Abstract
Efficient skeletal muscle repair and regeneration require coordinated remodeling of the extracellular matrix (ECM). Previous reports have indicated that matrix metalloproteinases (MMPs) play the pivotal role in ECM remodeling during muscle regeneration. The goal of the current study was to determine if the interstitial collagenase MMP-13 was involved in the muscle repair process. Using intramuscular cardiotoxin injections to induce acute muscle injury, we found that MMP-13 expression and activity transiently increased during the regeneration process. In addition, in muscles from mdx mice, which exhibit chronic injury, MMP-13 expression and protein levels were elevated. In differentiating C2C12 cells, a murine myoblast cell line, Mmp13 expression was most pronounced after myoblast fusion and during myotube formation. Using pharmacological inhibition of MMP-13 to test whether MMP-13 activity is necessary for the proliferation, differentiation, migration, and fusion of C2C12 cells, we found a dramatic blockade of myoblast migration, as well as a delay in differentiation. In contrast, C2C12 cells with stable overexpression of MMP-13 showed enhanced migration, without affecting myoblast maturation. Taken together, these results support a primary role for MMP-13 in myoblast migration that leads to secondary effects on differentiation.
Keywords: collagenase, matrix metalloproteinase, muscle repair, myoblast maturation
the extracellular matrix (ECM) environment undergoes tightly regulated remodeling during development and tissue repair. The enzymes responsible for the process include the matrix metalloproteinases (MMPs), a large family of proteinases whose primary targets are components of the ECM. Control of MMP activity occurs at several levels: transcription, activation of the precursor zymogen, and inhibition by endogenous inhibitors (tissue inhibitors of metalloproteinases). Without regulation, active MMPs can cause extensive ECM remodeling and result in a spectrum of pathological conditions, including arthritis, cancer, atherosclerosis, and fibrosis (21, 24, 27). However, the absence of MMP activity results in unresolved repair in bone (16), stalled development (14), and impaired wound healing (reviewed in Ref. 11). Therefore, appropriately coordinated activity of MMPs is essential for proper ECM remodeling during development and tissue repair.
Remodeling of the ECM by MMPs is a major feature of skeletal muscle repair, which is required to remove damaged tissue, recreate the ECM scaffold, and release reservoirs of stored growth factors, to ultimately result in the reconstruction of functioning muscle fibers. MMP-2 (gelatinase A, or 72-kDa type IV collagenase) and MMP-9 (gelatinase B, or 92-kDa type IV collagenase) have been examined extensively, although other MMPs are expressed at lower levels. In healthy adult muscle, the activities of MMP-2 and MMP-9 are minimal, for there is little need for ECM remodeling. However, upon muscle injury, MMP activation is an essential part of the repair process. Examination of an experimental model of muscle regeneration [cardiotoxin (CTX) injection] has documented a tightly regulated time course of MMP activation that is consistent with the resolution of damage (12, 15, 35). Generalized MMP inhibition impairs muscle repair (3), supporting the idea that these proteases are required to resolve muscle damage. Because the ECM of muscle contains many proteins, the need for other MMPs in addition to MMP-2 and MMP-9 is warranted, particularly for degradation of the abundant native fibrillar collagens in skeletal muscle. We recently found that muscle expresses MMP-13 in response to viral delivery of IGF-I, but that it is normally absent in healthy muscle (2). MMP-13, an interstitial collagenase and a member of a protease family that includes MMP-1 and MMP-8, was initially discovered in a breast carcinoma (10). MMP-13 is a very potent ECM-degrading enzyme and a key activator of other MMPs. It cleaves the interstitial collagens I, II, III, and IV. It also has potent activity against aggrecan, perlecan, fibronectin, fibrillin, and, potentially, biglycan (reviewed in Ref. 17). In addition to its role in ECM degradation, MMP-13 is pivotal in the MMP activation cascade. Specifically, MMP-13 is activated by MMP-2, MMP-3, and MMP-14 (a membrane-bound MMP) and, in turn, can activate MMP-2 and MMP-9.
In other cell types, MMP-13 activity is central to cell migration, most evident in breast carcinoma metastasis (32). In addition, it plays an important role in cutaneous wound healing (31) and bone formation (14). While different cell types are involved in these processes, the steps parallel those of skeletal muscle repair, where cells must proliferate and migrate to sites of damage and then coordinate the restoration of functional intact tissues. Consistent with this observation, in muscle from mdx mice, a model for Duchenne muscular dystrophy with heightened regeneration and degeneration, expression of Mmp13 is elevated (25). The upregulation of MMP-13 in dystrophic muscle could be a response to and indicative of the heightened inflammation and degeneration in diseased muscle. As such, MMP-13 could be beneficial for repair and increased to resolve muscle damage, or, alternatively, increased MMP-13 activity could impair repair. Because little is known about the actions of MMP-13 in muscle, the goal of the current study is to determine the time course of MMP-13 production and activity in vivo and in vitro and to assess whether it is necessary for the processes underlying muscle formation using myoblast cell lines.
MATERIALS AND METHODS
Muscle injury models.
All animal experiments were approved by the University of Pennsylvania Institutional Animal Care and Use Committee. Ten- to 12-wk-old male C57BL/6 mice were lightly anesthetized with ketamine and xylazine; then one anterior hindlimb was injected with 50 μl of 10 μM CTX (Sigma-Aldrich), targeting the tibialis anterior. This instigates acute damage and a well-documented time course of regeneration. The contralateral limb served as a control. Muscles were harvested from euthanized mice at 1, 2, 5, 7, and 11 days postinjection (n = 3 per time point). Muscles were rapidly frozen and stored in liquid nitrogen for subsequent assays. In addition to an acute injury model, male mdx mice of the same age were used to represent chronic injury. Upon euthanasia, muscles were rapidly frozen as described above.
Myoblast cultures.
Murine C2C12 cells, a myoblast cell line, were cultured directly on plastic in growth medium (GM: DMEM supplemented with 10% FBS and 0.2% gentamicin) at 37°C and 5% CO2. At 80% confluence, differentiation was induced by replacement of GM with differentiation medium (DM: DMEM supplemented with 2% horse serum and 0.2% gentamicin). Medium was replaced daily, and cells were harvested after 6, 24, 48, and 72 h of incubation in DM for subsequent molecular measurements.
To inhibit MMP-13 activity, MMP-13 inhibitor [IUPAC name pyrimidine-4,6-dicarboxylic acid (catalog no. 444283, Calbiochem), or bis-(4-fluoro-3-methyl-benzylamide) (CAS no. 544678-85-5)] was dissolved in DMSO and added with each medium replacement to a final concentration of 10 μM; the same volume of DMSO without inhibitor was added to the control cells. This inhibitor has high specificity for MMP-13 and binds to the catalytic domain (8). To inhibit MMP-2 activity, MMP-2 inhibitor [IUPAC name 2-((isopropoxy)-(1,1′-biphenyl-4-ylsulfonyl)-amino))-N-hydroxyacetamide (catalog no. 444288, Calbiochem), CAS no. 704888-90-4] was utilized as described above.
To increase MMP-13 levels, stable cell lines were generated following transfection of pCMV.IRES.eGFP (Clontech, Mountain View, CA) containing the cDNA for murine Mmp13 (accession no. NM_008607) using previously published methods (26). This vector contains the cytomegalovirus (CMV) promoter to drive high expression of genes of interest, an internal ribosomal entry site (IRES) to afford bicistronic expression, and enhanced green fluorescent protein (eGFP) to help identify cells harboring the plasmid and expressing the transgenes. Transfection was performed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). At 48 h after transfection, the cells were passaged and selected with G418 (900 μg/ml). Ten single colonies from each construct were isolated and expanded. Clonal expression of MMP-13 was confirmed by PCR, immunoblotting, and green fluorescent protein (GFP) staining. As a control, stable lines containing vector only (GFP) were also generated. Stable cell lines were maintained in GM + 200 μg/ml G418.
Cell proliferation assay.
Proliferation was measured by 5-bromo-2P-deoxyuridine (BrdU) incorporation, as previously described (4). C2C12 cells (1 × 105) were seeded onto 24-well plates with glass coverslips in GM with and without MMP-13 inhibitor. Stable cell lines were seeded in the same manner, and after 24 h to allow attachment, serum was removed. After 24 h of incubation in serum-free medium, the cells were incubated for 60 min with BrdU-labeling medium containing 10 μM BrdU dissolved in DMEM (BrdU Detection Kit I Immunofluorescence Assay, catalog no. 11296736001, Roche Diagnostics). Cells were stained with an antibody to BrdU and with 4′,6-diaminido-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA) to visualize nuclei. Images were acquired using a Leica DMR epifluorescence microscope and OpenLab imaging software (Improvision, PerkinElmer, Waltham, MA). Experiments were done in triplicate for each condition.
Cell Transwell assay.
The effects of MMP-13 on myoblast cell movement were tested using 24-well Transwell plate assays, as previously described (4), with the following modifications. For MMP-13 and MMP-2 inhibition, 2 × 104 C2C12 cells were seeded in the upper chamber, and GM + inhibitor for MMP-13, MMP-2, or both or DMSO only was added to the lower chamber prior to transfer of the upper chamber onto the Transwell plate. Cells were allowed to migrate for 6 or 12 h. For increased MMP-13, stable cell lines expressing Mmp13 or GFP were seeded on the upper chamber with serum-free medium and allowed to migrate for 6 h. Nonmigrated cells on the upper side of the filter were wiped with Q-tips; the migrated cells attached to the lower side of the filter were fixed with 4% formaldehyde for 10 min, washed three times with PBS, stained with crystal violet solution for 30 min, and rinsed with PBS. The filters were cut out from the Transwell plates, mounted onto glass slides with mounting medium, and viewed under a Leica DFC300 light microscope. Four high-power (×100 magnification) fields were randomly chosen from each preparation, and the total number of cells per field was determined. Each condition was performed at least in triplicate.
Gene expression analysis.
Total RNA was isolated from tissues and cells using the TRIzol reagent isolation method (Invitrogen), and RNA concentration and purity were determined. For cDNA synthesis, a 20-μl RT reaction was carried out on 1 μg of total RNA with the GeneAmp RNA PCR kit (Applied Biosystems) according to the product protocol. Real-time PCR was performed on resultant cDNA using Power SYBR Green Master Mix (Applied Biosystems) and the Applied Biosystems 7300 Real Time PCR System. Expression levels of markers of differentiation, as well as members of the MMP family, were determined, with 18S expression serving as the reference housekeeping gene. Primers are listed in Table 1.
Table 1.
Primers used for PCR
| Gene | Primer (5′–3′) |
|
|---|---|---|
| Sense | Antisense | |
| 18S | CTCTGTTCCGCCTAGTCCTG | AATGAGCCATTCGCAGTTTC |
| MyoD | GGCTCTCTCTGCTCCTTTGA | AGTAGGGAAGTGTGCGTGCT |
| Myogenin | GGGCCCCTGGAAGAAAAG | AGGAGGCGCTGTGGGAGT |
| Myo3 | GCATAGCTGCACCTTTCCTC | CGTGTATCGGTCCTTGAGGT |
| Mmp13 | AGTTGACAGGCTCCGAGAAA | CACATCAGGCACTCCACATC |
| Mmp2 | ACCCTGGGAGAAGGACAAGT | ATCACTGCGACCAGTGTCTG |
| Mmp9 | CGTCGTGATCCCCACTTACT | AACACACAGGGTTTGCCTTC |
Mmp, matrix metalloproteinase.
Collagen zymography.
Collagen gels were prepared in the laboratory following published methods (13) using Gel Sol (UltraPure ProtoGel, National Diagnostics), resolving buffer (ProtoGel Resolving Buffer, National Diagnostics), 1 mg/ml collagen (rat tail collagen type I, BD Biosciences), stacking buffer (ProtoGel Stacking Buffer, National Diagnostics), and distilled water. Tetramethylethylenediamine (Bio-Rad, Hercules, CA) and ammonium persulfate (Fischer Scientific) were used to catalyze polymerization of the polyacrylamide gel.
Frozen tissues were lysed with zymogen extraction buffer (100 mM Tris·HCl, 200 mM NaCl, 100 mM CaCl2, and 1% Triton X-100, pH 7.6). The protein concentrations were measured by the Bradford method (Bio-Rad protein assay). Samples were electrophoresed on collagen gels and then renatured with zymogram renaturing buffer (2.5% Triton X-100 in distilled water) under gentle agitation for 1 h at room temperature. Gels were equilibrated for 30 min at room temperature with zymogram developing buffer (50 mM Tris·HCl, 0.2 M NaCl, 5 mM CaCl2, and 0.02% Brij 35) and incubated with fresh zymogram developing buffer at 37°C for 24 h. After 24 h, the gels were stained with Coomassie blue (0.25% Coomassie blue, 50% methanol, 30% acetic acid, and 10% glycerol) for 1 h at room temperature and incubated in destaining solution (10% glycerol, 10% acetic acid, and 10% methanol) until areas of protease activity were resolved.
Immunoblotting.
Total protein was obtained from each sample with RIPA lysis buffer [50 mM Tris·HCl (pH 7.4), 1% (wt/vol) Triton X-100, 0.25% sodium deoxycholate, 150 mM NaCl, and protease inhibitor cocktail; catalog no. P8340, Sigma] or zymogen extract buffer (see above). Tissue homogenates and cell lysates were centrifuged to pellet debris, and total protein in the supernatant was measured using the Bradford procedure (Bio-Rad). The samples were electrophoresed on 10% SDS-PAGE precast commercial gels (Bio-Rad) and then electrotransferred onto Immobilon-P polyvinylidene difluoride transfer membranes (Millipore). After inhibition of nonspecific binding by incubation with 5% nonfat dry milk in Tris-buffered saline-Tween 20, the membrane was incubated overnight at 4°C with mouse anti-MMP-13 (1:400 dilution; catalog no. IM78, Calbiochem). The membrane was washed and then incubated with horseradish peroxidase-linked secondary antibody (anti-mouse or anti-rabbit, 1:2,000 dilution; Cell Signaling Technology) for 90 min at room temperature. Protein was detected using enhanced chemiluminescence (Western Lightning ECL, PerkinElmer) and the ImageQuant detection system (GE Healthcare Biosciences, Pittsburgh, PA). Equal protein loading was confirmed by immunoblotting for GAPDH (catalog no. sc-32233, Santa Cruz Biotechnology, Santa Cruz, CA).
MMP activity assays.
Zymogen extracts from C2C12 cell culture were tested for MMP-13 activity. Samples were diluted to 50–150 μg/ml in assay buffer (50 mM Tris·HCl-10 mM CaCl2). The specificity of the MMP-13 inhibitor was determined by addition of 100 nM–10 μM inhibitor to samples containing 50 ng/ml recombinant MMP-2 or MMP-13 (AnaSpec, San Jose, CA) diluted in assay buffer. Trypsin (10 μg/ml) was added for cleavage of the inhibiting proregion of recombinant MMP-13. All samples were incubated at 37°C for 20 min to facilitate MMP activation. Soybean inhibitor (80 μM) was added to inactivate trypsin and to prevent nonspecific cleavage.
The activity of samples was assessed using fluorogenic MMP-13- or MMP-2-specific substrates (EMD Millipore, Billerica, MA). Substrate stock consisted of 1 mg/ml substrate dissolved in DMSO, which was diluted in assay buffer to produce substrate buffer. Twenty microliters of prepared sample cocktail were mixed with 80 μl of substrate buffer and added to clear-bottom black 96-well plates, and fluorescence was read using a fluorescence plate reader (SpectraMax M5, Molecular Devices). The fluorogenic portion of the substrate is excited after disassociation from the quencher by cleavage of the substrate by MMP. Measurements were taken at wavelengths of 325 nm (excitation) and 393 nm (emission). Fluorescence was measured every 10–30 min for a total of 120 min.
For calculation of MMP activity, each measurement was background-corrected to the average of the controls containing substrate only at the same time point. The slope of background-corrected fluorescence vs. time was calculated as a measure of MMP activity for each sample. MMP activity was normalized to the level of activity without MMP-13 inhibitor for recombinant MMP-13 and MMP-2.
Immunocytochemistry.
C2C12 cells were cultured in 24-well plates on coverslips in GM or DM for 0–3 days. At each time point, cells were fixed in 4% formaldehyde. Cells were washed with 0.5% Triton X-100 in PBS under gentle shaking and then incubated at 37°C for 1 h with a primary antibody: mouse anti-myosin (MF20, 1:5 dilution; Iowa Developmental Studies Hybridoma Bank) or mouse anti-MMP-13 (catalog no. IM78, Calbiochem). Cells were then incubated for 1 h at 37°C with phalloidin (1:50 dilution; Alexa Fluor 488, Invitrogen) and an anti-mouse secondary antibody (1:500 dilution; Alexa Fluor 555, Invitrogen). Finally, cells were washed with 0.5% Triton X-100 in PBS under gentle shaking. The coverslips with cells were mounted on glass slides with Vectashield mounting medium for fluorescence with DAPI. Image analysis was performed on a Leica DMR epifluorescence microscope. Three high-power (×200–400 magnification) fields were randomly chosen in each preparation. Negative control slides were subjected to the same treatment, but without primary antibody.
Fusion assay.
Cells that stained positive for MF20 and contained three or more nuclei were considered myotubes and were taken into account for the fusion assay. The number of myotubes per microscopic field was tallied for each day of differentiation.
Statistics.
Analysis for proliferation and a subset of migration assays was performed using a two-tailed unpaired t-test. For all other assays, one- and two-way ANOVA was followed by Bonferroni's post hoc analysis. Statistical significance was accepted at P < 0.05.
RESULTS
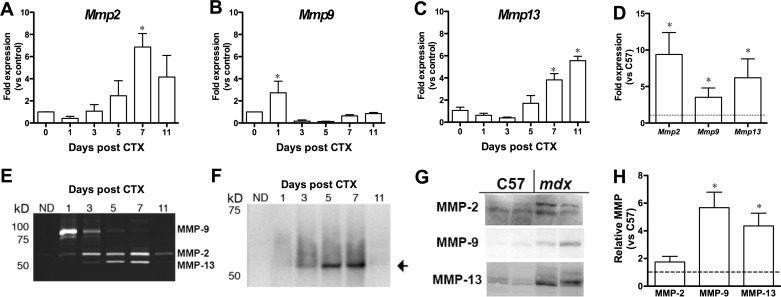
Skeletal muscle regeneration requires timely expression of genes to coordinate activation, proliferation, migration, and differentiation of satellite cells, as well as to regulate ECM remodeling. To determine the time course of MMP expression in regenerating muscle, CTX was injected into the anterior muscle compartment of adult C57 mice. This is a very reproducible model of muscle regeneration after acute injury. Tibialis anterior muscles were harvested 1–11 days after CTX injection to capture the time course of regeneration and processed for quantitative RT-PCR, collagen zymography (13), and immunoblotting (Fig. 1). Mmp2 expression was elevated transiently 1 wk after CTX injection (Fig. 1A). Mmp9 expression was elevated early in the repair process but fell to below control muscle values by day 3 (Fig. 1B). This is similar to previous measurements of Mmp2 and Mmp9 expression during muscle regeneration (1, 15). In contrast, Mmp13 expression did not increase until ∼1 wk after CTX injection and remained high (Fig. 1C). Collagen zymography results reflected the levels of expression (Fig. 1E), where increased MMP-9 activity was observed in the early phases of regeneration, MMP-2 activity was detectable throughout regeneration with a maximum level at 7 days after CTX injection. The band for MMP-13 was confirmed by immunoblotting of the zymogen extracts (Fig. 1F). Thus each MMP had a distinctive time course during the muscle regeneration process following acute injury.
Fig. 1.
Matrix metalloproteinase (MMP) levels in acute and chronic muscle damage. A–C: results from quantitative RT-PCR show different patterns of Mmp2, Mmp9, and Mmp13 expression during repair following cardiotoxin (CTX) injection. *Significantly different (P < 0.05, by 1-way ANOVA followed by Bonferroni's post hoc analysis) from no damage (day 0). D: elevation of Mmp2, Mmp9, and Mmp13 expression in muscles from mdx compared with C57BL/6 (C57) mice (dashed line). *P < 0.05, mdx vs. C57 (by unpaired t-test). E: collagen zymography showing activity of MMP-2, MMP-9, and MMP-13. Activity follows a time course similar to that of transcriptional changes during repair following CTX injection. Samples consist of 15 μg of protein from zymogen extracts of CTX-injected and noninjected (ND) muscles. F: immunoblots of MMP-13 in zymogen extracts of regenerating muscle. Results further confirm that the 52-kDa band is MMP-13. G: immunoblots of MMP-2, MMP-9, and MMP-13 in muscles from mdx and C57 mice. H: results from G showing significantly higher MMP-9 and MMP-13 levels in mdx muscles than in C57 (dashed line). *P < 0.05, mdx vs. C57 (by unpaired t-test).
To extend our analysis to chronic damage associated with disease, we measured expression and protein levels of MMP-2, MMP-9, and MMP-13 in muscles from mdx mice, a murine model for Duchenne muscular dystrophy. Expression of all three Mmps was significantly higher in dystrophic muscles (Fig. 1D), consistent with previous reports (15, 25). Furthermore, protein levels of MMP-9 and MMP-13 were significantly higher in tissue from mdx than wild-type control mice (Fig. 1, G and H). On the basis of acute and chronic damage models, MMP-13 expression and activity were correlated with muscle regeneration.
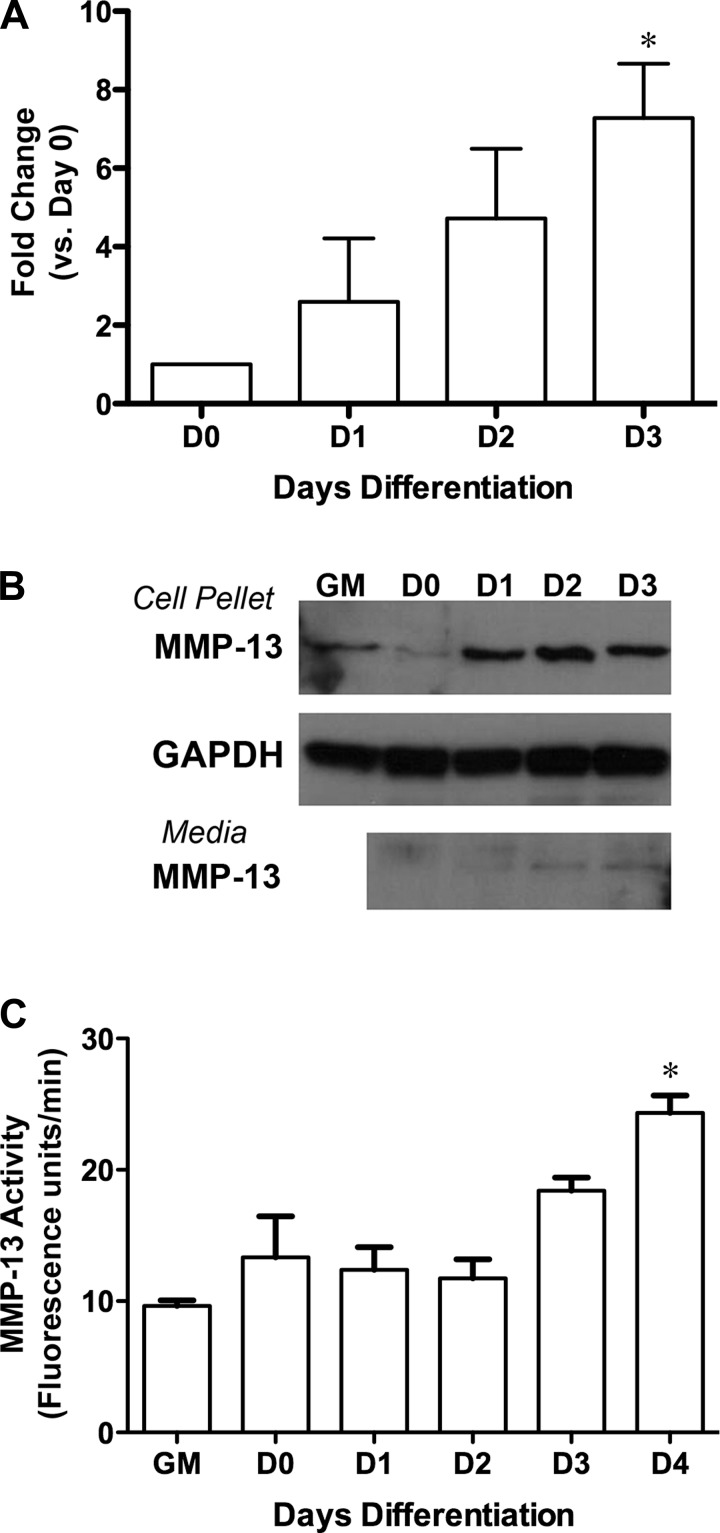
Many cell types within muscle contribute to its repair. To clarify if skeletal muscle could produce MMP-13, we used C2C12 cells, a murine myoblast cell line, to examine expression, protein level, and activity of MMP-13 during differentiation. Mmp13 expression was most pronounced after myoblast fusion and during myotube generation, at days 2–3 of differentiation in the cultures (Fig. 2A). Immunoblotting for MMP-13 using zymogen extracts of cell pellets showed a robust increase in protein by day 1 of differentiation (Fig. 2B). Furthermore, evidence of MMP-13 secretion into the medium was detected by day 1 of differentiation, although detection required a fivefold concentration of the medium. To determine if MMP-13 pools were active, a fluorogenic activity assay was utilized. We found that MMP-13 activity in zymogen extracts of cell pellets increased during differentiation (Fig. 2C) but was delayed with respect to protein levels. MMP-13 activity was also tested in the medium but was below the limit of detection for our assay and was not pursued further.
Fig. 2.
Change in MMP-13 in differentiating C2C12 cells. A: progressive increase in Mmp13 expression during differentiation. Values are means ± SE of 3 separate experiments. *P < 0.05 (by 1-way ANOVA followed by Bonferroni's post hoc analysis) vs. day 0 (D0). B: increase in MMP-13 protein levels by day 1 of differentiation and elevation of MMP-13 in proliferating cells [growth medium (GM) condition]. Serum-free medium collected every 24 h from differentiating myoblasts shows secreted MMP-13 by day 1 of differentiation. C: increased MMP-13 activity in zymogen extracts of cell pellets as myoblasts differentiate. Values are means ± SE of 3 separate experiments. *P < 0.05 vs. D0 (by 1-way ANOVA followed by Bonferroni's post hoc analysis).
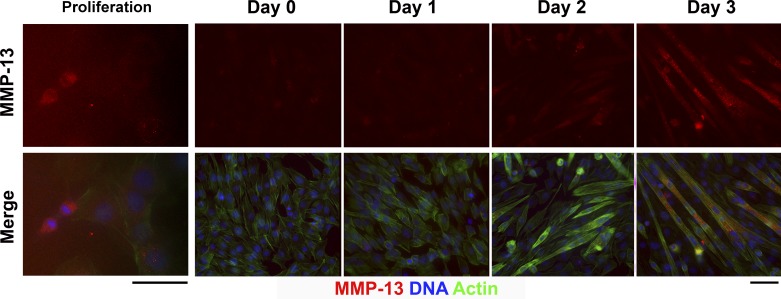
To determine the localization of MMP-13, immunocytochemistry for MMP-13 was also performed throughout the differentiation process. As shown in Fig. 3, differentiated myotubes exhibited robust staining for MMP-13 intracellularly, although faint staining could be detected in single myoblasts. MMP-13 expression was most pronounced in single myoblasts undergoing cell division. Therefore, myotubes and proliferating myoblasts produced MMP-13.
Fig. 3.
Immunocytochemistry for MMP-13 in proliferating and differentiating C2C12 cells. Top: MMP-13 staining alone (red). Bottom: merged image with phalloidin-Alexa 488 (staining actin, green) and 4′,6-diaminido-2-phenylindole (DAPI, blue) to reveal nuclei. Note increased MMP-13 in the daughter nuclei upon cell division (Proliferation, left). MMP-13 is not evident until day 2 of differentiation, after which myotubes exhibit positive cytoplasmic staining. Scale bars, 50 μm.
To determine whether MMP-13 was necessary for myoblast maturation, we used pharmacological inhibition to block activity and stable overexpression of Mmp13 to increase activity. As shown in Fig. 4A, the inhibitor caused 90% reduction of MMP-13 activity in an in vitro assay at an inhibitor concentration of 10 μM. To ensure that the inhibitor was specific for MMP-13 at this concentration, we also examined MMP-2 activity in the presence of increasing inhibitor concentration. There was no significant decrease in MMP-2 activity, supporting the specificity of the inhibitor.
Fig. 4.
Modulation of MMP-13 in myoblasts. A: increasing concentrations of MMP-13 inhibitor were added to samples of recombinant MMP-13 or MMP-2 (rMMP-13 and rMMP-2), and enzyme activity was measured for each MMP. MMP-13 activity was blocked in a dose-dependent manner, with significant decreases in activity at 1 and 10 μM inhibitor. MMP-2 activity was not affected by the inhibitor at any concentration. *P < 0.05 vs. 0 μM (by 1-way ANOVA followed by Bonferroni's post hoc analysis). B: immunoblot of stable C2C12 cells expressing MMP-13 and/or green fluorescent protein (GFP). Note heightened levels of MMP-13 in cell extracts from proliferating myoblasts (Cell Pellet). Secretion of MMP-13 is increased in serum-free medium (Medium) following 24 h of incubation with cells. GFP blotting is a control for transfection efficiency, and GAPDH is a loading control. Blot represents 1 of 3 clonal lines.
To determine the effects of increased MMP-13 in myoblasts, we generated C2C12 stable cell lines overexpressing MMP-13, where we measured mRNA and protein levels. Expression of Mmp13 was 103 ± 54 fold higher in stable cell line clones than in a GFP-expressing cell line (n = 3 cell lines). Immunoblotting of cell pellets and conditioned medium showed robust increases in MMP-13 in both compartments (Fig. 4B), consistent with the heightened expression levels.
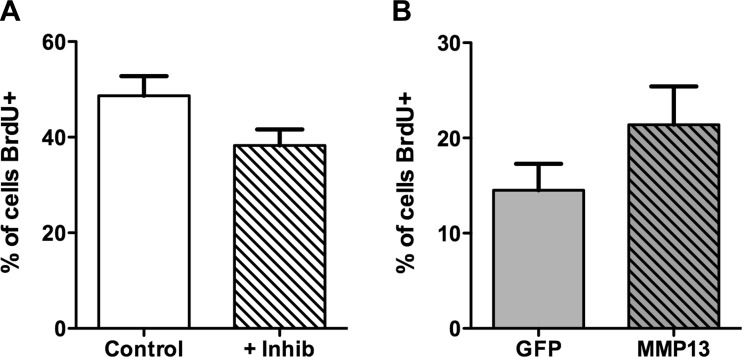
We utilized BrdU incorporation to measure myoblast proliferation while manipulating MMP-13. Over the 24-h measurement period, we found that inhibition of MMP-13 did not have a significant effect on the proliferation of C2C12 cells. Correspondingly, increased expression of Mmp13 in C2C12 cells did not affect cell proliferation (Fig. 5). Thus MMP-13 levels were not a modulating factor in myoblast proliferation.
Fig. 5.
Effect of MMP-13 on myoblast proliferation measured by 5-bromo-2P-deoxyuridine (BrdU) incorporation. A: MMP-13 inhibitor does not impair proliferation in C2C12 myoblasts in GM. B: stable expression of MMP-13 does not alter proliferation in C2C12 cells in the absence of serum compared with cells stably expressing GFP vector only. Values are means ± SE for 3 separate experiments.
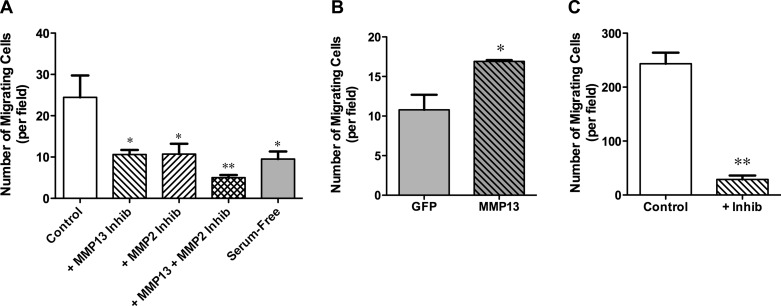
MMP-13 is known to regulate cell migration through its proteolytic activity. We used a Transwell migration assay to investigate if C2C12 cell migration is affected by MMP-13. MMP-13 inhibition resulted in a significant 50% reduction in cells passing through the Transwell membrane during a 6-h incubation period (Fig. 6A). Because MMP-2 can also regulate cell movement and has cross-activation with MMP-13, Transwell assays were performed with MMP-2 inhibition. This resulted in a similar reduction in Transwell migration (Fig. 6A), raising the possibility that MMP-2 or MMP-13 was directly regulating cell movement and that the other MMP regulated cell movement indirectly via activation of the former. Application of both MMP-13 and MMP-2 inhibitors further reduced migration to ∼20%, indicating a role for MMP-13 in myoblast migration independent of MMP-2. Stable cell lines expressing MMP-13 or GFP only were also tested in a Transwell assay, but in the absence of serum to uncover the specific effects of MMP-13. Stable cell lines exhibited the same degree of migration as native C2C12 cells over 6 h of incubation (Fig. 6, A and B). Migration was more efficient for MMP-13-overexpressing cells than for GFP cells (Fig. 6B). When incubation time was extended to 12 h, the MMP-13 inhibitor prevented passage of 90% of cells through the Transwell membrane (Fig. 6C). Taken together, these results indicate that MMP-13 is required for cell migration and that the endogenous levels of MMP-13 in myoblasts can be increased to enhance cell movement.
Fig. 6.
Effect of MMP-13 activity on myoblast migration measured by Transwell assays. A: MMP-13 or MMP-2 inhibition in GM significantly impairs movement of C2C12 myoblasts allowed to migrate for 6 h. The combination of both inhibitors further impairs migration of C2C12 myoblasts. Serum is a primary source for MMPs, because migration is significantly reduced in its absence. Values are means ± SE for 7 separate experiments. B: stable expression of MMP-13 in the absence of serum significantly increases migration of C2C12 myoblasts allowed to migrate for 6 h compared with cells stably expressing GFP vector only. Note values for cells stably expressing GFP vector only are equivalent to values for native C2C12 myoblasts in serum-free medium. Values are means ± SE for 3 separate experiments. C: MMP-13 inhibition significantly impairs migration of C2C12 myoblasts in GM allowed to migrate for 12 h. Values are means ± SE for 3 separate experiments. For each experiment, the average number of cells passing the Transwell filter from 5 random fields was determined. *P < 0.05, **P < 0.001 (by Bonferroni's post hoc tests and unpaired t-tests).
Myoblast differentiation indexes were measured to determine the effects of MMP-13 on this process. In both sets of experiments, control cells showed a progressive increase of myogenin and embryonic myosin expression, consistent with the patterns of myoblast maturation and differentiation. MMP-13 inhibition blocked the normal increase in myogenin and embryonic myosin expression in the cultures during differentiation (Fig. 7, A and C). Overexpression of MMP-13 in myoblasts caused no significant delay in the expression pattern of these differentiation markers compared with myoblasts expressing GFP only (Fig. 7, B and D). Thus, increased MMP-13 does not alter differentiation, but loss of MMP-13 activity suppresses this process.
Fig. 7.
Effect of MMP-13 activity on C2C12 differentiation. A: MMP-13 inhibition significantly impairs expression of myogenin in differentiating C2C12 cells. B: increased production of MMP-13 in stable C2C12 myoblasts undergoing differentiation does not alter myogenin expression. C: embryonic myosin heavy chain expression is reduced, evident at day 2 of differentiation in the presence of MMP-13 inhibitor. D: embryonic myosin heavy chain expression is not significantly altered in C2C12 cells with stable expression of MMP-13 compared with GFP vector controls. Values are means ± SE for 3 separate experiments. *P < 0.05 vs. D0 for each condition (by 2-way ANOVA followed by Bonferroni's post hoc analysis). †P < 0.05 vs. control (or GFP) at each time point(by 2-way ANOVA followed by Bonferroni's post hoc analysis).
A fusion assay was conducted to determine if MMP-13 manipulation altered myotube formation. Myosin-positive myotubes with three or more nuclei were counted. In each experimental condition, myotube formation was significantly increased by days 2 and 3 of differentiation (Fig. 8). Neither inhibition nor overexpression of MMP-13 altered the myotube accumulation, suggesting that myoblast fusion is not directly affected by MMP-13 activity.
Fig. 8.
Effect of MMP-13 activity on myoblast fusion. Myotubes expressing embryonic myosin and with ≥3 nuclei were counted. A: MMP-13 inhibition does not impair myoblast fusion during differentiation. B: stable expression of MMP-13 does not alter C2C12 myoblast fusion compared with cells stably expressing GFP vector only. Values are means ± SE for 3 separate experiments for each condition. *P < 0.05 vs. D0 for each condition (by 2-way ANOVA followed by Bonferroni's post hoc analysis).
DISCUSSION
This study adds MMP-13 to the family of MMPs involved in skeletal muscle repair. MMP-13 is expressed during the late phases of muscle regeneration in vivo, which is distinct from the patterns of MMP-2 and MMP-9. Accumulation of MMP-13 occurs in myotubes during differentiation, and levels of MMP-13 in myoblasts are also heightened at the point of cell division. Myoblast cultures enabled us to examine the direct effects of MMP-13 on their maturation and to isolate the steps that required this MMP. Pharmacological inhibition of MMP-13 resulted in a dramatic decrease in migration rates and delayed differentiation of C2C12 cells but little effect on cell proliferation or myoblast fusion. Similarly, MMP-13 overexpression altered only myoblast migration, where movement of stable cell lines expressing high levels of MMP-13 was enhanced compared with control cell lines. Thus, increasing MMP-13 activity in muscle may induce more efficient satellite cell migration to sites of damage and accelerate muscle repair.
To examine the role of MMP-13 during the phases of myoblast maturation, we implemented two strategies. 1) Using pharmacological inhibition of MMP-13 activity, we were able to inhibit the activity of MMP-13 by ∼90%, with negligible effects on other MMPs. Although the myoblasts under differentiating conditions did not secrete significant levels of active MMP-13 until day 4 of differentiation, the normal cellular environment within muscle may contain MMP-13 from other sources. Therefore, the inhibition experiments were performed in the presence of serum to ensure that there was an MMP-13 source to inhibit and to provide a more physiologically relevant context. 2) Stable overexpression of MMP-13 was used in experiments performed without serum to limit the effects of additional MMPs and to unmask the effects of heightened MMP-13 levels. Therefore, while we could not directly compare the results from inhibition and overexpression, given the differences in culture conditions, both strategies were required to determine the necessity and sufficiency of MMP-13 actions.
The primary effect of MMP-13 modulation was on cell migration. MMP-13 is commonly implicated in driving migration in a variety of cell types and is a highly prognostic biomarker for metastatic cancers (29). Similar enhancements to migration have been described for C2C12 cells and mesenchymal stem cells following exposure to the interstitial collagenase MMP-1 (30, 34). Because mice do not express MMP-1 (19) and because we observed dynamic changes in MMP-13 muscle tissues and myoblasts, we manipulated this protein directly. We limited our study to MMP-13, and so while the other MMPs may also regulate cell migration, we are confident that MMP-13 is important for this process.
What other MMPs contribute to myoblast behavior? A broad-spectrum MMP inhibitor was used in muscle, where not only was migration of C2C12 and mesenchymal stem cells impaired, but differentiation and skeletal muscle healing were also negatively affected (3). Conversely, boosting MMP activity can enhance muscle cell migration. For example, increased MMP-2 activity in muscles improves the transplantation efficiency of injected myoblasts (28). Furthermore, MMP-7, a matrilysin, enhances the migration of myoblasts and their engraftment when the myoblasts themselves express high levels of MMP-7 (6) or when the myoblasts are treated with the COOH-terminal extension of IGF-I, called the E-peptide, which in turn upregulates MMP-7 (22). Because the ECM has a multitude of different proteins, it is reasonable to assume that many different proteases, including the MMPs, are required to degrade the variety of protein substrates. MMPs can directly degrade a target protein, but because the MMPs are known to activate each other, one MMP could mediate degradation indirectly through activation of another MMP. This possibility motivated us to test the interactions of MMP-2 and MMP-13 in cell movement. If MMP-13 acted on substrates indirectly through MMP-2, then inhibition of MMP-13, MMP-2, or both would cause the same extent of blockade. While we found that individual inhibition MMP-2 or MMP-13 caused similar reductions in cell movement, the combined treatment caused an even more significant decrease. This supports the notion that MMP-2 and MMP-13 act directly on their respective substrates.
Despite expression of MMP-13 during cell division, there was no significant change in proliferation rates of C2C12 cells. Furthermore, there was no impairment of cell fusion in the absence of MMP-13. While cell migration must occur for myoblasts to fuse to each other, our cultured cells were 80% confluent, such that cells could fuse to neighboring myoblasts without extensive movement. Thus we assert that there is no direct effect of MMP-13 on myoblast fusion, per se. In contrast, we did observe a delay in expression of the differentiation markers myogenin and embryonic myosin when MMP-13 was inhibited, even though these delays were not sufficient to disrupt myoblast fusion. The changes in gene expression could be through direct actions of MMPs on transcription or the result of MMP-mediated release of growth factors. Direct actions on gene expression by MMP-1, i.e., upregulation of VEGF receptor-2 in endothelial cells following MMP-1 stimulation, were recently reported (20). Alternatively, MMPs can cleave IGF-binding proteins to release IGF-I (9), leading to indirect regulation of differentiation via IGF-I and, potentially, a regulatory feedback loop with IGF-I activity and several MMPs (2, 33). Increased MMP-13 did not alter differentiation markers, suggesting that the effect is already saturated in control cells, but a more thorough comparison of the changes in expression across additional genes is warranted.
Our finding of MMP-13 in proliferating myoblasts and in mature myotubes suggests two areas where its effect on muscle repair might be harnessed. The myoblasts, upon division, showed increased staining, which suggests that MMP-13 is secreted from these cells to move them away from each other, or toward a site of damage. This is consistent with the effects on cell migration by MMP-13 we report here. However, because it is also found in mature myotubes and in the later phases of muscle regeneration, additional actions of MMP-13 could occur. 1) MMP-13 activity from the muscle fibers could simply resolve scar formation to enable more efficient fiber formation and fiber growth. 2) MMP-13 secretion from fibers or myotubes could degrade the surrounding matrix to enable more efficient movement of myoblasts toward the fibers/myotubes. 3) MMP-13 activity could release growth factors from the matrix for the later phases of muscle repair, including VEGF to promote angiogenesis (5) and IGF-I to enhance fiber growth. These investigations will require in vivo models to address whether any of the proposed actions occur.
MMP-13 is not the only MMP in muscle, and others have clearly shown that modulation of MMP-2 and MMP-9 significantly affects skeletal muscle. For example, gene targeting of MMP-2 impairs neovascularization in muscles after ischemia and also causes diminished regenerative capacity of muscles of the mdx mouse (a model for Duchenne muscular dystrophy) (7, 23). The loss of interaction between MMP-2 and VEGF expression underlies these deficits. In contrast, MMP-9 ablation in mdx mice improves the phenotype (18). The transient appearance of MMP-9 early in the muscle repair phase is consistent with the heightened presence of immune cells, which produce it. However, in dystrophic tissue, where MMP-9 is constantly upregulated due to heightened inflammation, its actions become pathological, and not beneficial to the regeneration process. Therefore, it is clear that the process of muscle repair requires many different MMPs, and timing of their activities must be tightly coordinated for the complete and efficient resolution of muscle damage.
Whether modulation of MMP-13 will alter the regeneration process is an open question. The primary effect on myoblast migration may provide a regulatory step that can be enhanced if cells must undergo extensive movement to damaged sites. Furthermore, the presence of scar tissue could be resolved with heightened MMP-13 activity and afford efficient migration and better muscle integrity. Future studies will investigate the role of MMP-13 as a potential therapy based on the mechanistic insights established in myoblasts.
GRANTS
This study was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant AR-057363 to E. R. Barton. L. R. Smith was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases Training Grant AR-053461. D. Leong was supported by the Pennsylvania School of Dental Medicine Dean's Scholarship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.L., D.L., and L.R.S. performed the experiments; H.L., D.L., L.R.S., and E.R.B. analyzed the data; H.L., D.L., L.R.S., and E.R.B. interpreted the results of the experiments; H.L., D.L., L.R.S., and E.R.B. prepared the figures; H.L., L.R.S., and E.R.B. edited and revised the manuscript; H.L., D.L., L.R.S., and E.R.B. approved the final version of the manuscript; D.L. and E.R.B. drafted the manuscript; E.R.B. is responsible for conception and design of the research.
ACKNOWLEDGMENTS
The authors are grateful to members of the Barton lab for many helpful discussions.
REFERENCES
- 1.Bani C, Lagrota-Candido J, Pinheiro DF, Leite PE, Salimena MC, Henriques-Pons A, Quirico-Santos T. Pattern of metalloprotease activity and myofiber regeneration in skeletal muscles of mdx mice. Muscle Nerve 37: 583–592, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Barton ER, DeMeo J, Lei H. The insulin-like growth factor (IGF)-I E-peptides are required for isoform-specific gene expression and muscle hypertrophy after local IGF-I production. J Appl Physiol 108: 1069–1076, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellayr I, Holden K, Mu X, Pan H, Li Y. Matrix metalloproteinase inhibition negatively affects muscle stem cell behavior. Int J Clin Exp Pathol 6: 124–141, 2013 [PMC free article] [PubMed] [Google Scholar]
- 4.Brisson BK, Barton ER. Insulin-like growth factor-I E-peptide activity is dependent on the IGF-I receptor. PLos One 7: e45588, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown MD, Hudlicka O. Modulation of physiological angiogenesis in skeletal muscle by mechanical forces: involvement of VEGF and metalloproteinases. Angiogenesis 6: 1–14, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Caron NJ, Asselin I, Morel G, Tremblay JP. Increased myogenic potential and fusion of matrilysin-expressing myoblasts transplanted in mice. Cell Transplant 8: 465–476, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Cheng XW, Kuzuya M, Nakamura K, Maeda K, Tsuzuki M, Kim W, Sasaki T, Liu Z, Inoue N, Kondo T, Jin H, Numaguchi Y, Okumura K, Yokota M, Iguchi A, Murohara T. Mechanisms underlying the impairment of ischemia-induced neovascularization in matrix metalloproteinase 2-deficient mice. Circ Res 100: 904–913, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Engel CK, Pirard B, Schimanski S, Kirsch R, Habermann J, Klingler O, Schlotte V, Weithmann KU, Wendt KU. Structural basis for the highly selective inhibition of MMP-13. Chem Biol 12: 181–189, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Fowlkes JL, Thrailkill KM, Serra DM, Suzuki K, Nagase H. Matrix metalloproteinases as insulin-like growth factor binding protein-degrading proteinases. Prog Growth Factor Res 6: 255–263, 1995 [DOI] [PubMed] [Google Scholar]
- 10.Freije JM, Diez-Itza I, Balbin M, Sanchez LM, Blasco R, Tolivia J, Lopez-Otin C. Molecular cloning and expression of collagenase-3, a novel human matrix metalloproteinase produced by breast carcinomas. J Biol Chem 269: 16766–16773, 1994 [PubMed] [Google Scholar]
- 11.Gill SE, Parks WC. Metalloproteinases and their inhibitors: regulators of wound healing. Int J Biochem Cell Biol 40: 1334–1347, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goetsch SC, Hawke TJ, Gallardo TD, Richardson JA, Garry DJ. Transcriptional profiling and regulation of the extracellular matrix during muscle regeneration. Physiol Genomics 14: 261–271, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Gogly B, Groult N, Hornebeck W, Godeau G, Pellat B. Collagen zymography as a sensitive and specific technique for the determination of subpicogram levels of interstitial collagenase. Anal Biochem 255: 211–216, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Inada M, Wang Y, Byrne MH, Rahman MU, Miyaura C, Lopez-Otin C, Krane SM. Critical roles for collagenase-3 (Mmp13) in development of growth plate cartilage and in endochondral ossification. Proc Natl Acad Sci USA 101: 17192–17197, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kherif S, Lafuma C, Dehaupas M, Lachkar S, Fournier JG, Verdiere-Sahuque M, Fardeau M, Alameddine HS. Expression of matrix metalloproteinases 2 and 9 in regenerating skeletal muscle: a study in experimentally injured and mdx muscles. Dev Biol 205: 158–170, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Kosaki N, Takaishi H, Kamekura S, Kimura T, Okada Y, Minqi L, Amizuka N, Chung UI, Nakamura K, Kawaguchi H, Toyama Y, D'Armiento J. Impaired bone fracture healing in matrix metalloproteinase-13 deficient mice. Biochem Biophys Res Commun 354: 846–851, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Leeman MF, Curran S, Murray GI. The structure, regulation, and function of human matrix metalloproteinase-13. Crit Rev Biochem Mol Biol 37: 149–166, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Li H, Mittal A, Makonchuk DY, Bhatnagar S, Kumar A. Matrix metalloproteinase-9 inhibition ameliorates pathogenesis and improves skeletal muscle regeneration in muscular dystrophy. Hum Mol Genet 18: 2584–2598, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mariani TJ, Sandefur S, Roby JD, Pierce RA. Collagenase-3 induction in rat lung fibroblasts requires the combined effects of tumor necrosis factor-α and 12-lipoxygenase metabolites: a model of macrophage-induced, fibroblast-driven extracellular matrix remodeling during inflammatory lung injury. Mol Biol Cell 9: 1411–1424, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mazor R, Alsaigh T, Shaked H, Altshuler AE, Pocock ES, Kistler EB, Karin M, Schmid-Schonbein GW. Matrix metalloproteinase-1-mediated up-regulation of vascular endothelial growth factor-2 in endothelial cells. J Biol Chem 288: 598–607, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller MC, Manning HB, Jain A, Troeberg L, Dudhia J, Essex D, Sandison A, Seiki M, Nanchahal J, Nagase H, Itoh Y. Membrane type 1 matrix metalloproteinase is a crucial promoter of synovial invasion in human rheumatoid arthritis. Arthritis Rheum 60: 686–697, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mills P, Lafreniere JF, Benabdallah BF, El Fahime M, Tremblay JP. A new pro-migratory activity on human myogenic precursor cells for a synthetic peptide within the E domain of the mechano growth factor. Exp Cell Res 313: 527–537, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Miyazaki D, Nakamura A, Fukushima K, Yoshida K, Takeda S, Ikeda S. Matrix metalloproteinase-2 ablation in dystrophin-deficient mdx muscles reduces angiogenesis resulting in impaired growth of regenerated muscle fibers. Hum Mol Genet 20: 1787–1799, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Nistico P, Bissell MJ, Radisky DC. Epithelial-mesenchymal transition: general principles and pathological relevance with special emphasis on the role of matrix metalloproteinases. Cold Spring Harbor Perspect Biol 4: a011908, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Percival JM, Whitehead NP, Adams ME, Adamo CM, Beavo JA, Froehner SC. Sildenafil reduces respiratory muscle weakness and fibrosis in the mdx mouse model of Duchenne muscular dystrophy. J Pathol 228: 77–87, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfeffer LA, Brisson BK, Lei H, Barton ER. The insulin-like growth factor-I E-peptides modulate cell entry of the mature IGF-I protein. Mol Biol Cell 20: 3810–3817, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siefert SA, Sarkar R. Matrix metalloproteinases in vascular physiology and disease. Vascular 20: 210–216, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Torrente Y, El Fahime E, Caron NJ, Bresolin N, Tremblay JP. Intramuscular migration of myoblasts transplanted after muscle pretreatment with metalloproteinases. Cell Transplant 9: 539–549, 2000 [PubMed] [Google Scholar]
- 29.Vihinen P, Kahari VM. Matrix metalloproteinases in cancer: prognostic markers and therapeutic targets. Int J Cancer 99: 157–166, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Wang W, Pan H, Murray K, Jefferson BS, Li Y. Matrix metalloproteinase-1 promotes muscle cell migration and differentiation. Am J Pathol 174: 541–549, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu N, Opalenik S, Liu J, Jansen ED, Giro MG, Davidson JM. Real-time visualization of MMP-13 promoter activity in transgenic mice. Matrix Biol 21: 149–161, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Zhang B, Cao X, Liu Y, Cao W, Zhang F, Zhang S, Li H, Ning L, Fu L, Niu Y, Niu R, Sun B, Hao X. Tumor-derived matrix metalloproteinase-13 (MMP-13) correlates with poor prognoses of invasive breast cancer. BMC Cancer 8: 83, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang D, Samani AA, Brodt P. The role of the IGF-I receptor in the regulation of matrix metalloproteinases, tumor invasion and metastasis. Horm Metab Res 35: 802–808, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Zheng Z, Leng Y, Zhou C, Ma Z, Zhong Z, Shi XM, Zhang W. Effects of matrix metalloproteinase-1 on the myogenic differentiation of bone marrow-derived mesenchymal stem cells in vitro. Biochem Biophys Res Commun 428: 309–314, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Zimowska M, Brzoska E, Swierczynska M, Streminska W, Moraczewski J. Distinct patterns of MMP-9 and MMP-2 activity in slow and fast twitch skeletal muscle regeneration in vivo. Int J Dev Biol 52: 307–314, 2008 [DOI] [PubMed] [Google Scholar]