Abstract
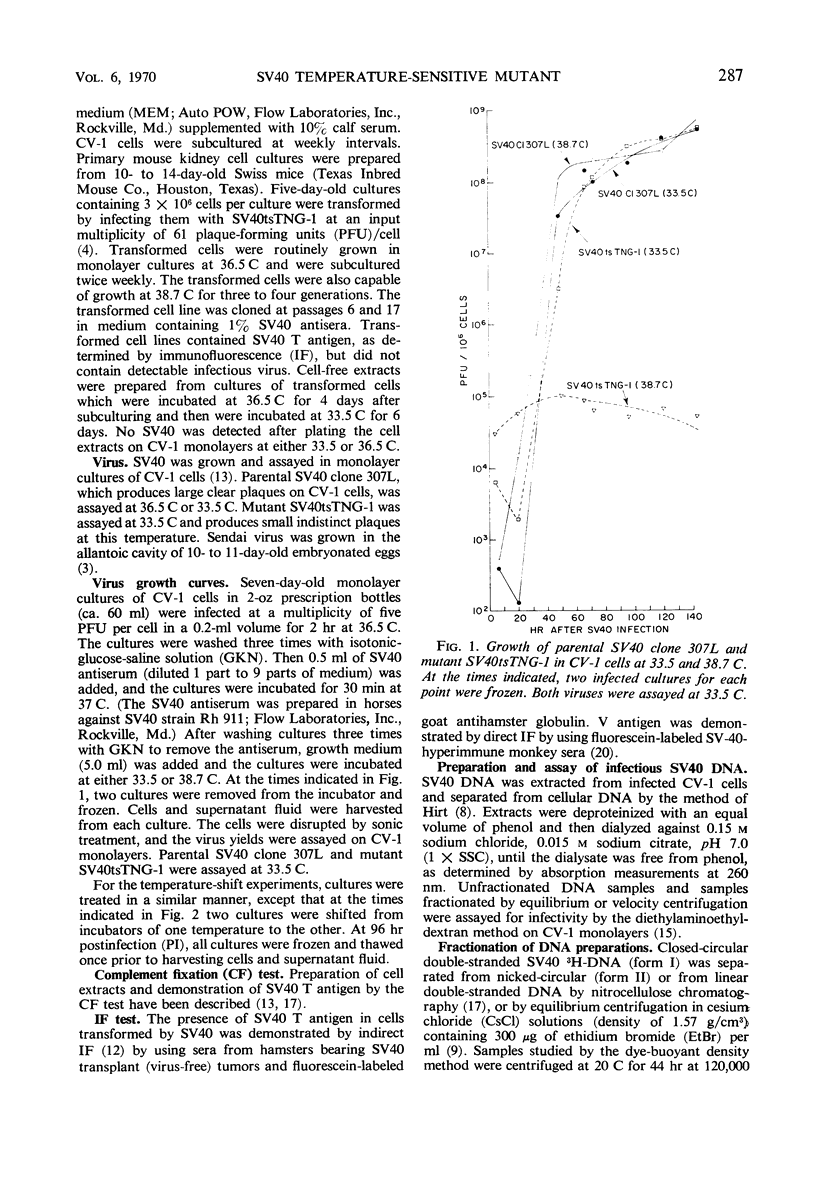
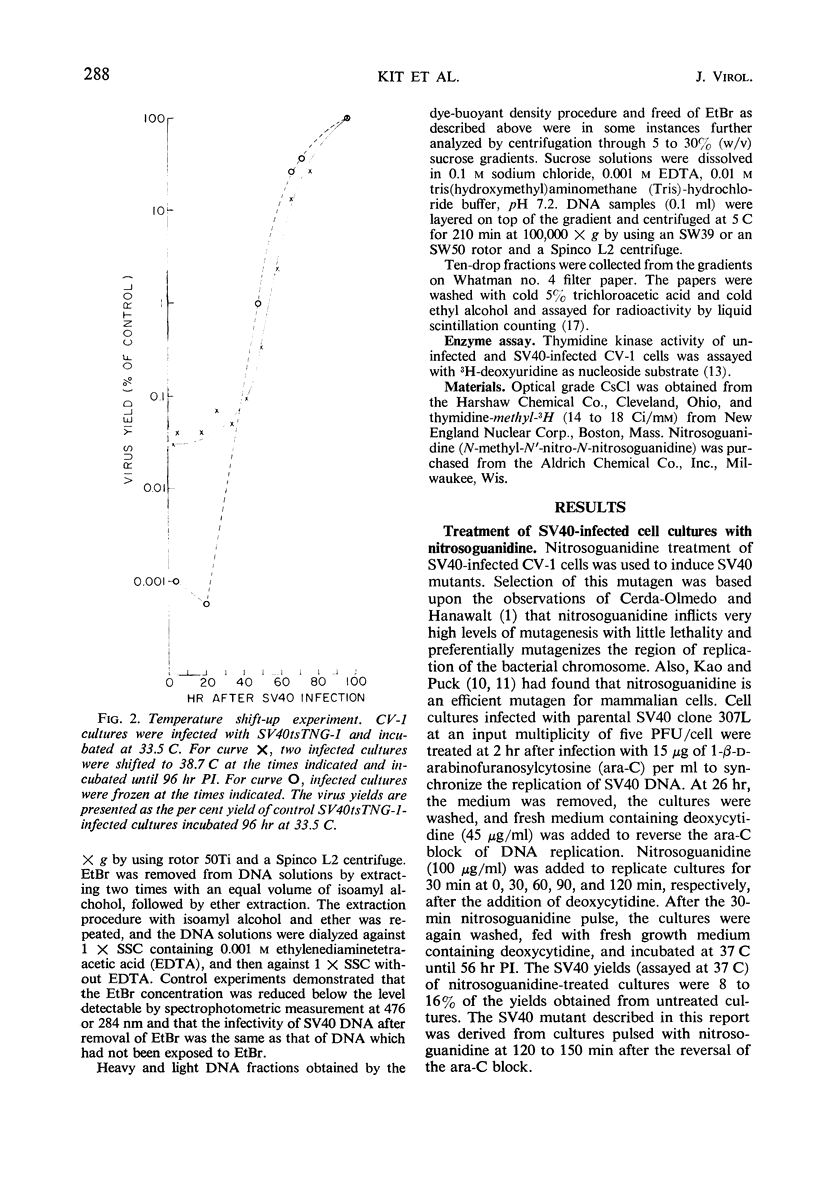
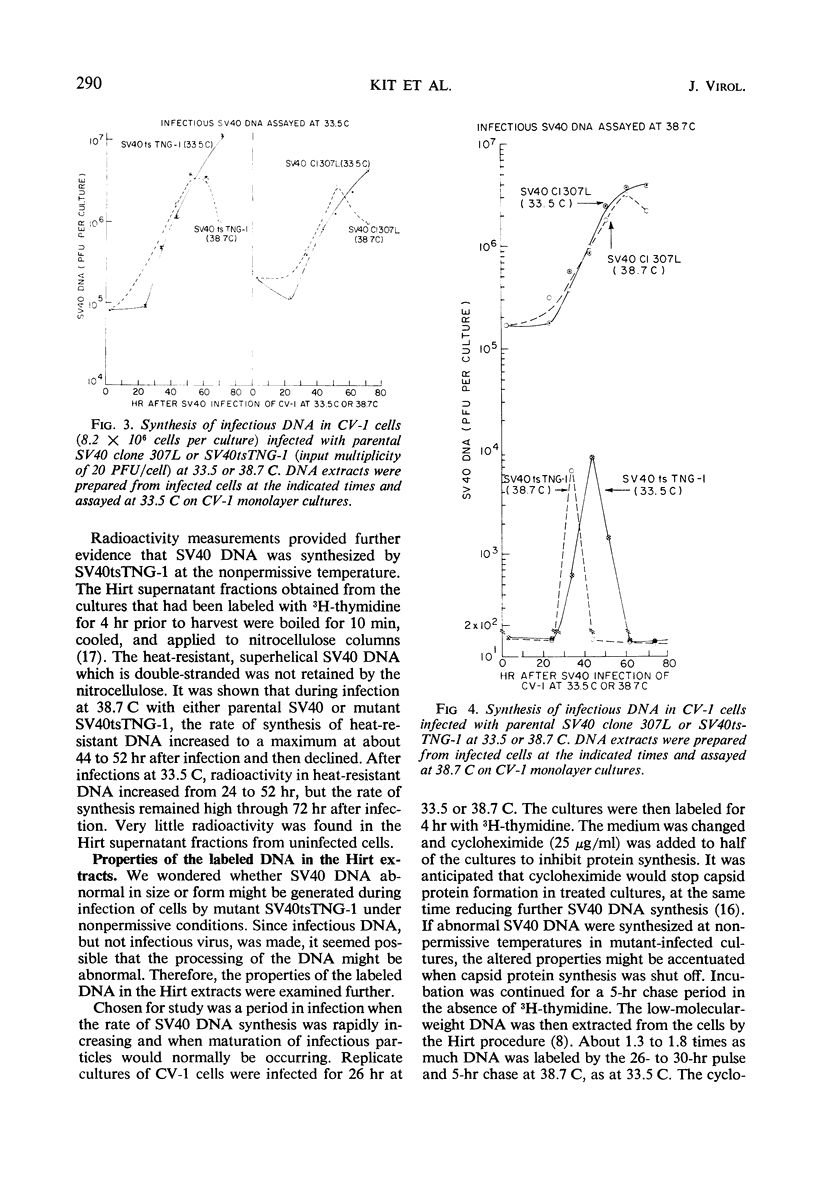
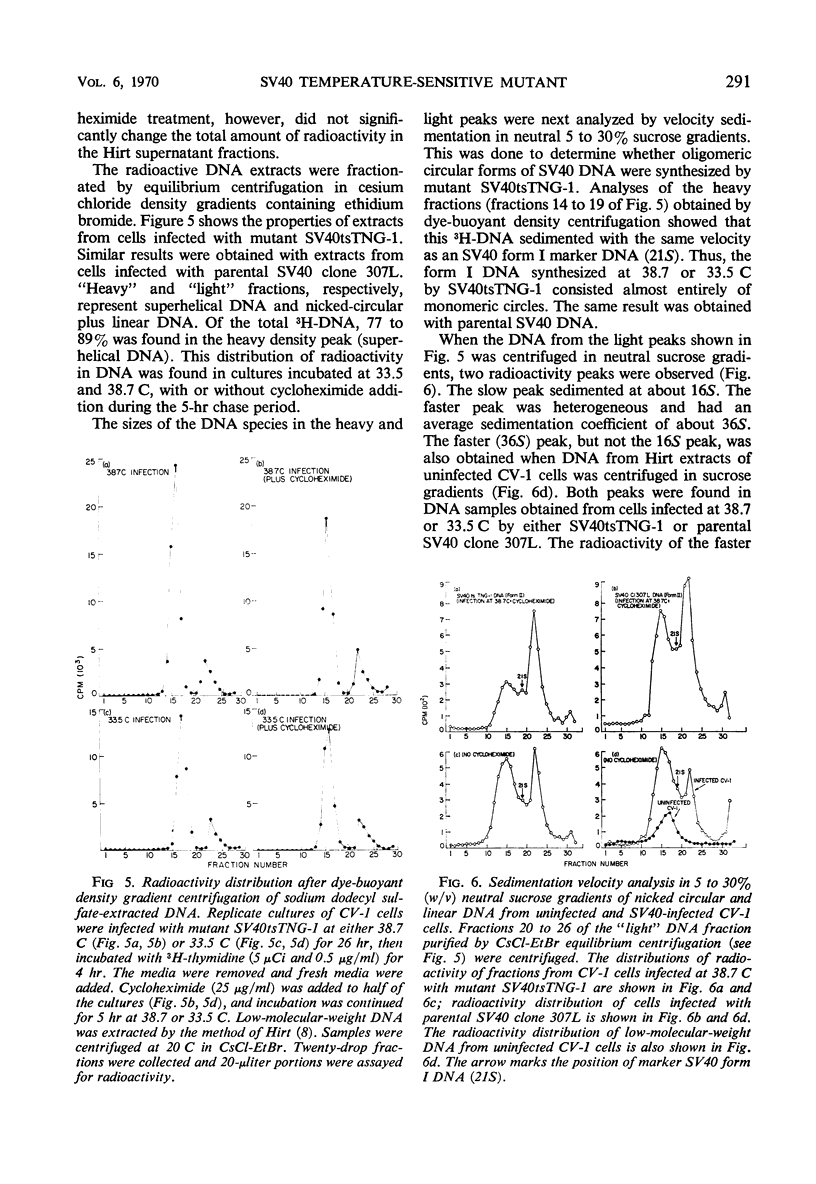
A temperature-sensitive simian virus 40 (SV40) mutant, tsTNG-1, has been isolated from nitrosoguanidine-treated and SV40-infected African green monkey kidney (CV-1) cultures. Replication of virus at the nonpermissive temperature (38.7 C) was 3,000-fold less than at the permissive temperature (33.5 C). Plaque formation by SV40tsTNG-1 deoxyribonucleic acid (DNA) on CV-1 monolayers occurred normally at 33.5 C but was grossly inhibited at 38.7 C. The time at which virus replication was blocked at 38.7 C was determined by temperature-shift experiments. In shift-up experiments, cultures infected for various times at 33.5 C were shifted to 38.7 C. In shift-down experiments, cultures infected for various times at 38.7 C were shifted to 33.5 C. All cultures were harvested at 96 hr postinfection (PI). No virus growth occurred when the shift-up occurred before 40 hr PI. Maximum virus yields were obtained at 96 hr PI when the shift-down occurred at 66 hr, but only about 15% of the maximum yield was obtained when the shift-down occurred at 76 hr PI. These results indicate that SV40tsTNG-1 contains a conditional lethal mutation in a late viral gene function. Mutant SV40tsTNG-1 synthesized T antigen, viral capsid antigens, and viral DNA, and induced thymidine kinase activity at either 33.5 or 38.7 C. The properties of the SV40 DNA synthesized in mutant-infected CV-1 cells at 33.5 or 38.7 C were very similar to those of SV40 DNA made in parental virus-infected cells, as determined by nitrocellulose column chromatography, cesium-chloride-ethidium bromide equilibrium centrifugation, and by velocity centrifugation in neutral sucrose gradients. Mutant SV40tsTNG-1 enhanced cellular DNA synthesis in primary cultures of mouse kidney cells at 33.5 and 38.7 C and also transformed mouse kidney cultures at 36.5 C. SV40tsTNG-1 was recovered from clonal lines of transformed cells after fusion with susceptible CV-1 cells and incubation of heterokaryons at 33.5 C, but not at 38.7 C.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cerdá-Olmedo E., Hanawalt P. C. The replication of the Escherichia coli chromosome studied by sequential nitrosoguanidine mutagenesis. Cold Spring Harb Symp Quant Biol. 1968;33:599–607. doi: 10.1101/sqb.1968.033.01.066. [DOI] [PubMed] [Google Scholar]
- Di Mayorca G., Callender J., Marin G., Giordano R. Temperature-sensitive mutants of polyoma virus. Virology. 1969 May;38(1):126–133. doi: 10.1016/0042-6822(69)90134-2. [DOI] [PubMed] [Google Scholar]
- Dubbs D. R., Kit S., De Torres R. A., Anken M. Virogenic properties of bromodeoxyuridine-sensitive and bromodeoxyuridine-resistant simian virus 40-transformed mouse kidney cells. J Virol. 1967 Oct;1(5):968–979. doi: 10.1128/jvi.1.5.968-979.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubbs D. R., Kit S. Isolation of defective lysogens from Simian virus 40-transformed mouse kidney cultures. J Virol. 1968 Nov;2(11):1272–1282. doi: 10.1128/jvi.2.11.1272-1282.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhart W. Cell transformation by polyoma virus and SV40. Nature. 1969 Dec 13;224(5224):1069–1071. doi: 10.1038/2241069a0. [DOI] [PubMed] [Google Scholar]
- Eckhart W. Complementation and transformation by temperature-sensitive mutants of polyoma virus. Virology. 1969 May;38(1):120–125. doi: 10.1016/0042-6822(69)90133-0. [DOI] [PubMed] [Google Scholar]
- FRIED M. CELL-TRANSFORMING ABILITY OF A TEMPERATURE-SENSITIVE MUTANT OF POLYOMA VIRUS. Proc Natl Acad Sci U S A. 1965 Mar;53:486–491. doi: 10.1073/pnas.53.3.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hudson B., Upholt W. B., Devinny J., Vinograd J. The use of an ethidium analogue in the dye-buoyant density procedure for the isolation of closed circular DNA: the variation of the superhelix density of mitochondrial DNA. Proc Natl Acad Sci U S A. 1969 Mar;62(3):813–820. doi: 10.1073/pnas.62.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao F. T., Puck T. T. Genetics of somatic mammalian cells, VII. Induction and isolation of nutritional mutants in Chinese hamster cells. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1275–1281. doi: 10.1073/pnas.60.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao F. T., Puck T. T. Genetics of somatic mammalian cells. IX. Quantitation of mutagenesis by physical and chemical agents. J Cell Physiol. 1969 Dec;74(3):245–258. doi: 10.1002/jcp.1040740305. [DOI] [PubMed] [Google Scholar]
- Kit S., Brown M. Rescue of Simian Virus 40 from Cell Lines Transformed at High and at Low Input Multiplicities by Unirradiated or Ultraviolet-irradiated Virus. J Virol. 1969 Sep;4(3):226–230. doi: 10.1128/jvi.4.3.226-230.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kit S., De Torres R. A., Dubbs D. R., Salvi M. L. Induction of cellular deoxyribonuleic acid synthesis by simian virus 40. J Virol. 1967 Aug;1(4):738–746. doi: 10.1128/jvi.1.4.738-746.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kit S., Dubbs D. R., Frearson P. M., Melnick J. L. Enzyme induction in SV40-infected green monkey kidney cultures. Virology. 1966 May;29(1):69–83. doi: 10.1016/0042-6822(66)90197-8. [DOI] [PubMed] [Google Scholar]
- Kit S., Kurimura T., De Torres R. A., Dubbs D. R. Simian virus 40 deoxyribonucleic acid replication. I. Effect of cycloheximide on the replication of SV40 deoxyribonucleic acid in monkey kidney cells and in heterokaryons of SV40-transformed and susceptible cells. J Virol. 1969 Jan;3(1):25–32. doi: 10.1128/jvi.3.1.25-32.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kit S., Kurimura T., Dubbs D. R. Properties of simian virus 40 rescued from cell lines transformed by ultraviolet-irradiated simian virus 40. J Virol. 1969 Nov;4(5):585–595. doi: 10.1128/jvi.4.5.585-595.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kit S., Kurimura T., Salvi M. L., Dubbs D. R. Activation of infectious SV40 synthesis in transformed cells. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1239–1246. doi: 10.1073/pnas.60.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozer H. L., Takemoto K. K., Kirschstein R. L., Axelrod D. Immunochemical characterization of plaque mutants of simian virus 40. J Virol. 1969 Jan;3(1):17–24. doi: 10.1128/jvi.3.1.17-24.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozer H. L., Takemoto K. K. Site of host restriction of simian virus 40 mutants in an established African green monkey kidney cell line. J Virol. 1969 Oct;4(4):408–415. doi: 10.1128/jvi.4.4.408-415.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAPP F., MELNICK J. L., KITAHARA T. TUMOR AND VIRUS ANTIGENS OF SIMIAN VIRUS 40: DIFFERENTIAL INHIBITION OF SYNTHESIS BY CYTOSINE ARABINOSIDE. Science. 1965 Feb 5;147(3658):625–627. doi: 10.1126/science.147.3658.625. [DOI] [PubMed] [Google Scholar]
- Stoker M., Dulbecco R. Abortive transformation by the Tsa mutant of polyoma virus. Nature. 1969 Jul 26;223(5204):397–398. doi: 10.1038/223397a0. [DOI] [PubMed] [Google Scholar]
- Takemoto K. K., Kirschstein R. L., Habel K. Mutants of simian virus 40 differing in plaque size, oncogenicity, and heat sensitivity. J Bacteriol. 1966 Oct;92(4):990–994. doi: 10.1128/jb.92.4.990-994.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto K. K., Todaro G. J., Habel K. Recovery of SV40 virus with genetic markers of original inducing virus from SV40-transformed mouse cells. Virology. 1968 May;35(1):1–8. doi: 10.1016/0042-6822(68)90299-7. [DOI] [PubMed] [Google Scholar]
- Vogt M. Induction of virus multiplication in 3T3 cells transformed by a thermosensitive mutant of polyoma virus. I. Isolation and characterization of Ts-a-3T3 cells. J Mol Biol. 1970 Feb 14;47(3):307–316. doi: 10.1016/0022-2836(70)90304-9. [DOI] [PubMed] [Google Scholar]



