Abstract
The mechanisms governing maintenance of quiescence during pregnancy remain largely unknown. The current study characterizes a stretch-activated, tetraethylammonium-insensitive K+ current in smooth muscle cells isolated from pregnant human myometrium. This study hypothesizes that these K+ currents can be attributed to TREK-1 and that upregulation of this channel during pregnancy assists with the maintenance of a negative cell membrane potential, conceivably contributing to uterine quiescence until full term. The results of this study demonstrate that, in pregnant human myometrial cells, outward currents at 80 mV increased from 4.8 ± 1.5 to 19.4 ± 7.5 pA/pF and from 3.0 ± 0.8 to 11.8 ± 2.7 pA/pF with application of arachidonic acid (AA) and NaHCO3, respectively, causing intracellular acidification. Similarly, outward currents were inhibited following application of 10 μM fluphenazine by 51.2 ± 9.8% after activation by AA and by 73.9 ± 4.2% after activation by NaHCO3. In human embryonic kidney (HEK-293) cells stably expressing TREK-1, outward currents at 80 mV increased from 91.0 ± 23.8 to 247.5 ± 73.3 pA/pF and from 34.8 ± 8.9 to 218.6 ± 45.0 pA/pF with application of AA and NaHCO3, respectively. Correspondingly, outward currents were inhibited 89.5 ± 2.3% by 10 μM fluphenazine following activation by AA and by 91.6 ± 3.4% following activation by NaHCO3. Moreover, currents in human myometrial cells were activated by stretch and were reduced by transfection with small interfering RNA or extracellular acidification. Understanding gestational regulation of expression and gating of TREK-1 channels could be important in determining appropriate maintenance of uterine quiescence during pregnancy.
Keywords: human, uterus, pregnancy myometrium, smooth muscle, stretch-activated potassium channels, TREK-1
premature birth is the leading cause of death for newborns worldwide (7), accounts for 12% of all live births in the United States, appears to be increasing in frequency (29, 47), and disproportionately impacts African American mothers (42). Despite decades of interest, the majority of cases of preterm labor are unexplained, and no US Food and Drug Administration-approved tocolytic is available to prevent uterine contractions at the time of labor. Moreover, there is no satisfactory animal model in which to propose studies of spontaneous preterm labor mechanisms, since there is no animal that naturally experiences this uniquely human problem, notwithstanding the recent description of preterm delivery in mice harboring a p53 knockout (33). The Institute of Medicine estimated the cost to the US economy at a staggering $26.2 billion annually in 2005 (17). There is a pressing need to learn more about the mechanisms that normally keep the uterus quiescent during gestation until full term and to gain understanding of the mechanisms involved in normal and abnormal transitioning from the quiescent to the laboring state. Understanding the mechanisms responsible for maintenance of quiescence and possible dysregulation in some patients could yield novel insights into screening, diagnosis, and treatment of preterm labor, thereby reducing the significant impacts of preterm birth.
Multiple K+ channel subtypes have been reported to contribute to uterine function, and several of these channels have been proposed to facilitate uterine quiescence to full term during pregnancy. Large-conductance K+, or BKCa, channels are the predominant K+ channels in pregnant and nonpregnant myometrium (28). These BKCa channels are voltage- and Ca2+-sensitive and have been described to have a profound effect on myometrial relaxation during midgestation in rodents. BKCa channels have been extensively studied in myometrium because of their relatively high density and the significant repolarizing current induced by the considerable ∼200- to 300-pS conductance of these channels (10). Another category of ion channels, voltage-gated K+ (Kv) channels, are widely expressed in uterine smooth muscle cells, and their activation has been reported to contribute to basal uterine tone prior to labor (36). Kv channels appear to have an integral role in controlling basal rhythmicity in rat myometrium that is unaffected by inhibition of BKCa channels (1). Electrophysiological measurements have identified two principal Kv currents in human myometrium: rapidly inactivating and delayed rectifying currents (37, 64). A role for Kv channels in uterine contraction has been suggested on the basis of differential responsiveness of pregnant and nonpregnant mouse myometrium to the potent Kv channel blockers 4-aminopyridine and phrixotoxin-2 (59). Among Kv channels, the Kv4 subfamily has been hypothesized to play the most predominant role during pregnancy and parturition (10), and a significant reduction in Kv4.3 α-subunit expression was observed in full-term-pregnant mouse myometrium (59). Kv7 channels, which are encoded by the KCNQ and KCNE genes, have been reported in murine and human pregnant myometrium and could represent a novel target for tocolytics (44). Kv10.1 channels, encoded by the KCNH1 gene, are reported to be regulated as a precursor to late-pregnancy physiological activity in conjunction with upregulation of KCNE gene expression (30). ATP-sensitive inward rectifying K+ (KATP) channels have been implicated in myometrial relaxation (10). Transcripts of the predominant isoform of the KATP channel in myometrium, Kir6.1/SUR2B, were found to be downregulated late in pregnancy, representing a possible contributing mechanism to the onset of labor (19). Another KATP channel in rat uterus, Kir1.1/ROMK1, has been shown to undergo dynamic gestational changes at the transcript level, arguing for a role in contractile quiescence of the gravid uterus (40). Small-conductance Ca2+-sensitive and voltage-insensitive K+ (SK) channels have also been discovered in myometrium. SK channels have been proposed to contribute to regulation of uterine contractility by decreasing influx through L-type Ca2+ channels and inhibiting the generation of contractions (11). Transgenic mice that overexpressed isoform 3 of small-conductance Ca2+-sensitive K+ (SK3Ca) channels demonstrate a phenotype that may result in inadequate contractions during labor, supporting the hypothesis that SK channels are regulators of myometrial contractility (9). Additionally, SK3Ca gene expression is known to be downregulated at full term in human myometrium (43). Although several distinct classes of K+ channels have been discovered in myometrium, the role of two-pore domain K+ channels has yet to be fully elucidated following the discovery of their presence in myometrium.
TREK-1 has recently been shown to be expressed in human myometrium (5, 13, 62) in a manner that is regulated by gestation, with higher expression in pregnant than nonpregnant human myometrium (13). Interestingly, TREK-1 protein expression was reduced in preterm laboring human myometrium (13), consistent with the hypothesis that TREK-1 participates in uterine quiescence during gestation. The role of TREK-1 has yet to be fully defined; however, activation of this outwardly rectifying K+ channel aids in cell hyperpolarization and, therefore, decreases myometrial excitability. It was found that treatment of immortalized pregnant human myometrial cells with the potent TREK-1 activator arachidonic acid (AA) increased channel activity, thereby decreasing excitability, an effect that was blocked by fluphenazine and methionine derivatives, known inhibitors of TREK-1 channels (6, 13).
TREK-1 is a member of the two-pore-domain background K+ channel protein family and is encoded by the KCNK2 gene (39). Unlike other K+ channels such as TASK-1 and TASK-3, which remain open at rest, opening of TREK-1 requires a chemical or mechanical stimulus (20). TREK-1 channels are particularly abundant in heart, brain, and neurons and are thought to be critical to many physiological processes. The pharmacological profile of TREK-1 is unique, in that it is insensitive to tetraethylammonium (TEA) and other classic blockers of one-pore domain K+ channels, while it demonstrates inhibition by selective serotonin reuptake inhibitors (26, 34). Also of pharmacological significance is the finding that TREK-1 is opened by the volatile anesthetics, such as chloroform and isoflurane (52). TREK-1 displays dose-dependent inhibition by antipsychotic drugs, such as haloperidol, fluphenazine, and chlorpromazine, a characteristic unique to TREK-1 compared with TRAAK, another two-pore domain background K+ channel (60) in human myometrium (13). In mice, deletion of the KCNK2 gene results in a depression-resistant phenotype (31). Researchers have determined that TREK-1-knockout mice are more sensitive to low-threshold mechanical stimuli and also display an increase in thermal and mechanical responses to pain (2). In the vascular system, TREK-1 plays an important role in cutaneous endothelium-dependent vasodilation (50).
The aim of the present study was to characterize a membrane current that could help explain the regulation of human myometrial excitability (13) and provide a basis for examining the role of TREK-1 variants discovered in preterm laboring patients (67). Here, using patch-clamp electrophysiology, we report the presence of a TEA-insensitive K+ current that demonstrates characteristics unique to TREK-1 in cells isolated from pregnant human myometrium. Understanding TREK-1-mediated K+ movement may lead to a better mechanistic understanding of uterine smooth muscle cell contraction/relaxation and provide a novel therapeutic target. TREK-1 could significantly impact smooth muscle relaxation, as well as the induction of labor, since TREK-1 gene variants are known to be correlated with patients who deliver spontaneously preterm (67). Our results expand previous investigations that have described the presence and gestational regulation of TREK-1 in human myometrium by providing evidence that supports a role for TREK-1 currents in uterine quiescence until full term in human pregnancy.
MATERIALS AND METHODS
Pregnant human uterine smooth muscle cells.
With informed consent obtained in writing and approval from the Biomedical Institutional Review Board at the University of Nevada, Reno, samples of near-term-pregnant (nonlaboring) uterine tissue from the superior aspect of the incision were obtained from women undergoing cesarean section. Women were selected on the basis of the surgery schedule when a clinical decision was made to deliver a normal-term pregnancy by cesarean section. Exclusion criteria were as follows: patient <21 yr of age, multiple pregnancy, known illicit drug use, or HIV or hepatitis C infection. Within 20 min of their removal, fresh tissue samples were transported to the laboratory in cold physiological buffer containing (in mM) 120 NaCl, 5 KCl, 0.587 KH2PO4, 0.589 Na2HPO4, 2.5 MgCl2, 20 dextrose, 2.5 CaCl2, 25 Tris, and 5 NaHCO3, with pH adjusted to 7.4. Uterine smooth muscle (myometrium) was dissected under the microscope from tissue samples and prepared as thin strips (2 × 2 × 8 mm) devoid of blood vessels in a modified Krebs-HEPES-buffered solution (in mM: 118 NaCl, 4.75 KCl, 1.2 KH2PO4, 0.25 NaHCO3, 1.2 MgSO4, 20 dextrose, and 25 Na-HEPES, with pH adjusted to 7.4). Minced tissue underwent three 30-min incubation periods in a 50-ml conical tube containing 2 mg/ml collagenase type II and 1 mg/ml trypsin at 37°C. Each successive supernatant was collected in growth medium with 10% FBS and antibiotics [penicillin (60 μg/ml), streptomycin (100 μg/ml), and amphotericin B (Fungizone, 5 μg/ml)], at pH 7.4, with trypsin inhibitor (1 mg/ml). Cells were centrifuged at 400 g, resuspended in Dulbecco's growth medium containing 10% steroid-free FBS (FBSSF) with estrogen (15 ng/ml)-progesterone (200 ng/ml) to mimic third-trimester human pregnant plasma concentrations, and plated on tissue culture dishes. For freshly isolated patch-clamp studies, the cells were immediately plated on glass coverslips coated with type I collagen from rat tail (Sigma) and employed in electrophysiological experiments within 8–12 h. Other cells were grown through passage 5 in primary culture and combined in equal numbers from three Caucasian donors at full term (24–29 yr) and telomerized to establish a pregnant myometrial cell model.
Immortalization of myometrial smooth muscle cells.
Myometrial cells in the combined culture were infected after one passage with a retroviral vector encoding the human telomerase reverse transcriptase (hTRT) gene. A plasmid (pGRN145) containing hTRT cDNA expression vector was a gift from Geron (Menlo Park, CA). The hTRT expression cassette was cloned into pLXIN (Clontech), and replication-incompetent Moloney murine leukemia virus retrovirus was generated in HEK-293 retroviral packaging cells. Primary and first-passage cultures of human uterine muscle cells were infected with the hTRT retrovirus and selected with 100 mg/ml G418 for 1 wk. Expression of hTRT was verified in immortalized cells by RT-PCR using telomerase-specific primers. For all experiments, myocytes grown on uncoated glass coverslips in DMEM supplemented with 50 U/ml streptomycin, 50 μg/ml penicillin, estrogen (15 ng/ml)-progesterone (200 ng/ml), and 10% FBSSF were used. Immortalized cells have been developed in a similar fashion by others (18). Our cells express smooth muscle actin, thin filament proteins such as caldesmon, oxytocin receptors, and proteins such as myosin phosphatase isozymes that are expressed in human uterine smooth muscle (not shown). The contractile state of the immortalized myometrial smooth muscle cells in culture has not been determined.
Patch-clamp techniques.
Cells were plated on glass coverslips 4–48 h before experiments and placed in a chamber mounted on top of an inverted microscope for recording, and currents typically were recorded in the standard whole cell variant of voltage clamp using pCLAMP software (version 9.2, Axon Instruments/Molecular Devices, Sunnyvale, CA). Currents were amplified with an Axopatch 200B amplifier (Axon Instruments/Molecular Devices), digitized using a computer interfaced with a Digi-Data 1322A acquisition system (Axon Instruments/Molecular Devices), and filtered at 1 kHz and digitized at 2 kHz for whole cell recording and filtered at 1 kHz and digitized at 4 kHz for inside-out patch recordings. The standard external solution contained (in mM) 140 NaCl, 5.4 KCl, 1.8 CaCl2, 10 HEPES, 1 MgCl2, and 2 TEA, with pH adjusted to 7.4 with NaOH and osmolarity adjusted to 310 mosmol/l with d-mannitol (measured with a model 3320 osmometer, Advanced Instruments, Norwood, MA). The standard pipette solution contained (in mM) 140 KCl, 3 K2ATP, 0.2 NaGTP, 5 HEPES, 1 MgCl2, and 10 BAPTA (to minimize BKCa currents), with pH adjusted to 7.4 with KOH and osmolarity adjusted to 310 mosmol/l with d-mannitol as needed. Solutions were delivered by gravity through a manifold perfusion system. Pipettes were made of borosilicate glass (Sutter Instrument, Novato, CA) pulled on a two-stage vertical puller (model pp-83, Narishige International USA, East Meadow, NY) and had a resistance of 2–4 mΩ when filled with standard pipette solution. Cell capacitance and series resistance were measured using the membrane test feature of pCLAMP. Series resistance was then compensated ∼70%. Cell capacitance was later used for normalization of whole cell current to capacitance to yield current density (pA/pF) for each cell. Whole cell currents were monitored by running a pulse-ramp protocol every 15 s, with stepping to +80 mV for 100 ms, ramping from +80 to −80 mV over 1 s, and finally stepping to −80 mV for 100 ms. For experiments employing AA, cells were held at −80 mV between pulse-ramp protocols (45). For all other whole cell experiments, cells were held at 0 mV between pulse-ramp protocols. For inside-out patch experiments, the pipette was filled with standard (5 mM KCl) bath solution containing 2 mM TEA, 100 μM DIDS, and 100 μM GdCl3 to block voltage-gated K+, Cl−, and nonselective cation channels, respectively. The bath solution for inside-out experiments contained (in mM) 140 KCl, 1 EGTA, 5 HEPES, 1 MgCl2, 2 TEA, and 5 4-aminopyridine, with pH adjusted to 7.4 with KOH and osmolarity of 310 mosmol/l. Negative pressure was applied via pipette suction. Suction was measured using a pressure transducer calibrated to mmHg. Currents were digitized at 4 kHz and filtered at 1 kHz. Channel activity was measured by taking the mean value of current of ∼10 s of recording and subtracting the mean value of baseline current (current when no channels were open).
Polymerase chain reaction.
Total RNA was extracted from hTRT pregnant human myometrial cells scraped from three 100-mm dishes in TRIzol (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol and resuspended in 30 μl of nuclease-free H2O. DNA contamination was removed by treatment at 37°C with 10 U of RNase-free DNase I (Promega, Madison, WI). DNase was inactivated by addition of 25 mM EDTA with heating at 55°C for 10 min. cDNA was synthesized from 1 μg of total RNA using 250 ng of random primers (Invitrogen), 0.125 mM each dNTPs, 10 mM DTT, and 200 U of SuperScript II reverse transcriptase (Invitrogen). Gene-specific primers for human TREK-1 (13) were designed from areas of high homology using Primer Quest software (Integrated DNA Technologies, Coralville, IA). Basic local alignment search tool (BLAST) searches were performed to confirm that primer sequences had no homology with any other known gene products, and transcripts were sequenced for product identity.
β-Actin primers were designed to amplify genomic (750 bp), as well as nongenomic (500 bp), products to control for genomic DNA contamination, while nontemplate controls ensured the integrity of the PCR. PCR amplification within the linear range was carried out as recently described (67).
Western blotting.
Stable TREK-1, HEK-293, and human TREK-1 (hTREK-1) small interfering RNA (siRNA)-transfected pregnant human uterine smooth muscle cell (pHUSMC) and nonsilencing negative control pHUSMC were grown to 80% confluence in 100-mm2 dishes and then washed with ice-cold PBS. Total protein was extracted and processed for Western blotting using standard methods, as recently described (12).
Cloning of human TREK-1.
Human TREK-1 (GenBank accession no. NM_001017424.2) gene was generated from total human myometrial RNA isolated from 2–5 mg of pregnant myometrial homogenate, as described elsewhere (67).
Development of stable TREK-1-transfected HEK-293 and hTREK-1 siRNA-transfected pHUSMC cell lines.
The TREK-1 cDNA was inserted into the lentiviral vector pCDH-CMV-GFP by restriction cloning from pcDNA 3.1V5-His. Green fluorescent protein (GFP) with elongation factor-1 promoter on the vector was chosen as an indicator for efficiency of infection and TREK-1 expression in the cell. Replication-incompetent lentivirus was generated in HEK-293 cell lines with pPACKH1 Lentivector Packaging Kit for packaging HIV-based lentivector expression constructs (System Biosciences, Mountain View, CA). HEK-293 cells were infected with the TREK-1 lentivirus and selected with 2 μg/ml puromycin for 2 wk. Expression of TREK-1 was verified in immortalized cells by Western blotting. The lentiviral shRNAmir clone targeting hTREK-1 (NM_001017424.2, Thermo Scientific, Rockford, IL) sequence was confirmed by the Nevada Genomics Center. Lentivirus was packaged in HEK-293FT cells transfected with 10 µg second-generation packaging mix (Applied Biological Material, Richmond, BC, Canada) and 10 µg of the shRNAmir lentiviral clone using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). The infected pHUSMC were selected with 2 μg/ml puromycin, and GFP on pGIPZ vector was the visual indicator. Decreases in TREK-1 expression were verified in immortalized cells by Western blotting.
Statistics.
Values are means ± SE. All data were normally distributed. We calculated the number of observations needed to meet 95% power to reject the null hypothesis. Student's t-tests were used to compare mean values. Paired tests were used when both conditions were measured on the same cell. Unpaired tests were used when conditions were measured on different cells. One-tailed tests were used when the direction of changes was hypothesized. P < 0.05 was considered statistically significant.
RESULTS
TEA-insensitive K+ current in freshly isolated myocytes.
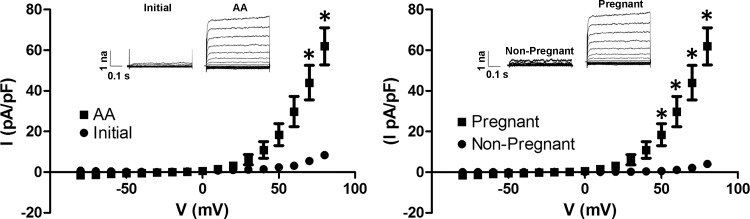
To investigate channel properties under conditions that most closely resemble the physiological environment in which we hypothesize a role for TREK-1, whole cell recordings were performed in myocytes freshly isolated from pregnant and nonpregnant subjects. In freshly isolated myocytes from pregnant subjects, we observed an outwardly rectifying TEA-insensitive current that could be activated by AA (n = 4; Fig. 1). Moreover, in freshly isolated myocytes from nonpregnant women, AA could not stimulate the same channel activity. Only a small residual outward basal current was detectable in pregnant myocytes in the presence of TEA. The AA-induced current was consistent with the properties of the TEA-insensitive K+ channel TREK-1, displaying outwardly rectifying properties, voltage-dependent activation, and lack of inactivation at positive potentials.
Fig. 1.
Arachidonic acid (AA) activates tetaethylammonium (TEA)-insensitive current in freshly isolated pregnant uterine myocytes. Whole cell recordings from freshly isolated pregnant myocytes demonstrate a current elicited using AA (left). AA activates pregnant, but not nonpregnant, myocytes in freshly isolated cells (right). I, current; V, voltage. *P < 0.05.
Expression of hTREK-1 in pHUSMC and HEK293-hTREK-1 cells.
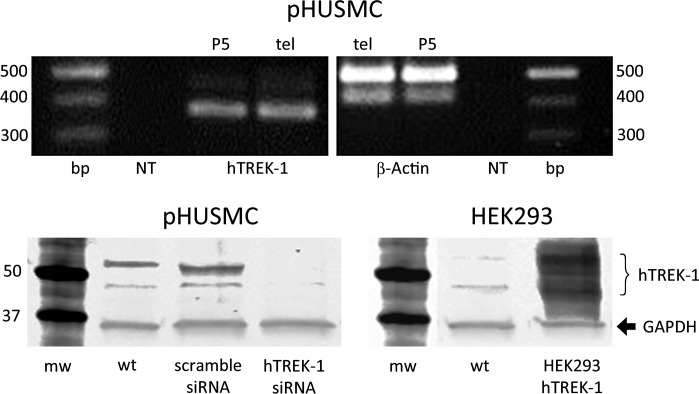
hTREK-1 expression was verified in telomerized pHUSMC by PCR (Fig. 2, top) and Western blotting (Fig. 2, bottom). These results are consistent with previously published results (5, 13, 62) demonstrating the expression of TREK-1 in human myometrium. Expression of hTREK-1 was similar before and after telomerization of primary cultured pHUSMC, as determined by PCR (Fig. 2, top) and similar to expression in human myometrial tissue (data not shown). Stable expression of siRNA targeting hTREK-1 in telomerized pHUSMC resulted in a decrease in hTREK-1 expression of 73% compared with wild-type telomerized pHUSMC (Fig. 2, bottom left). For comparisons with the currents found in native myocytes and telomerized pHUSMC under similar conditions, hTREK-1 was cloned from pregnant human myometrium and stably overexpressed in HEK-293 (HEK293-hTREK-1) cells. Expression of myometrial hTREK-1 in HEK293-hTREK-1 cells was also verified by Western blotting (Fig. 2, bottom right).
Fig. 2.
TREK-1 expression in telomerized pregnant human uterine smooth muscle cells (pHUSMC) and HEK293-hTREK-1 cells. Top: PCR amplification showing similar expression of human TREK-1 (hTREK-1) in cultured pHUMSC before (P5) and after (tel) telomerization (left) and similar expression of β-actin (right). NT, nontemplate control. Bottom: Western blot showing expression of hTREK-1 in telomerized pHUSMC (left) wild-type (wt) or stably expressing scrambled small interfering RNA (siRNA) or siRNA targeted to hTREK-1 and showing expression of human hTREK-1 in HEK293 cells (right) in untransfected [wild-type (wt)] cells or cells stably transfected with myometrial hTREK-1 (HEK293-hTREK-1). mw, Molecular weight.
AA activates TEA-insensitive K+ current in pHUSMC and HEK-hTREK-1 cells.
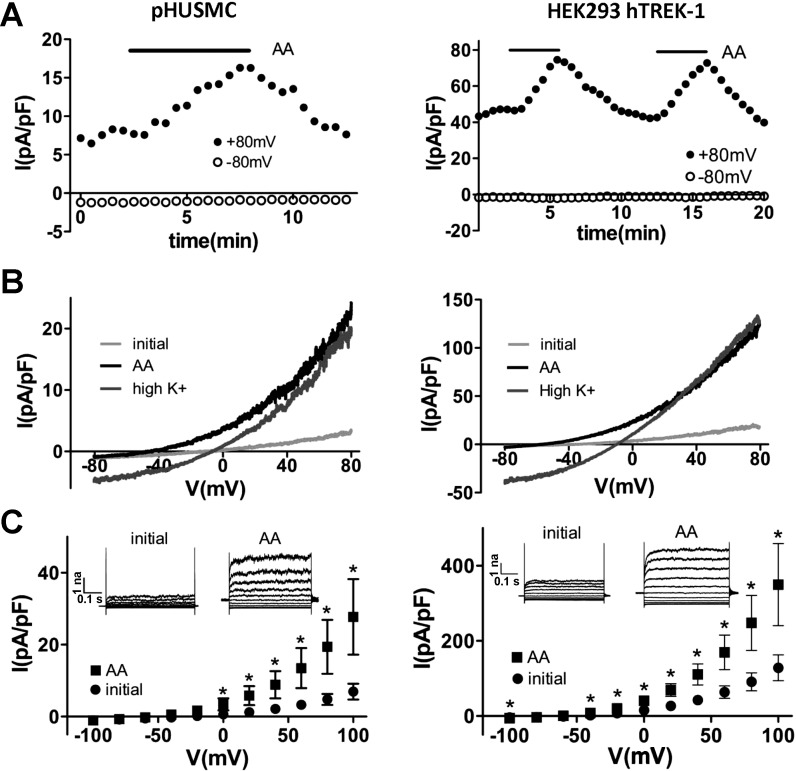
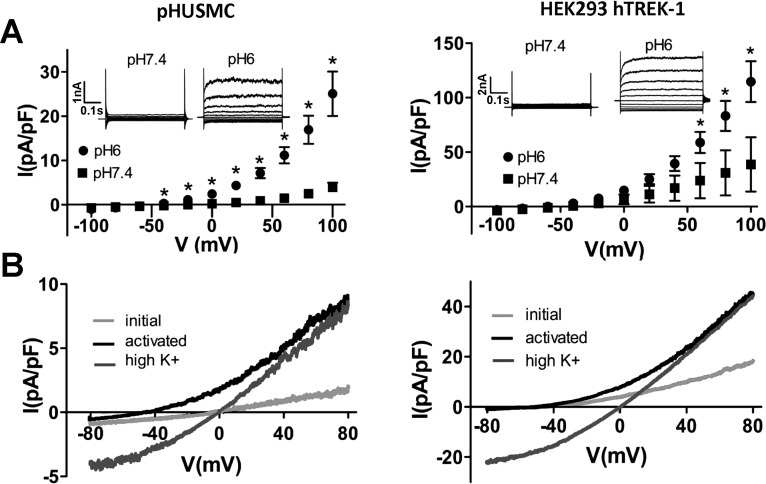
TREK-1 K+ channels have been shown to be activated by AA (14, 45, 48) and to be largely insensitive to the classical K+ channel blocker TEA (53). To test for the presence of an AA-activated and TEA-insensitive K+ current in pHUSMC, AA (10 μM) was applied during recording of whole cell currents in pHUSMC and HEK293-hTREK-1 cells in the presence of TEA (2 mM). Application of AA resulted in a significant increase in outward current at +80 mV, from 4.8 ± 1.5 to 19.4 ± 7.5 pA/pF (∼4 fold) in pHUSMC (n = 7) and from 91.0 ± 23.8 to 247.5 ± 73.3 pA/pF (∼3-fold) in HEK293-hTREK-1 cells (n = 6), that was reversible upon washout (Fig. 3). In addition, activation of current by AA resulted in a negative shift in reversal potential toward the equilibrium potential for K+ from 4.3 ± 5.8 to −33.1 ± 2.8 mV (n = 7) in pHUSMC and from −50.3 ± 7.5 to −62.8 ± 4.7 mV (n = 6) in HEK293-hTREK-1 cells. Under the recording conditions used, only K+ had a reversal potential negative to 0 mV. Replacing the standard bath with a high-K+ bath solution under AA activation increased inward current and moved the reversal potential near 0 mV (Fig. 3B) in both cell types, indicating that a large portion of the current was carried by K+. The AA-activated current in both cell types showed outward rectification in physiological K+, slight outward rectification in symmetrical K+, time-dependent activation at more positive membrane potentials, lack of inactivation, and a flickering appearance. All these properties have been previously reported for TREK-1. These data demonstrate the presence of a TEA-insensitive K+ current activated by AA in pHUSMC and HEK293-hTREK-1 cells with properties similar to those previously reported for TREK-1 channels (14, 45). Importantly, in response to AA, freshly isolated myocytes, pHUSMC, and HEK293-hTREK-1 cells demonstrated similar current activation.
Fig. 3.
AA activates a TEA-insensitive K+ current in pHUSMC and HEK293-hTREK-1 cells. A: time course of whole cell voltage-clamp recording from pHUSMC and HEK293-hTREK-1 cells at +80 and −80 mV showing reversible activation of outward current by AA (10 μM). B: representative whole cell current density traces in response to voltage ramps from +80 to −80 mV before treatment (initial) and in the presence of AA in standard and high-K+ bath solutions in pHUSMC and HEK293-hTREK-1 cells. C: mean current density in response to 20-mV voltage steps from −100 to +100 mV before (initial) and after AA treatment in pHUSMC and HEK293-hTREK-1 cells. *P < 0.05. Insets: representative whole cell current traces before and after AA treatment.
Intracellular acidification activates TEA-insensitive K+ current in pHUSMC and HEK293-hTREK-1 cells.
AA has been shown to activate several two-pore K+ channels, including TREK-1, TREK-2, and TRAAK (27, 35, 45). In addition to TREK-1, TRAAK expression has also been demonstrated in human myometrium, while expression of TREK-2 is absent, and no gestational regulation of TRAAK channels was found (13). Intracellular acidification has been shown to activate TREK-1, but not TRAAK, currents (41). The ability of intracellular acidification to activate TEA-insensitive K+ currents in pHUSMC was thus tested by measuring whole cell currents in the presence of TEA (2 mM) under two recording conditions resulting in intracellular acidification: 1) after exchange of 90 mM NaCl for 90 mM NaHCO3 in the bath solution (41) and 2) after adjustment of the pipette solution from pH 7.4 to pH 6 (46). Both approaches yielded an increase in outward current in pHUSMC and HEK293-hTREK-1 cells that resulted in a shift in current reversal potential toward that for K+.
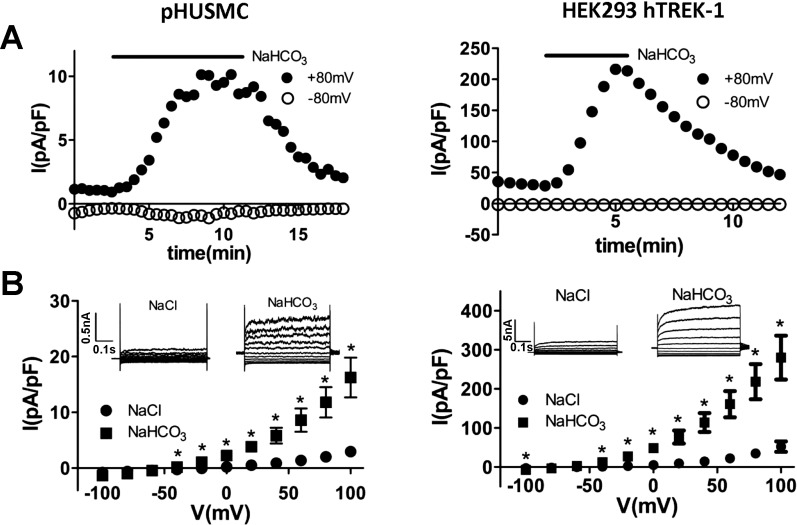
Intracellular acidification by NaHCO3 yielded an increase in outward current at +80 mV from 3.0 ± 0.8 to 11.8 ± 2.7 pA/pF (∼4-fold) in pHUSMC (n = 4) and from 34.8 ± 8.9 to 218.6 ± 45.0 pA/pF (∼6-fold) in HEK293-hTREK-1 cells (n = 8) that was reversible on return to standard bath solution (Fig. 4). NaHCO3 bath solutions also shifted the reversal potential toward that predicted for K+ (∼−85 mV), with a shift from 0.5 ± 6.0 mV in standard solution to −40.6 ± 3.0 mV in 90 mM NaHCO3 bath solution in pHUSMC (n = 4) and from −46.1 ± 4.4 mV in standard solution to −65.1 ± 3.6 mV in 90 mM NaHCO3 bath solution in HEK293-hTREK-1 cells (n = 8).
Fig. 4.
Intracellular acidification by 90 mM NaHCO3 activates a TEA-insensitive K+ current in pHUSMC and HEK293-hTREK-1 cells. A: time course of whole cell voltage-clamp recording at +80 and −80 mV showing reversible activation of TEA-insensitive outward current after exchange of 90 mM NaCl for 90 mM NaHCO3 in bath solution to cause intracellular acidification in pHUSMC and HEK293-hTREK-1 cells. B: mean current density in response to 20-mV voltage steps from −100 to +100 mV before (NaCl) and after NaHCO3 treatment in pHUSMC and HEK293-hTREK-1 cells. *P < 0.05. Insets: representative whole cell current traces before and after NaHCO3 treatment.
Intracellular acidification using pH 6 pipette solutions resulted in activation of an outward current that typically took 5–10 min after membrane rupture to reach a maximal steady state. Acidification by a pH 6 pipette solution resulted in significantly higher outward currents in pHUSMC 10 min after membrane rupture (16.9 ± 3.2 pA/pF, n = 5) compared with pH 7.4 pipette solutions at the same time point (2.5 ± 0.7 pA/pF, n = 5; Fig. 5). A similar increase was seen in HEK293-hTREK-1 cells 10 min after membrane rupture with pH 6 pipette solution: 83.2 ± 13.8 (n = 8) vs. 30.9 ± 20.7 pA/pF (n = 4) in pH 7.4 pipette solution. Intracellular acidification by pH 6 pipette solution also resulted in a negative shift in current reversal potential toward that predicted for K+ (ca. −85 mV) in pH 7.4 vs. pH 6 pipette solutions from 1.0 ± 5.1 to −46.0 ± 6.0 mV (n = 5) and from −46.1 ± 4.4 (n = 4) to −53.7 ± 4.2 mV (n = 8) in pHUSMC and HEK293-hTREK-1 cells, respectively. Replacing the standard bath with a high-K+ bath solution after activation of current with pH 6 pipette solution increased inward current and moved the reversal potential near 0 mV (Fig. 5B) in both cell types.
Fig. 5.
pH 6 pipette solution activates a TEA-insensitive K+ current. A: mean current density in response to 20-mV voltage steps from −100 to +100 mV with pH 7.4 and pH 6 pipette solutions in pHUSMC and HEK293-hTREK-1 cells. *P < 0.05. B: representative whole cell current density traces in response to voltage ramps from +80 to −80 mV shortly after gaining whole cell access (initial), after 10 min (activated), and after activation and switch to high-K+ bath solution (high K+). Insets: representative whole cell current traces in pH 6 and pH 7.4 solution.
Currents in pHUSMC and HEK293-hTREK-1 cells resulting from intracellular acidification by 90 mM NaHCO3 or pH 6 pipette solution showed the following properties similar to those seen by activation with AA: outward rectification in physiological K+, modest rectification in symmetrical K+, time-dependent activation at more positive membrane potentials, lack of inactivation, and a flickering appearance. These data demonstrate the presence of a TEA-insensitive K+ current activated by intracellular acidification in pHUSMC and HEK293-hTREK-1 cells with properties as reported for TREK-1 and are consistent with TREK-1, and not TRAAK, channels as the primary K+ current carrier under these conditions.
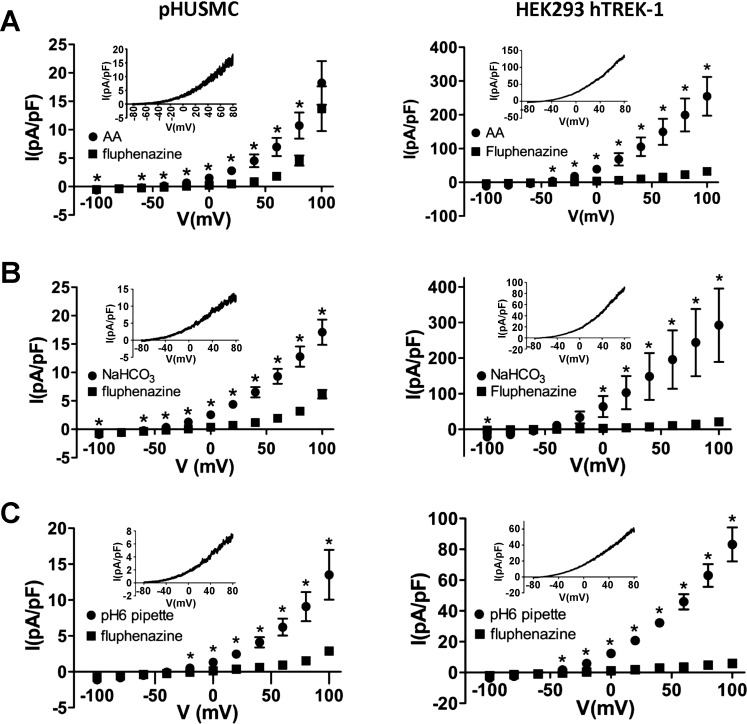
Fluphenazine blocks TEA-insensitive K+ currents in pHUSMC and HEK293-hTREK-1 cells.
The antipsychotic fluphenazine has been shown to block hTREK-1 and hTREK-2, but not hTRAAK, currents (60). To test the ability of fluphenazine to block the TEA-insensitive K+ currents reported here, currents were activated with 10 μM AA, a 90 mM NaHCO3 bath, and pH 6 pipette solution. In each case, the current was allowed to fully activate, and then 10 μM fluphenazine was applied in the continued presence of each agonist to observe blockade. Fluphenazine significantly blocked current stimulated by all three treatments (Fig. 6) in pHUSMC- and TREK-1-overexpressing HEK cells. Currents in pHUSMC at +80 mV were reduced by 51.2 ± 9.8% (n = 6), 73.9 ± 4.2% (n = 5), and 75.6 ± 4.0% (n = 6) after activation by 10 μM AA, 90 mM NaHCO3 bath, and pH 6 pipette solution, respectively. Currents in HEK293-hTREK-1 cells at +80 mV were reduced by 89.5 ± 2.3% (n = 3), 91.6 ± 3.4% (n = 3), and 89.8 ± 2.2% (n = 7) after activation by 10 μM AA, 90 mM NaHCO3 bath, and pH 6 pipette solution, respectively. The somewhat smaller block in the presence of AA in pHUSMC was likely due to the presence of a contaminating current that reversed at ∼0 mV and sometimes developed in these experiments. Subsequent experiments were carried out in the presence of 100 μM GdCl3 and 100 μM DIDS to reduce nonselective cation and Cl− currents, respectively. The fluphenazine-sensitive currents (Fig. 6, insets) for all three agonists showed outward rectification in physiological K+ and reversal potentials [−71.0 ± 3.9 mV (n = 6), −77.1 ± 1.3 mV (n = 5), and −67 ± 7.6 mV (n = 6) for AA-, NaHCO3-, and pH 6 pipette solution-activated currents, respectively, in pHUSMC] near that predicted for K+ (ca. −85 mV). HEK293-hTREK-1 cells showed similar fluphenazine-sensitive currents, with reversal potentials of −62.4 ± 11.8 mV (n = 3), −66.0 ± 5.7 mV (n = 6), and −63.2 ± 12.4 mV (n = 3) for AA-, NaHCO3-, and pH 6 pipette solution-activated currents, respectively. These data show that the TEA-insensitive K+ currents activated in pHUSMC and HEK293-hTREK-1 cells by AA and intracellular acidification are significantly blocked by fluphenazine, a known blocker of TREK-1 channels (60). These findings are consistent with hTREK-1, and not hTRAAK, being the primary K+ current carrier under these conditions.
Fig. 6.
Fluphenazine block of TEA-insensitive K+ currents. A–C: mean current density in response to 20-mV voltage steps from −100 to +100 mV before and after 10 μM fluphenazine in pHUSMC and HEK293-hTREK-1 cells. Currents were first activated by AA (A), 90 mM NaHCO3 (B), or pH 6 pipette solution (C). *P < 0.05. Insets: representative whole cell current density traces in response to voltage ramps from +80 to −80 mV of the fluphenazine-sensitive current (fully activated − fluphenazine-blocked).
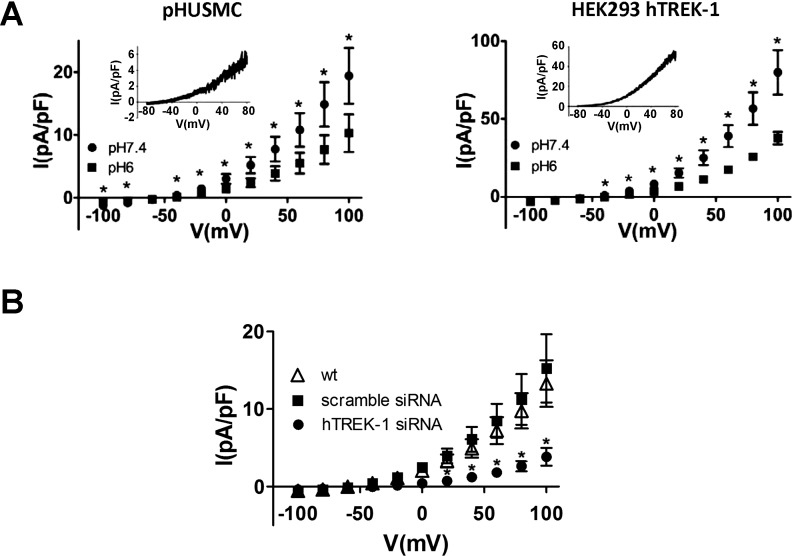
Extracellular acidification inhibits TEA-insensitive K+ current in pHUSMC and HEK293-hTREK-1 cells.
Extracellular acidification has been shown to inhibit TREK-1 channels, in contrast to increasing TREK-2 channel activity (56). To test the ability of external acidification to inhibit TEA-insensitive K+ currents in pHUSMC, outward currents were first activated by pH 6 pipette solution for ∼10 min, and then the standard bath solution (pH 7.4) was exchanged for the same bath solution adjusted to pH 6. This resulted in a significant decrease in outward current (51.3 ± 4.8% at +80 mV, n = 9; Fig. 7A) in pHUSMC that showed a reversal potential in physiological K+ of −61 ± 4.3 mV (n = 9), indicating that it was largely carried by K+ (Fig. 7A). A similar inhibition of pH 6 pipette solution-activated outward current by pH 6 bath solution was seen in HEK293-hTREK-1 cells (52.8 ± 4.0% at +80 mV; Fig. 7A), with the pH 6 bath-sensitive current showing a reversal potential of 64.5 ± 14.5 mV (n = 4). These data show that the TEA-insensitive K+ current activated by intracellular acidification in pHUSMC and HEK293-hTREK-1 cells is inhibited by extracellular acidification and is consistent with the hTREK-1, and not hTREK-2, channels being the primary K+ current carrier under these conditions.
Fig. 7.
Extracellular acidification inhibits TEA-insensitive K+ currents in pHUSMC and HEK293-hTREK-1 cells, and siRNA knockdown of hTREK-1 reduces NaHCO3-activated current in pHUSMC. A: mean whole cell current density in response to 20-mV voltage steps from −100 to +100 mV in pH 7.4 and pH 6 bath solution in pHUSMC and HEK293-hTREK-1 cells. *P < 0.05. Insets: representative whole cell current density traces in response to voltage ramps from +80 to −80 mV of the pH 6 bath-sensitive current (fully activated − pH 6 bath-blocked). B: mean NaHCO3-activated (after NaHCO3 − before NaHCO3) current density in response to 20-mV voltage steps from −100 to +100 mV in wild-type pHUSMC and pHUSMC stably expressing scrambled siRNA or hTREK-1-targeted siRNA. *P < 0.05, hTREK-1 siRNA vs. wt.
TREK-1 knockdown by siRNA in pHUSMC decreases TEA-insensitive K+ current activated by intracellular acidification.
To directly assess the contribution of hTREK-1 to the TEA-insensitive K+ currents in pHUSMC in the current study, pHUSMC cell lines that stably expressed siRNA targeted to hTREK-1 or a scrambled siRNA as a control were generated. Whole cell current densities for each of these three cell types were measured in the presence of 2 mM TEA, 100 μM DIDS, and 100 μM GdCl3 in response to intracellular acidification by exchange of 90 mM NaHCO3 for 90 mM NaCl in the bath solution (Fig. 7B) in a manner concurrent with the data of Fig. 3. The whole cell current density activated in response to NaHCO3 (NaHCO3-activated − initial) was significantly reduced at all voltages measured positive to −60 mV in response to NaHCO3 in hTREK-1 siRNA-expressing pHUSMC vs. wild-type pHUSMC (Fig. 7B). The decrease of 71% in NaHCO3-activated current between wild-type and hTREK-1 siRNA-expressing pHUSMC measured at +100 mV was similar to the 73% decrease in hTREK-1 protein expression between the same cell types. There were no significant differences between NaHCO3-activated current densities in scramble siRNA control pHUSMC and wild-type pHUSMC at any voltage measured. These data indicate that a significant portion of the outward current in pHUSMC activated in response to intracellular acidification was carried by hTREK-1 and could be reduced using interfering RNA.
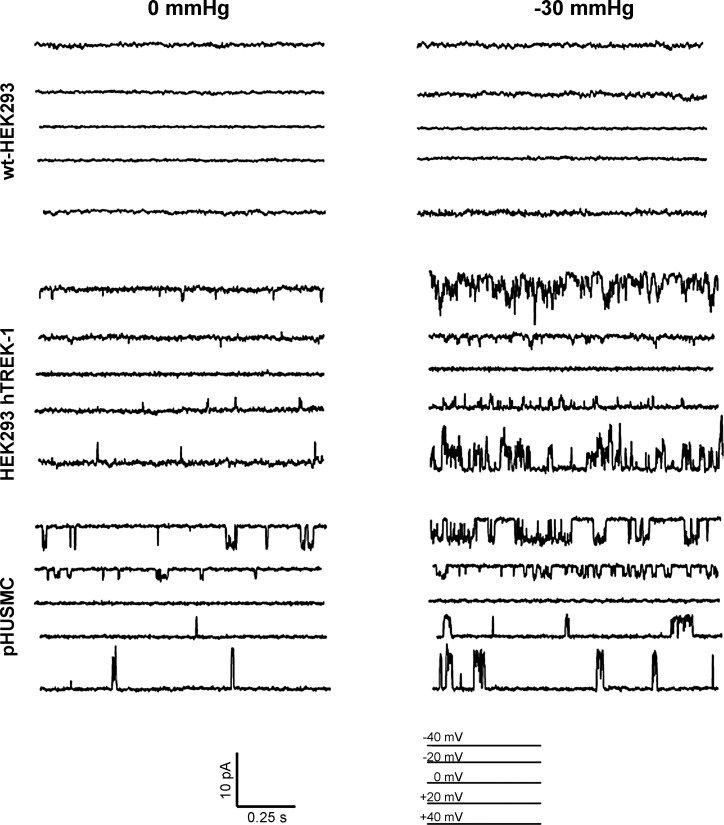
Inside-out patch clamp of HEK293-hTREK-1 cells and pHUSMC shows similar currents not observed in wild-type HEK-293 (wt-HEK293) cells and stretch activation.
In addition to being activated by AA and intracellular acidification, TREK-1 channels are known to be activated by membrane stretch (53). To test for the presence of stretch-activated, TEA-insensitive K+ channels in pHUSMC, inside-out patch recordings were made in the presence of TEA (2 mM), while negative pressure was applied to the membrane via pipette suction. In symmetrical high-K+ solutions (140:140 mM), the unitary conductance of TREK-1 has been reported to be 95–130 pS (25). To measure and calculate the unitary conductance of the TEA-insensitive K+ channel in pHUSMC, inside-out recordings were made with GdCl3 (100 μM) and DIDS (100 μM) in the pipette solution, 4-aminopyridine (5 mM) in the bath solution, and TEA (2 mM) in the bath and pipette solutions to minimize all potential contaminating currents. During the experiments, cells were held at 0, −20, +20, −40, and +40 mV for 30 s while undergoing 0-mmHg stretch; then negative pressure (−30 mmHg) was applied, and the cells were held at the same voltages. This experiment was also performed on wt-HEK293 cells to establish a baseline and HEK293-hTREK-1 cells for comparison with pHUSMC cells. While adopting the inside-out patch configuration, the pipette potential is equal in magnitude but opposite in polarity to the membrane potential for the channels being measured. Figure 8, top, demonstrates a representative trace at each voltage for wt-HEK cells; in this cell type, there were no distinguishable currents (n = 6). Figure 8, middle, illustrates representative traces at each input voltage in HEK293-hTREK-1 cells (n = 5); at 0 mmHg an increase in channel activity was observed compared with wt-HEK cells, but channel activity was significantly increased when negative pressure (−30 mmHg) was applied to the pipette. Because of the presence of symmetrical K+, the current reversal potential of TREK-1 would be expected to be close to 0 mV. The channel activity for the inside-out patch HEK293-hTREK-1 cells exhibited no measurable activity at 0 mV and reversed polarity when the input voltage was changed from positive to negative values. Figure 8, bottom, shows the representative channel activity (n = 5) in the pHUSMC. The channels in HEK293-hTREK-1 cells and pHUSMC were very similar. In both cell types, a TEA-insensitive K+ current that reversed at ∼0 mV in symmetrical K+ that was activated by stretch was observed. Calculation of the single-channel conductance for the channel in pHUSMC resulted in 119 pS, which is in the range of reported conductances for TREK-1. On the other hand, Ca2+-dependent K+ (BK) channels exhibit unitary conductances of ∼250–300 pS in symmetrical K+ (15), and delayed rectifying K+ channels exhibit conductances of <50 pS.
Fig. 8.
Inside-out patch-clamp recording of HEK293-hTREK-1 cells and pHUSMC show similar currents not observed in wt-HEK293 and stretch activation. Representative traces are from cells in symmetrical K+ solution (140:140 mM) that were held at 0, −20, +20, −40, and +40 mV without stretch; then cells were stretched using −30-mmHg pipette suction and held at the same voltage potential. Top: almost no current was observed at 0 or 30 mmHg for wt-HEK293 cells. Middle: HEK293-hTREK-1 demonstrated a significant increase in channel activity after undergoing membrane stretch. Bottom: inside-out recordings of pHUSMC show channel activity very similar to HEK293-hTREK-1 cells and stretch activation.
DISCUSSION
This work characterized in detail a TEA-insensitive K+ current in freshly isolated uterine smooth muscle myocytes, a stable human myometrial cell line, and HEK-293 cells stably transfected with hTREK-1. This current showed activation by AA, intracellular acidification, and membrane stretch, in addition to inhibition by extracellular acidification, treatment with the antipsychotic drug fluphenazine, or siRNA knockdown. Activated currents exhibited outward rectification in physiological K+, modest rectification in symmetrical K+, time-dependent activation at more positive membrane potentials, a lack of inactivation, and a flickering appearance. These properties are consistent with those previously reported for TREK-1 (45, 48, 56, 60) and channels in mouse myometrium (55). These data illustrate the functional presence of TREK-1 K+ channels and currents in smooth muscle cells isolated from pregnant human myometrium.
The TREK-1 channel shows current rectification that is largely dependent on the concentration gradient for K+ across the cell membrane, as predicted by the Goldman-Hodgkin-Katz current equation (32), in which current is carried more effectively from the side of the membrane with higher K+ concentration. This was seen in the present study, as evidenced by the significant reduction in rectification upon switching from physiological to symmetrical K+ gradients (Figs. 3B and 5B). This indicates that the TEA-insensitive K+ current found here in pHUSMC shows properties of a “leak”-type K+ channel, despite some voltage-dependent gating, as previously described for TREK-1 (8, 41, 53). These channels could thus be expected to be similarly active across the range of membrane potentials experienced by a uterine smooth muscle cell in vivo. TREK-1 channels typically show little basal activity (49), but activation of these channels by stimuli such as stretch, pH changes, or AA (14, 41, 45, 53) would result in a tendency to shift cell membrane potential toward the equilibrium potential for K+ from essentially any membrane potential experienced by uterine smooth muscle cells. This would decrease excitability by helping return a depolarized cell to a more negative resting potential and by helping maintain a resting cell near the K+ equilibrium potential. The result would be a reduction in the activity of voltage-gated (particularly Na+ and Ca2+) channels that lead to uterine smooth muscle contraction (12, 58), primarily through Ca2+ entry through L-type Ca2+ channels (65).
The activity of the TREK-1 channel has been shown to be regulated by numerous factors, including AA, pH, stretch, phosphorylation, nitric oxide, temperature, and others (20, 22–24, 57). Here we provide provocative evidence that pregnancy regulates the channel, such that cells from nonpregnant women do not activate when stimulated by AA, despite expression of the channel (13), albeit at a lower level than during pregnancy. Although the nature of this regulation in the native myocyte has not been explained, it provides an intriguing question. Because of the polymodal control of TREK-1 activity, the channel is ideally suited for a role in regulating contractility of human myometrium over the many changes present during the course of human pregnancy. Changes in many of the factors that regulate TREK-1 channel activity, such as membrane stretch, AA, pH, nitric oxide, and others, could be experienced by uterine smooth muscle cells during pregnancy (3, 21, 38). Myocyte signaling domains may be acidic on the basis of their construction (4). Relative acidification at the myocyte membrane activating TREK-1 during gestation would be consistent with hyperpolarization of the membrane and decreased uterine activity. An abundance of AA in pregnant myometrium (21) also would signal TREK-1 activation. In addition, the physical stretch of the myometrium itself through pregnancy is also a likely activator of TREK-1 channels (53). Increases in intracellular Ca2+ and resting membrane potential have been shown to inhibit TREK-1 channels (23, 57) and to take place over the course of pregnancy (51).
A complex interplay of these and other factors could be important for proper regulation of TREK-1 K+ channels during pregnancy. If these stimuli combine to increase activity of TREK-1 K+ channels, this would tend to decrease contractile activity, even in the face of increasing tension on uterine smooth muscle cells. Conversely, if the balance of these factors combined to decrease TREK-1 K+ activity, this would tend to shift the uterus away from quiescence and toward labor. The proper coordination of these regulatory factors and the appropriate TREK-1 response could thus be of critical importance in appropriate maintenance of uterine quiescence during pregnancy.
In addition to factors that could alter the activity of TREK-1 channels already discussed, recently published data show that the expression of TREK-1 protein is higher in pregnant than nonpregnant myometrium in human (13) and mouse (55). Ovariectomized mice also demonstrated a decrease in TREK-1 expression compared with wild-type mice (55). Interestingly, TREK-1 expression was reduced in full-term and preterm laboring vs. nonlaboring human myometrium (13), consistent with the notion that TREK-1 contributes to myometrial quiescence during gestation. Membrane stretch has also been shown to increase TREK-1 expression in mouse cardiac muscle (68). TREK-1 expression changes in myometrium are consistent with a role for TREK-1 in maintaining quiescence during pregnancy, with upregulation potentially contributing to maintenance of uterine quiescence and downregulation contributing to the critical transition from quiescent to laboring myometrium. In addition, because the TREK-1 channel is assembled as a dimer and may even form multimers (16), the differing expression of TREK-1 variants and even coexpression of variants with wild-type TREK-1 could have a significant impact on uterine excitability (66). Indeed, a dominant-negative, nonconducting splice variant of TREK-1 isolated from mouse brain was recently reported to decrease wild-type TREK-1 expression and currents when coexpressed in tsA201 cells (63). It is possible that a similar mechanism could take place in human myometrium, decreasing or eliminating TREK-1 K+ currents and shifting the balance from quiescent toward laboring tissue. The properly controlled expression of TREK-1 could be an important parameter in regulation of uterine quiescence during pregnancy.
Given the significant impact of preterm birth, it is of critical importance that a clear understanding of mechanisms regulating uterine quiescence and potential dysregulation be developed. Here we demonstrate the functional presence of the two-pore K+ channel TREK-1 in human myometrial cells. This work and our recent genetic studies in preterm laboring patients (66) have opened a new opportunity for investigation into maintenance of uterine quiescence. Further investigation is needed to define the specific role of TREK-1 channel expression and activity in proper regulation of myometrial quiescence during pregnancy and to determine the potential role of dysregulation of this current in the premature transition of pregnant uterus from the quiescent to the laboring state.
GRANTS
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant R01-HD-053028, a grant from the March of Dimes Foundation, and a Gates Grand Challenges Grant (to I. L. O. Buxton).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.S.H., C.L.C., C.A.S., and I.L.O.B. are responsible for conception and design of the research; N.S.H., C.L.C., S.D.B., Y.-Y.W., C.C., and N.L. performed the experiments; N.S.H., C.L.C., S.D.B., C.A.S., N.L., and I.L.O.B. analyzed the data; N.S.H., C.L.C., Y.-Y.W., and I.L.O.B. interpreted the results of the experiments; N.S.H., C.L.C., S.D.B., and I.L.O.B. prepared the figures; N.S.H. and I.L.O.B. drafted the manuscript; N.S.H., C.L.C., and I.L.O.B. approved the final version of the manuscript; C.L.C. and I.L.O.B. edited and revised the manuscript.
REFERENCES
- 1. Aaronson PI, Sarwar U, Gin S, Rockenbauch U, Connolly M, Tillet A, Watson S, Liu B, Tribe RM. A role for voltage-gated, but not Ca2+-activated, K+ channels in regulating spontaneous contractile activity in myometrium from virgin and pregnant rats. Br J Pharamcol 147: 815–824, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alloui A, Zimmermann K, Mamet J, Duprat F, Noel J, Chemin J, Guy N, Blondeau N, Voilley N, Rubat-Coudert C, Borsotto M, Romey G, Heurteaux C, Reeh P, Eschalier A, Lazdunski M. TREK-1, a K+ channel involved in polymodal pain perception. EMBO J 25: 2368–2376, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anderson AB, Turnbull AC, Murray AM. The relationship between amniotic fluid pressure and uterine wall tension in pregnancy. Am J Obstet Gynecol 97: 992–997, 1967 [DOI] [PubMed] [Google Scholar]
- 4. Anderson RG. The caveolae membrane system. Annu Rev Biochem 67: 199–225, 1998 [DOI] [PubMed] [Google Scholar]
- 5. Bai X, Bugg GJ, Greenwood SL, Glazier JD, Sibley CP, Baker PN, Taggart MJ, Fyfe GK. Expression of TASK and TREK, two-pore domain K+ channels, in human myometrium. Reproduction 129: 525–530, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Baker SA, Hennig GW, Han J, Britton FC, Smith TK, Koh SD. Methionine and its derivatives increase bladder excitability by inhibiting stretch-dependent K+ channels. Br J Pharamcol 153: 1259–1271, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beck S, Wojdyla D, Say L, Betran AP, Merialdi M, Requejo JH, Rubens C, Menon R, Van Look PF. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ 88: 31–38, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bockenhauer D, Zilberberg N, Goldstein SA. KCNK2: reversible conversion of a hippocampal potassium leak into a voltage-dependent channel. Nat Neurosci 4: 486–491, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Bond CT, Sprengel R, Bissonnette JM, Kaufmann WA, Pribnow D, Neelands T, Storck T, Baetscher M, Jerecic J, Maylie J, Knaus HG, Seeburg PH, Adelman JP. Respiration and parturition affected by conditional overexpression of the Ca2+-activated K+ channel subunit, SK3. Science 289: 1942–1946, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Brainard AM, Korovkina VP, England SK. Potassium channels and uterine function. Semin Cell Dev Biol 18: 332–339, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brown A, Cornwell T, Korniyenko I, Solodushko V, Bond CT, Adelman JP, Taylor MS. Myometrial expression of small conductance Ca2+-activated K+ channels depresses phasic uterine contraction. Am J Physiol Cell Physiol 292: C832–C840, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Bursztyn L, Eytan O, Jaffa AJ, Elad D. Mathematical model of excitation-contraction in a uterine smooth muscle cell. Am J Physiol Cell Physiol 292: C1816–C1829, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Buxton IL, Singer CA, Tichenor JN. Expression of stretch-activated two-pore potassium channels in human myometrium in pregnancy and labor. PLos One 5: e12372, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Caley AJ, Gruss M, Franks NP. The effects of hypoxia on the modulation of human TREK-1 potassium channels. J Physiol 562: 205–212, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carvacho I, Gonzalez W, Torres YP, Brauchi S, Alvarez O, Gonzalez-Nilo FD, Latorre R. Intrinsic electrostatic potential in the BK channel pore: role in determining single channel conductance and block. J Gen Physiol 131: 147–161, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chemin J, Patel AJ, Duprat F, Sachs F, Lazdunski M, Honore E. Up- and down-regulation of the mechano-gated K2P channel TREK-1 by PIP2 and other membrane phospholipids. Pflügers Arch 455: 97–103, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Committee on Understanding Premature Birth Institute of Medicine. Preterm Birth: Causes, Consequences, and Prevention. Washington, DC: National Academies Press, 2006 [Google Scholar]
- 18. Condon J, Yin S, Mayhew B, Word RA, Wright WE, Shay JW, Rainey WE. Telomerase immortalization of human myometrial cells. Biol Reprod 67: 506–514, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Curley M, Cairns MT, Friel AM, McMeel OM, Morrison JJ, Smith TJ. Expression of mRNA transcripts for ATP-sensitive potassium channels in human myometrium. Mol Hum Reprod 8: 941–945, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Dedman A, Sharif-Naeini R, Folgering JH, Duprat F, Patel A, Honore E. The mechano-gated K2P channel TREK-1. Eur Biophys J 38: 293–303, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Egarter CH, Husslein P. Biochemistry of myometrial contractility. Baillieres Clin Obstet Gynaecol 6: 755–769, 1992 [DOI] [PubMed] [Google Scholar]
- 22. Enyeart JA, Liu H, Enyeart JJ. cAMP analogs and their metabolites enhance TREK-1 mRNA and K+ current expression in adrenocortical cells. Mol Pharmacol 77: 469–482, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Enyeart JJ, Liu H, Enyeart JA. Calcium-dependent inhibition of adrenal TREK-1 channels by angiotensin II and ionomycin. Am J Physiol Cell Physiol 301: C619–C629, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Enyeart JJ, Liu H, Enyeart JA. Evidence for cAMP-independent bTREK-1 inhibition by ACTH and NPS-ACTH in adrenocortical cells. Mol Cell Endocrinol 348: 305–312, 2012 [DOI] [PubMed] [Google Scholar]
- 25. Enyedi P, Czirjak G. Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol Rev 90: 559–605, 2010 [DOI] [PubMed] [Google Scholar]
- 26. Fink M, Duprat F, Lesage F, Reyes R, Romey G, Heurteaux C, Lazdunski M. Cloning, functional expression and brain localization of a novel unconventional outward rectifier K+ channel. EMBO J 15: 6854–6862, 1996 [PMC free article] [PubMed] [Google Scholar]
- 27. Fink M, Lesage F, Duprat F, Heurteaux C, Reyes R, Fosset M, Lazdunski M. A neuronal two P domain K+ channel stimulated by arachidonic acid and polyunsaturated fatty acids. EMBO J 17: 3297–3308, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gao L, Cong B, Zhang L, Ni X. Expression of the calcium-activated potassium channel in upper and lower segment human myometrium during pregnancy and parturition. Reprod Biol Endocrinol 7: 27, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet 371: 75–84, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Greenwood IA, Yeung SY, Tribe RM, Ohya S. Loss of functional K+ channels encoded by ether-a-go-go-related genes in mouse myometrium prior to labour onset. J Physiol 587: 2313–2326, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Heurteaux C, Lucas G, Guy N, El YM, Thummler S, Peng XD, Noble F, Blondeau N, Widmann C, Borsotto M, Gobbi G, Vaugeois JM, Debonnel G, Lazdunski M. Deletion of the background potassium channel TREK-1 results in a depression-resistant phenotype. Nat Neurosci 9: 1134–1141, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Hille B. Ionic Channels of Excitable Membranes. Sunderland, MA: Sinauer, 1992 [Google Scholar]
- 33. Hirota Y, Daikoku T, Tranguch S, Xie H, Bradshaw HB, Dey SK. Uterine-specific p53 deficiency confers premature uterine senescence and promotes preterm birth in mice. J Clin Invest 120: 803–815, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kennard LE, Chumbley JR, Ranatunga KM, Armstrong SJ, Veale EL, Mathie A. Inhibition of the human two-pore domain potassium channel, TREK-1, by fluoxetine and its metabolite norfluoxetine. Br J Pharmacol 144: 821–829, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim D. Fatty acid-sensitive two-pore domain K+ channels. Trends Pharmacol Sci 24: 648–654, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Knock GA, Smirnov SV, Aaronson PI. Voltage-gated K+ currents in freshly isolated myocytes of the pregnant human myometrium. J Physiol 518: 769–781, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Knock GA, Tribe RM, Hassoni AA, Aaronson PI. Modulation of potassium current characteristics in human myometrial smooth muscle by 17β-estradiol and progesterone. Biol Reprod 64: 1526–1534, 2001 [DOI] [PubMed] [Google Scholar]
- 38. Larcombe-McDouall J, Buttell N, Harrison N, Wray S. In vivo pH and metabolite changes during a single contraction in rat uterine smooth muscle. J Physiol 518: 783–790, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lesage F, Lazdunski M. Mapping of human potassium channel genes TREK-1 (KCNK2) and TASK (KCNK3) to chromosomes 1q41 and 2p23. Genomics 51: 478–479, 1998 [DOI] [PubMed] [Google Scholar]
- 40. Lundgren DW, Moore JJ, Chang SM, Collins PL, Chang AS. Gestational changes in the uterine expression of an inwardly rectifying K+ channel, ROMK. Proc Soc Exp Biol Med 216: 57–64, 1997 [DOI] [PubMed] [Google Scholar]
- 41. Maingret F, Patel AJ, Lesage F, Lazdunski M, Honore E. Mechano- or acid stimulation, two interactive modes of activation of the TREK-1 potassium channel. J Biol Chem 274: 26691–26696, 1999 [DOI] [PubMed] [Google Scholar]
- 42. Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Mathews TJ, Osterman MJ. Births: Final Data for 2008. National Vital Statistics Reports. Washington, DC: Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System, 2010, vol. 59, pt. 1, p. 3–71 [PubMed] [Google Scholar]
- 43. Mazzone J, Buxton IL. Changes in small conductance potassium channel expression in human myometrium during pregnancy measured by RT-PCR. Proc West Pharmacol Soc 46: 74–77, 2003 [PubMed] [Google Scholar]
- 44. McCallum LA, Pierce SL, England SK, Greenwood IA, Tribe RM. The contribution of Kv7 channels to pregnant mouse and human myometrial contractility. J Cell Mol Med 15: 577–586, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Meadows HJ, Benham CD, Cairns W, Gloger I, Jennings C, Medhurst AD, Murdock P, Chapman CG. Cloning, localisation and functional expression of the human orthologue of the TREK-1 potassium channel. Pflügers Arch 439: 714–722, 2000 [DOI] [PubMed] [Google Scholar]
- 46. Miller P, Peers C, Kemp PJ. Polymodal regulation of hTREK1 by pH, arachidonic acid, and hypoxia: physiological impact in acidosis and alkalosis. Am J Physiol Cell Physiol 286: C272–C282, 2004 [DOI] [PubMed] [Google Scholar]
- 47.March of Dimes White Paper on Preterm Birth: The Global and Regional Toll. New York: World Health Organization, 2009 [Google Scholar]
- 48. Mohaou Maati H, Peyronnet R, Devader C, Veyssiere J, Labbal F, Gandin C, Mazella J, Heurteaux C, Borsotto M. A human TREK-1/HEK cell line: a highly efficient screening tool for drug development in neurological diseases. PLos One 6: e25602, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ohara A, Saeki Y, Nishikawa M, Yamamoto Y, Yamamoto G. Single-channel recordings of TREK-1 K+ channels in periodontal ligament fibroblasts. J Dent Res 85: 664–669, 2006 [DOI] [PubMed] [Google Scholar]
- 50. Opazo Saez A, Zhang W, Wu Y, Turner CE, Tang DD, Gunst SJ. Tension development during contractile stimulation of smooth muscle requires recruitment of paxillin and vinculin to the membrane. Am J Physiol Cell Physiol 286: C433–C447, 2004 [DOI] [PubMed] [Google Scholar]
- 51. Parkington HC, Tonta MA, Brennecke SP, Coleman HA. Contractile activity, membrane potential, and cytoplasmic calcium in human uterine smooth muscle in the third trimester of pregnancy and during labor. Am J Obstet Gynecol 181: 1445–1451, 1999 [DOI] [PubMed] [Google Scholar]
- 52. Patel AJ, Honore E, Lesage F, Fink M, Romey G, Lazdunski M. Inhalational anesthetics activate two-pore-domain background K+ channels. Nat Neurosci 2: 422–426, 1999 [DOI] [PubMed] [Google Scholar]
- 53. Patel AJ, Honore E, Maingret F, Lesage F, Fink M, Duprat F, Lazdunski M. A mammalian two pore domain mechano-gated S-like K+ channel. EMBO J 17: 4283–4290, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Price SA, Bernal AL. Uterine quiescence: the role of cyclic AMP. Exp Physiol 86: 265–272, 2001 [DOI] [PubMed] [Google Scholar]
- 56. Sandoz G, Douguet D, Chatelain F, Lazdunski M, Lesage F. Extracellular acidification exerts opposite actions on TREK1 and TREK2 potassium channels via a single conserved histidine residue. Proc Natl Acad Sci USA 106: 14628–14633, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Segal-Hayoun Y, Cohen A, Zilberberg N. Molecular mechanisms underlying membrane-potential-mediated regulation of neuronal K2P2.1 channels. Mol Cell Neurosci 43: 117–126, 2010 [DOI] [PubMed] [Google Scholar]
- 58. Shmygol A, Blanks AM, Bru-Mercier G, Gullam JE, Thornton S. Control of uterine Ca2+ by membrane voltage: toward understanding the excitation-contraction coupling in human myometrium. Ann NY Acad Sci 1101: 97–109, 2007 [DOI] [PubMed] [Google Scholar]
- 59. Smith RC, McClure MC, Smith MA, Abel PW, Bradley ME. The role of voltage-gated potassium channels in the regulation of mouse uterine contractility. Reprod Biol Endocrinol 5: 41, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Thummler S, Duprat F, Lazdunski M. Antipsychotics inhibit TREK but not TRAAK channels. Biochem Biophys Res Commun 354: 284–289, 2007 [DOI] [PubMed] [Google Scholar]
- 62. Tichenor JN, Hansen ET, Buxton IL. Expression of stretch-activated potassium channels in human myometrium. Proc West Pharmacol Soc 48: 44–48, 2005 [PubMed] [Google Scholar]
- 63. Veale EL, Rees KA, Mathie A, Trapp S. Dominant negative effects of a non-conducting TREK1 splice variant expressed in brain. J Biol Chem 285: 29295–29304, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wang SY, Yoshino M, Sui JL, Wakui M, Kao PN, Kao CY. Potassium currents in freshly dissociated uterine myocytes from nonpregnant and late-pregnant rats. J Gen Physiol 112: 737–756, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wray S, Jones K, Kupittayanant S, Li Y, Matthew A, Monir-Bishty E, Noble K, Pierce SJ, Quenby S, Shmygol AV. Calcium signaling and uterine contractility. J Soc Gynecol Invest 10: 252–264, 2003 [DOI] [PubMed] [Google Scholar]
- 66. Wu YY, Singer CA, Buxton IL. Variants of stretch-activated 2-pore potassium channel TREK-1 associated with preterm labor in humans. Biol Reprod 87: 96, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wu YY, Buxton IL. TREK-1 splice variants may lead to failure of human myometrial relaxation and contribute to preterm labor. Proc West Pharmacol Soc 53: 2010 [Google Scholar]
- 68. Zhao F, Dong L, Cheng L, Zeng Q, Su F. Effects of acute mechanical stretch on the expression of mechanosensitive potassium channel TREK-1 in rat left ventricle. J Huazhong Univ Sci Technol Med Sci 27: 385–387, 2007 [DOI] [PubMed] [Google Scholar]