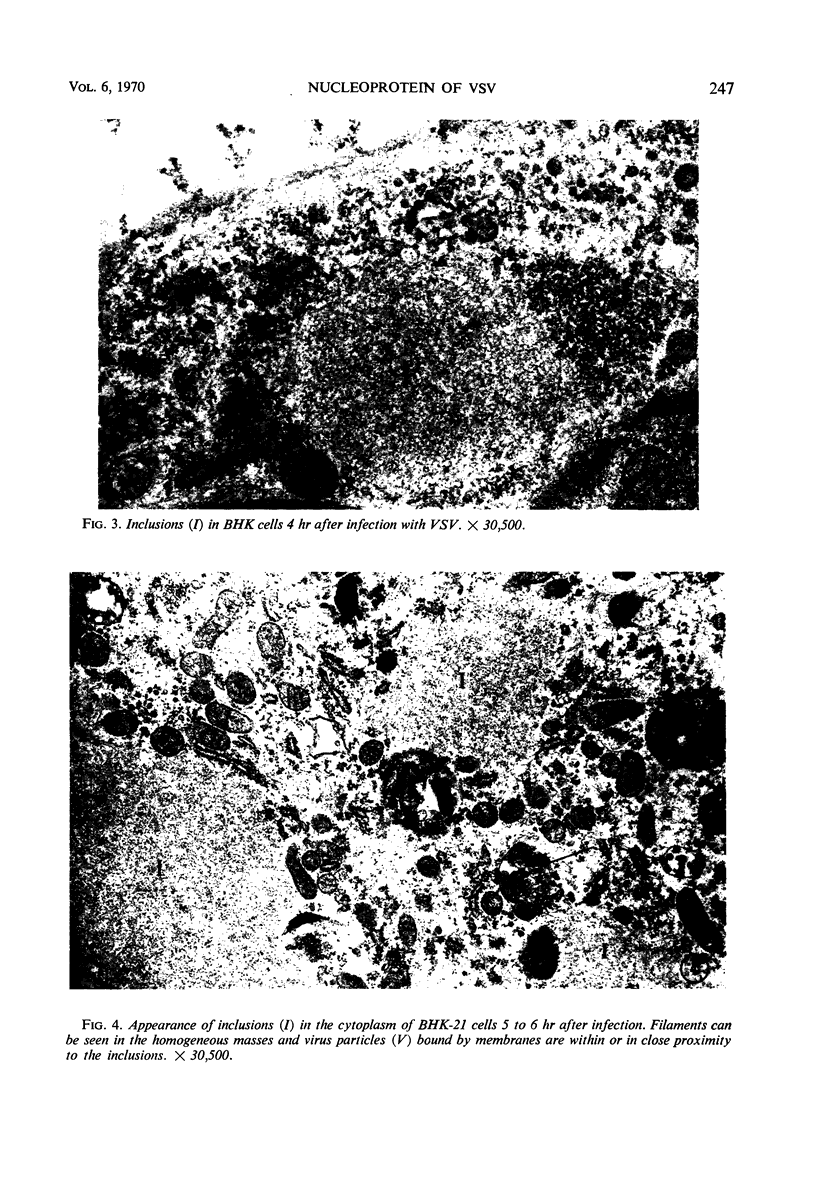
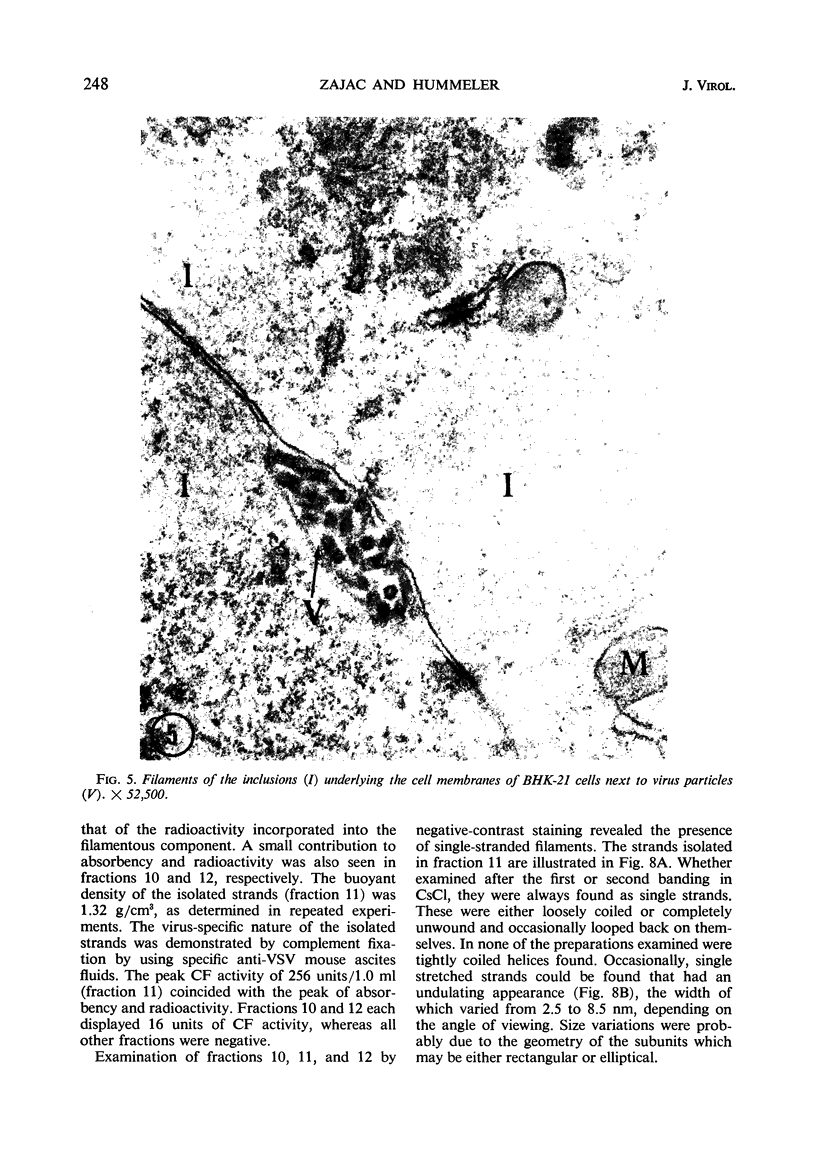
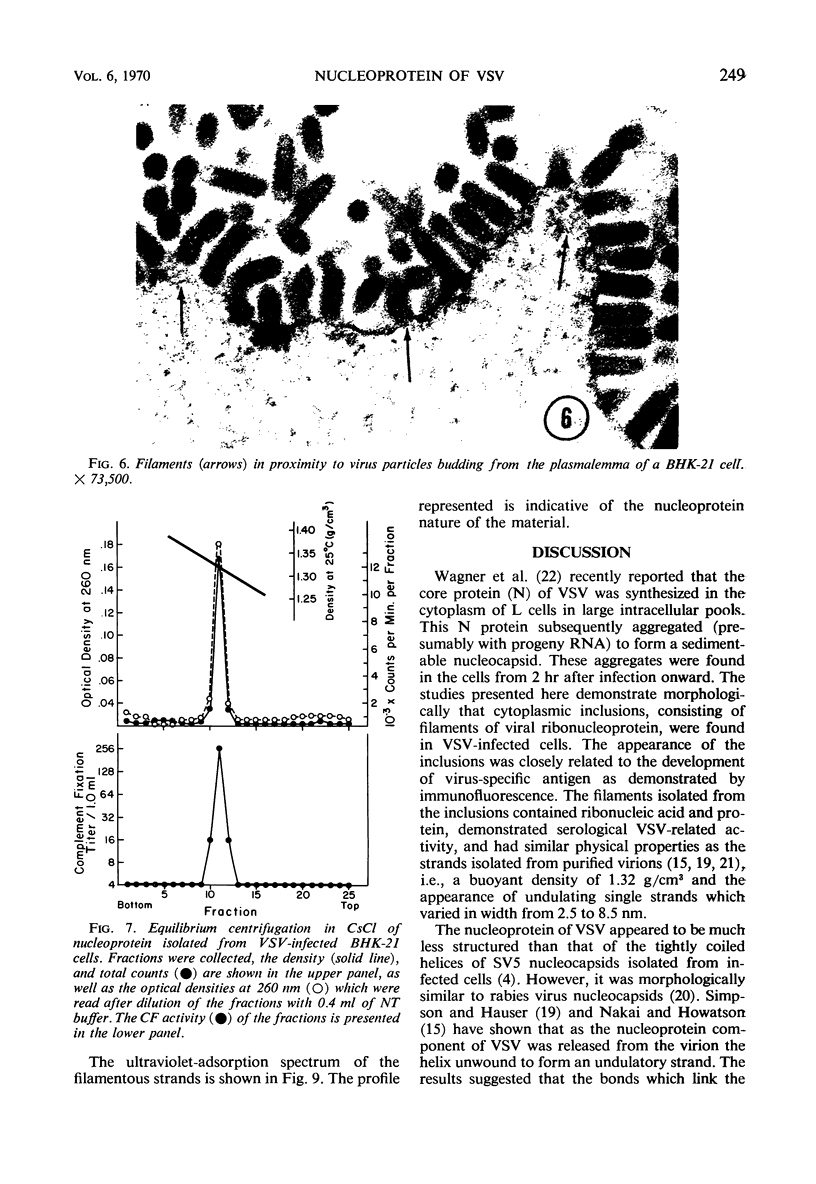
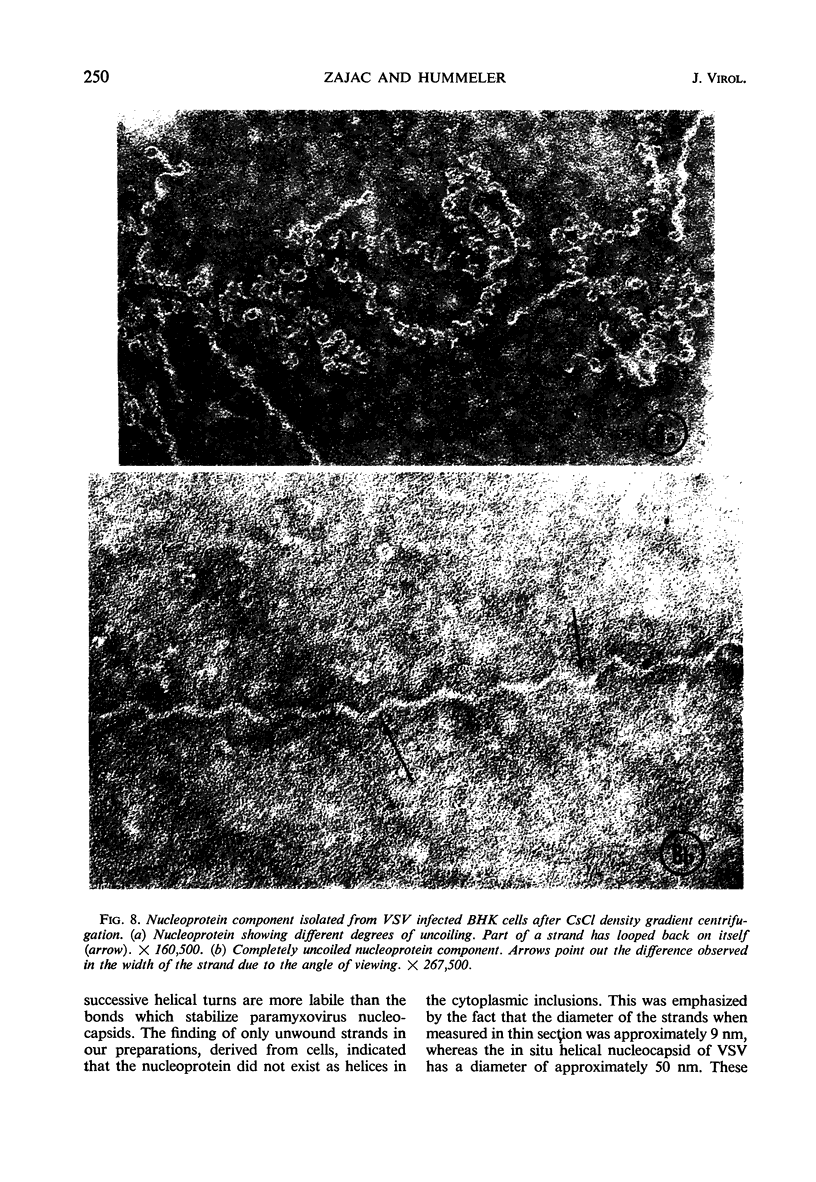
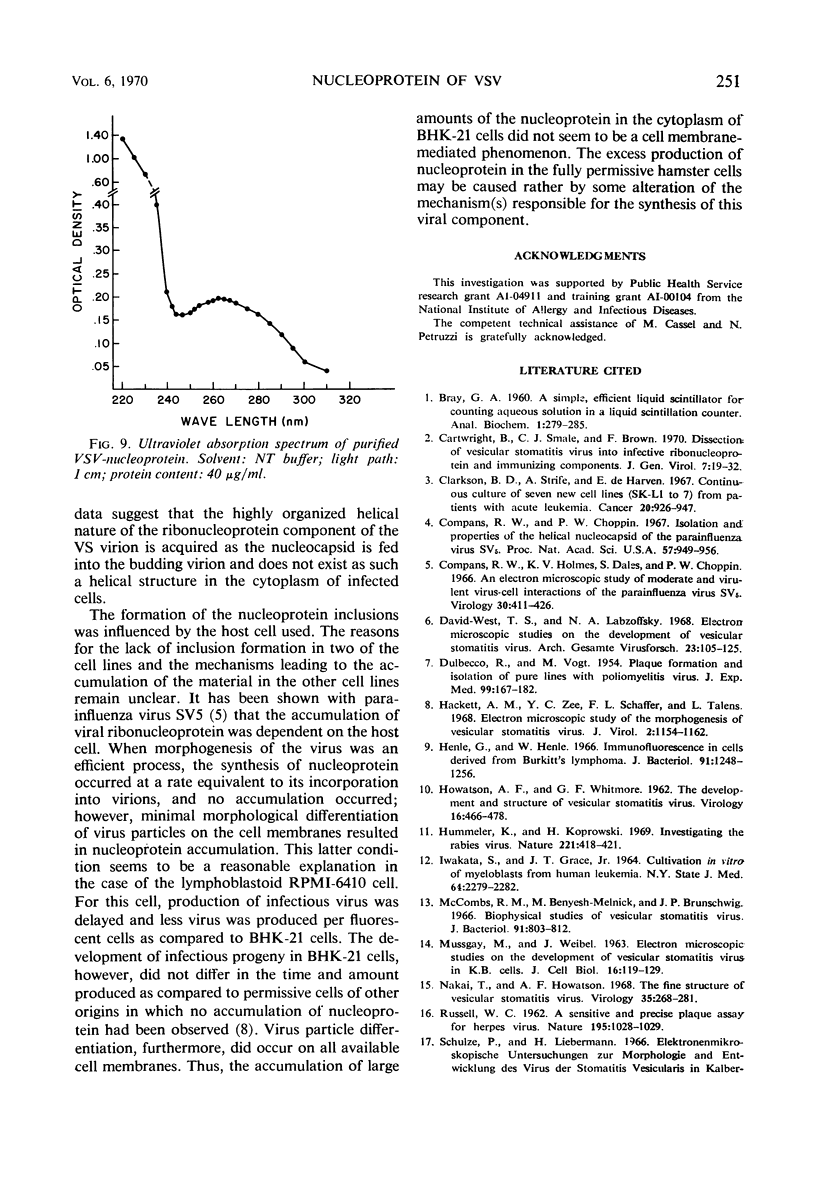
Accumulation of the nucleoprotein of vesicular stomatitis virus (VSV) in the cytoplasm of BHK-21 cells and in two of four human cell lines was demonstrated. Appearance and progression of the nucleoprotein inclusions paralleled development of virus-specific immunofluorescence and production of virus progeny. The inclusions appeared early as discrete foci of filamentous material which eventually increased in size to form large masses which replaced normal cytoplasmic constituents. The filamentous strands were found in close proximity to budding virions. The inclusion material was extracted from infected cells and purified in cesium chloride gradients. The isolated filaments resembled the ribonucleoprotein isolated from purified virions. They incorporated 3H-uridine, exhibited virus-specific complement-fixing activity, had a buoyant density of 1.32 g/cm3, and appeared as single wavy strands the width of which varied from 2.5 to 8.5 nm, depending on the angle of viewing.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cartwright B., Smale C. J., Brown F. Dissection of vesicular stomatitis virus into the infective ribonucleoprotein and immunizing components. J Gen Virol. 1970 Apr;7(1):19–32. doi: 10.1099/0022-1317-7-1-19. [DOI] [PubMed] [Google Scholar]
- Clarkson B., Strife A., De Harven E. Continuous culture of seven new cell lines (SK-L1 to 7) from patients with acute leukemia. Cancer. 1967 Jun;20(6):926–947. doi: 10.1002/1097-0142(196706)20:6<926::aid-cncr2820200603>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Compans R. W., Choppin P. W. Isolation and properties of the helical nucleocapsid of the parainfluenza virus SV5. Proc Natl Acad Sci U S A. 1967 Apr;57(4):949–956. doi: 10.1073/pnas.57.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compans R. W., Holmes K. V., Dales S., Choppin P. W. An electron microscopic study of moderate and virulent virus-cell interactions of the parainfluenza virus SV5. Virology. 1966 Nov;30(3):411–426. doi: 10.1016/0042-6822(66)90119-x. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David-West T. S., Labzoffsky N. A. Electron microscopic studies on the development of vesicular stomatitis virus. Arch Gesamte Virusforsch. 1968;23(1):105–125. doi: 10.1007/BF01242119. [DOI] [PubMed] [Google Scholar]
- HOWATSON A. F., WHITMORE G. F. The development and structure of vesicular stomatitis virus. Virology. 1962 Apr;16:466–478. doi: 10.1016/0042-6822(62)90228-3. [DOI] [PubMed] [Google Scholar]
- Hackett A. J., Zee Y. C., Schaffer F. L., Talens L. Electron microscopic study of the morphogenesis of vesicular stomatitis virus. J Virol. 1968 Oct;2(10):1154–1162. doi: 10.1128/jvi.2.10.1154-1162.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle G., Henle W. Immunofluorescence in cells derived from Burkitt's lymphoma. J Bacteriol. 1966 Mar;91(3):1248–1256. doi: 10.1128/jb.91.3.1248-1256.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummeler K., Koprowski H. Investigating the rabies virus. Nature. 1969 Feb 1;221(5179):418–421. doi: 10.1038/221418a0. [DOI] [PubMed] [Google Scholar]
- MUSSGAY M., WEIBEL J. Electron microscopic studies on the development of vesicular stomatitis virus in KB cells. J Cell Biol. 1963 Jan;16:119–129. doi: 10.1083/jcb.16.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCombs R. M., Melnick M. B., Brunschwig J. P. Biophysical studies of vesicular stomatitis virus. J Bacteriol. 1966 Feb;91(2):803–812. doi: 10.1128/jb.91.2.803-812.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai T., Howatson A. F. The fine structure of vesicular stomatitis virus. Virology. 1968 Jun;35(2):268–281. doi: 10.1016/0042-6822(68)90267-5. [DOI] [PubMed] [Google Scholar]
- RUSSELL W. C. A sensitive and precise plaque assay for herpes virus. Nature. 1962 Sep 8;195:1028–1029. doi: 10.1038/1951028a0. [DOI] [PubMed] [Google Scholar]
- Schulze P., Liebermann H. Elektronenmikroskopische Untersuchungen zur Morphologie und Entwicklung des Virus der Stomatitis vesicularis in Kälbernierenzellkulturen. Arch Exp Veterinarmed. 1966 Sep;20(4):713–729. [PubMed] [Google Scholar]
- Sedwick W. D., Sokol F. Nucleic acid of rubella virus and its replication in hamster kidney cells. J Virol. 1970 Apr;5(4):478–489. doi: 10.1128/jvi.5.4.478-489.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R. W., Hauser R. E. Structural components of vesicular stomatitis virus. Virology. 1966 Aug;29(4):654–667. doi: 10.1016/0042-6822(66)90289-3. [DOI] [PubMed] [Google Scholar]
- Sokol F., Schlumberger H. D., Wiktor T. J., Koprowski H. Biochemical and biophysical studies on the nucleocapsid and on the RNA of rabies virus. Virology. 1969 Aug;38(4):651–665. doi: 10.1016/0042-6822(69)90184-6. [DOI] [PubMed] [Google Scholar]
- Wagner R. R., Schnaitman T. C., Snyder R. M., Schnaitman C. A. Protein composition of the structural components of vesicular stomatitis virus. J Virol. 1969 Jun;3(6):611–618. doi: 10.1128/jvi.3.6.611-618.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. R., Snyder R. M., Yamazaki S. Proteins of vesicular stomatitis virus: kinetics and cellular sites of synthesis. J Virol. 1970 May;5(5):548–558. doi: 10.1128/jvi.5.5.548-558.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]