Abstract
Local shear stress sensed by arterial endothelial cells is occasionally altered by changes in global hemodynamic parameters, e.g., heart rate and blood flow rate, as a result of normal physiological events, such as exercise. In a recently study (41), we demonstrated that during the adaptive response to increased shear magnitude, porcine endothelial cells exhibited an unique phenotype featuring a transient increase in permeability and the upregulation of a set of anti-inflammatory and antioxidative genes. In the present study, we characterize the adaptive response of these cells to an increase in shear frequency, another important hemodynamic parameter with implications in atherogenesis. Endothelial cells were preconditioned by a basal-level sinusoidal shear stress of 15 ± 15 dyn/cm2 at 1 Hz, and the frequency was then elevated to 2 Hz. Endothelial permeability increased slowly after the frequency step-up, but the increase was relatively small. Using microarrays, we identified 37 genes that are sensitive to the frequency step-up. The acute increase in shear frequency upregulates a set of cell-cycle regulation and angiogenesis-related genes. The overall adaptive response to the increased frequency is distinctly different from that to a magnitude step-up. However, consistent with the previous study, our data support the notion that endothelial function during an adaptive response is different than that of fully adapted endothelial cells. Our studies may also provide insights into the beneficial effects of exercise on vascular health: transient increases in frequency may facilitate endothelial repair, whereas similar increases in shear magnitude may keep excessive inflammation and oxidative stress at bay.
Keywords: endothelial permeability, gene expression, adaptation, shear stress, frequency
the endothelial cells lining blood vessels are constantly exposed to shear stress, the friction force from blood flow, and their function is tightly regulated by this external mechanical stimulus. For decades, researchers have studied how shear stress profiles regulate endothelial permeability and gene expression and, consequently, affect the pathogenesis of atherosclerosis (4, 5, 9). Prolonged unidirectional shear stress is generally considered to be atheroprotective, since it leads to a better endothelial barrier function (6, 17, 19) and an anti-inflammatory endothelial phenotype (11). On the other hand, “disturbed” shear stress, a term that is still poorly defined, promotes a proinflammatory phenotype and increased permeability (9, 11). Our understanding of endothelial mechanobiology has been advanced by studies that have isolated the endothelial response to different parameters of the shear stress profile, including shear stress magnitude (13), frequency (15), temporal (1) and spatial (20) gradient, and flow reversal (15).
However, one often neglected component of endothelial mechanobiology is the transient response to temporal changes in shear stress with time constants considerably longer than the pulsatile cycle. Local shear stress sensed by endothelial cells can be altered in vivo by changes in global hemodynamic parameters, such as heart rate and blood flow rate, as a result of normal physiological events, such as exercise. When local shear stress is altered, endothelial cells transiently adapt to the new shear profile. It remains largely unknown how endothelial function is changed during these periods of adaptation and the extent to which this transient phenotype contributes to the pathogenesis of atherosclerosis. In a recent study (41), we stabilized porcine endothelial cells in a physiological shear environment and investigated their adaptive response to a physiologically realistic acute increase in shear stress magnitude. We characterized endothelial permeability, a key regulator in atherogenesis, and endothelial gene expression during the adaptation. We reported a near-immediate increase in endothelial permeability in response to an increase in shear stress magnitude, which was consistent with previous in vivo observations (12, 14). Using microarray techniques, we identified a set of genes that were uniquely sensitive to the step-up in shear magnitude. This study strongly supported the notion that endothelial cells exhibit a specific phenotype during adaptation which is different from that of fully adapted endothelial cells.
In addition to magnitude, the frequency of shear stress is also an important parameter of shear profiles. Himburg et al. (15) reported that higher-frequency (2 and 3 Hz) shear profiles evoked a proinflammatory endothelial phenotype not exhibited by cells cultured under 1 Hz. Studies in the porcine iliac arteries (16) show that there are regions in these vessels where the dominant shear harmonic is two or more times the heart rate and that these “high harmonic” regions colocalize with those experiencing a low shear magnitude, suggesting a possible contributing role of frequency content in atherosclerotic lesion development. Naturally, the frequency content of the shear profile is directly related to heart rate. Therefore, the focus of this study is to document the transient phenotype of endothelial cells during the adaptive response to shear frequency alteration and to test our hypothesis that such phenotype is different than that of fully adapted endothelial cells. Cultured porcine endothelial cells were preconditioned by a basal level sinusoidal shear of 15 ± 15 dyn/cm2 at 1 Hz for 24 h, and the frequency was then increased to 2 Hz for 6 h. Endothelial permeability and gene expression were measured at multiple time points after the step-up in frequency. This paper, together with our previous paper (41) and earlier animal studies (12, 14), are among the first efforts to document the transient endothelial phenotype during adaptation. We believe the genes and pathways identified in our study can serve as a basis for further studies to understand this important adaptive process and its implications.
MATERIALS AND METHODS
Porcine aortic endothelial cells were harvested from female swine aortae as previously described (41). Animal experiments were performed in accordance with a protocol approved by the Duke University Institutional Animal Care and Use Committee. Cells between passages 2 and 5 were used in all studies. Endothelial cells were preconditioned by a basal level shear stress (15 ± 15 dyn/cm2 at 1 Hz, sinusoidal waveform) for 24 h. The preconditioning time was confirmed to be sufficient for endothelial cells to reach a stable permeability level and transcriptional activity (41). The frequency of the applied shear stress was then increased to 2 Hz. The experimental set-up and protocols were similar to the previous work, and the following brief descriptions of methods are adapted from the previous paper (41).
Flow experiment setup.
The steady mean flow was provided by a peristaltic roller pump (Cole-Parmer) with a dampener. To superimpose the pulsatile flow, a computer-controlled stepping motor was used to drive a bellows pump. The volumetric flow rate was monitored using ultrasonic flow probes (Transonic Systems). The shear stress was calculated from the flow rate, culture media viscosity, and height of the flow domain. The flow circuit was placed in a cell culture incubator at 37°C and 5% CO2. The stepping motor was programmed to allow an increase in pulsatile frequency without altering shear stress levels.
Permeability study.
Endothelial permeability to bovine serum albumin (BSA) was measured using a custom-designed dual-chamber apparatus equipped with an optical-fiber based fluorescence detector. Fluorescein isothiocyanate-labeled BSA (FITC-BSA) was added to the perfusion media at a final concentration of 1 mg/ml. The fluorescence detection system was turned on to record the tracer concentration in the abluminal chamber, which was separated from the perfusion media by the endothelial monolayer cultured on a Transwell (Corning) membrane. The permeability of the preconditioned endothelial cells was obtained from the average rate of change in abluminal FITC-BSA concentration, Sp, found from a linear fit to the concentration profile over 5 min. After the permeability of the preconditioned endothelial cells was obtained, shear stress frequency was increased to 2 Hz. Endothelial permeability was then measured at multiple time points. At each time point, the average rate of change in concentration, S, was calculated as above during a time period of 5 min centered on the measurement time point. The endothelial permeability at each time point was normalized by the pre-step-up value (S/Sp). The integrity of the confluent endothelial monolayer was assessed at the end of each experiment. Samples with compromised monolayer integrity were not included.
Microarray study.
Endothelial cells in eight separate flow chambers were preconditioned simultaneously for 24 h. One control sample was obtained before the frequency step-up. At each designated time point (5, 15, 45, 90, 180, and 360 min after step-up), one randomly selected chamber was detached from the flow circuit and disassembled for RNA isolation. RNA isolation, amplification and DNA microarray hybridization were performed as described previously (41).
Microarray data analysis.
Quality filtering and normalization were performed using Genespring (Agilent). The expression levels at all time points after frequency step-up were normalized to the expression level of the corresponding pre-step-up control sample. The gene expression values presented in this paper are normalized values using a log2 scale. The differentially expressed genes at each time point were identified using significance analysis of microarrays (38) (SAM, Stanford). Ingenuity pathways analysis (IPA, Ingenuity) and GeneGo (GeneGo) were used for gene ontology and pathway analysis. The microarray data (28 arrays) are available at National Center for Biotechnology Information Gene Expression Omnibus (GSE26513).
Reverse transcription polymerase chain reaction.
Quantitative RT-PCR was performed to validate the microarray results for selected genes. GAPDH was used as the housekeeping gene. Primers used had previously been designed and tested for efficiency. The 2−ΔΔCT method was used to quantify expression levels of the target genes relative to the standard control sample. All expression values were normalized by the corresponding preconditioning values. Student's t-test was used for statistical interference.
RESULTS
Endothelial permeability increases slowly in response to the elevated shear frequency.
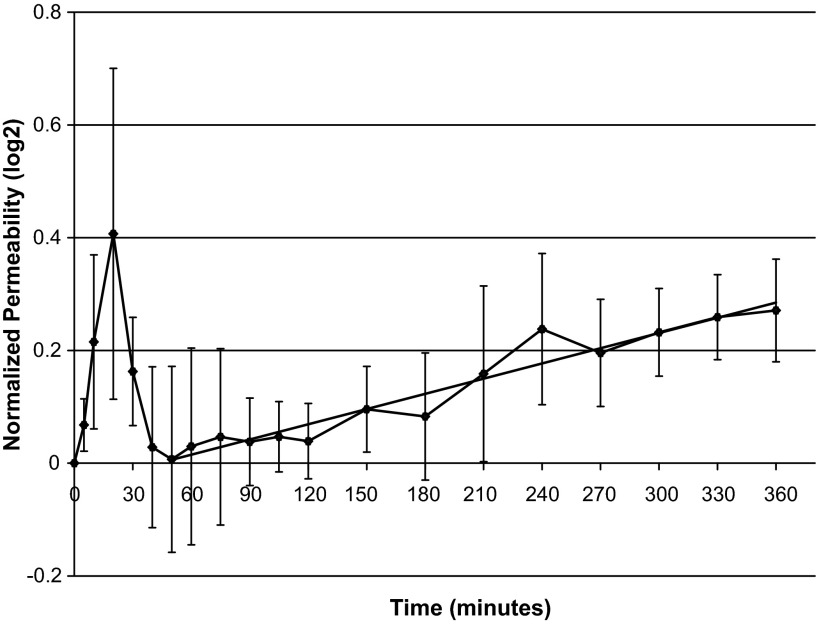
Figure 1 shows the normalized endothelial permeability at each time point during the adaptation process. The mean preconditioning value of endothelial permeability is 3.78 ± 0.47 × 10−6 cm/s. The elevated shear stress frequency had a modest effect on the endothelial permeability. The apparent large increase in permeability at 20 min came mostly from a single experiment, and the peak disappeared if that data set was removed. However, a clear increase in endothelial permeability over time was observed after 50 min exposure to the elevated shear frequency. A linear regression performed on data from 50 min to 360 min yields an R2 value of 0.94 and a highly significant slope (P < 10−8).
Fig. 1.
Normalized endothelial permeability during the adaptive response to the elevated shear frequency. Time 0, start of the frequency step-up. Endothelial permeability values were normalized to the preconditioning value. Error bars represent standard error of the mean, n = 4. A linear fit to the data from 50 to 360 min is shown (R2 = 0.94, P < 10−8).
Only a limited number of genes are sensitive to the increase in shear frequency.
Table 1 lists the number of genes identified as differentially expressed at each time point, relative to their expression in preconditioned cells, at different levels of false discovery rate (FDR). A total of 37 genes were identified by SAM to be upregulated by the frequency step-up at one or more time points when FDR was <10%. No gene was identified at any time point as downregulated by the frequency step-up. Table 2 is the complete list of identified genes. Some probes are not annotated with gene names since the corresponding porcine genes are unknown and no human or mouse homologous sequence was identified.
Table 1.
Numbers of identified genes at different time points after shear frequency step-up at different FDR levels
| Estimated FDR < 5% |
Estimated FDR < 10% |
|||
|---|---|---|---|---|
| Time | Upregulated | Downregulated | Upregulated | Downregulated |
| 5 min | 0 | 0 | 0 | 0 |
| 15 min | 2 | 0 | 2 | 0 |
| 45 min | 0 | 0 | 0 | 0 |
| 90 min | 5 | 0 | 5 | 0 |
| 180 min | 2 | 0 | 2 | 0 |
| 360 min | 12 | 0 | 33 | 0 |
FDR, false discovery rate.
Table 2.
Genes identified at each time point
| ID | Gene | Expression | Description |
|---|---|---|---|
| 15 min | |||
| SS00008885 | NUSAP1 | 0.59 | Nucleolar and spindle-associated protein 1 |
| SS00008707 | NDRG4 | 0.41 | NDRG family member 4 |
| 90 min | |||
| SS00008707 | NDRG4 | 1.08 | NDRG family member 4 |
| SS00009063 | SERTAD4 | 1.07 | SERTA domain containing 4 |
| SS00009402 | MBNL1 | 0.94 | Muscle blind-like (Drosophila) |
| SS00008885 | NUSAP1 | 0.86 | Nucleolar and spindle-associated protein 1 |
| SS00006968 | MGC17943 | 0.67 | Hypothetical protein MGC17943 |
| 180 min | |||
| SS00009402 | MBNL1 | 0.96 | Muscle blind-like (Drosophila) |
| SS00008883 | SHCBP1 | 0.78 | Likely ortholog of mouse Shc SH2-domain binding protein 1 |
| 360 min | |||
| SS00009479 | MUM1L1 | 1.28 | Melanoma-associated antigen (mutated) 1-like 1 |
| SS00009156 | CCNB3 | 1.17 | Cyclin B3 |
| SS00008885 | NUSAP1 | 1.16 | Nucleolar and spindle-associated protein 1 |
| SS00002311 | CENPA | 1.09 | Centromere protein A, 17 kDa |
| SS00009039 | DEK | 1.08 | DEK oncogene (DNA binding) |
| SS00008118 | STK38L | 1.08 | Serine/threonine kinase 38-like |
| SS00007619 | ID2 | 1.05 | Inhibitor of DNA binding 2 |
| SS00008745 | HNRPLL | 1.05 | Heterogeneous nuclear ribonucleoprotein L-like |
| SS00000455 | CCNB1 | 1.05 | Cyclin B1 |
| SS00010453 | 1.02 | ||
| SS00008883 | SHCBP1 | 0.95 | Likely ortholog of mouse Shc SH2-domain binding protein 1 |
| SS00009037 | FLJ20152 | 0.95 | Hypothetical protein FLJ20152 |
| SS00009945 | CDC2 | 0.93 | Cell division cycle 2, G1 to S and G2 to M |
| SS00005702 | MGC13017 | 0.91 | Similar to RIKEN cDNA A430101B06 gene |
| SS00008689 | VEGF | 0.87 | Vascular endothelial growth factor |
| SS00000123 | MCFP | 0.87 | Mitochondrial carrier family protein |
| SS00009449 | SRD5A2L | 0.85 | Steroid 5 α-reductase 2-like |
| SS00009189 | ZCCHC8 | 0.82 | Zinc finger, CCHC domain containing 8 |
| SS00009946 | NEK2 | 0.82 | NIMA (never in mitosis gene a)-related kinase 2 |
| SS00010472 | SNX16 | 0.80 | Sorting nexin 16 |
| SS00007910 | NCOA7 | 0.78 | Nuclear receptor coactivator 7 |
| SS00009514 | AMD1 | 0.78 | Adenosylmethionine decarboxylase 1 |
| SS00003461 | CCNA2 | 0.77 | Cyclin A2 |
| SS00007681 | FGFR2 | 0.76 | Fibroblast growth factor receptor 2 |
| SS00004188 | SCG2 | 0.76 | Secretogranin II (chromogranin C) |
| SS00008006 | ABCB1 | 0.74 | ATP-binding cassette, subfamily B (MDR/TAP), member 1 |
| SS00009066 | ARG99 | 0.74 | ARG99 protein |
| SS00010203 | 0.73 | ||
| SS00007312 | PSIP1 | 0.70 | PC4 and SFRS1-interacting protein 1 |
| SS00009132 | DNCLI1 | 0.69 | Dynein, cytoplasmic, light intermediate polypeptide 1 |
| SS00007957 | APPL | 0.69 | Adaptor protein containing pH domain, PTB domain, and leucine zipper motif |
| SS00007539 | USP1 | 0.65 | Ubiquitin-specific protease 1 |
| SS00009512 | 0.65 |
Expression values are in log2 scale, relative to the expression values before frequency step-up.
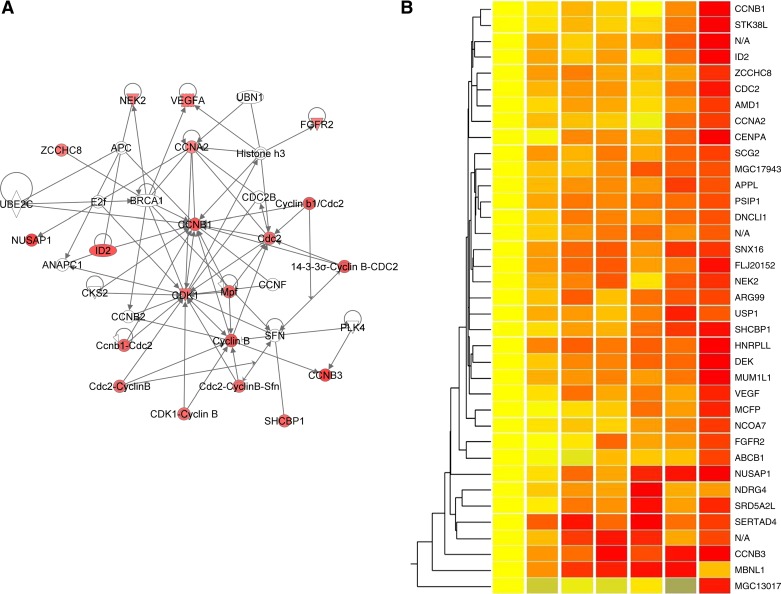
A total of 86 genes had been identified as responsive to a shear magnitude step-up at 10% FDR (41), so the endothelial cells appear to be somewhat less sensitive to changes in shear frequency. The regulation pattern of these genes is much simpler than that in the magnitude adaptation study, as shown by hierarchical clustering (Fig. 2). For most genes, the expression values increased throughout the time period. Only a few genes exhibited the pattern of early adaptation seen in the magnitude step-up study, where the expression values peaked at around 90 min and then dropped back to the pre-step-up values.
Fig. 2.
Gene network and clustering heat map generated from the identified genes. A: top ranked gene network generated by IPA. Genes are color-coded by the expression values at 360 min. B: hierarchical clustering of the identified genes. Red, overexpressed; yellow, unchanged; blue, underexpressed. See Table 2 for definitions of abbreviations.
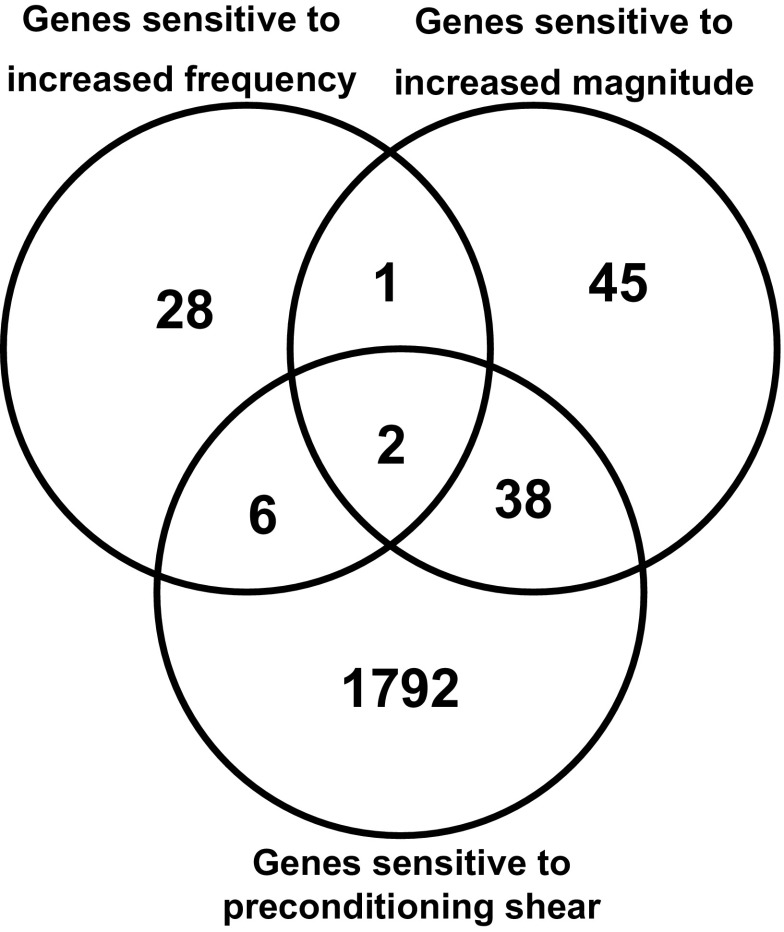
The previous study (41) identified 1,838 genes that were regulated by the preconditioning shear stress, compared with static culture. Figure 3 shows how these genes relate to the genes regulated by the two step-up exposures. Only 8 of these genes were sensitive to the frequency step-up. Only three genes were sensitive to both the frequency step-up and the magnitude step-up. Taken together, our data suggest that endothelial cells have distinct mechanotransduction mechanisms for shear frequency and shear magnitude.
Fig. 3.
Venn diagram displaying the overlap of genes sensitive to the increase in shear frequency (current study), genes sensitive to the increase in shear magnitude, and genes sensitive to the preconditioning shear compared with static cultured cells (41). False discovery rate = 10%.
Increased shear frequency upregulates a set of cell-cycle and mitosis regulation genes.
The list of identified genes was imported into IPA for network analysis. Interestingly, 11 of the 37 identified genes were found in a densely interconnected gene network as shown in Fig. 2, indicating their participation in a unique cellular process. Almost all genes in this network have important regulatory roles in cell cycle and mitotic events.
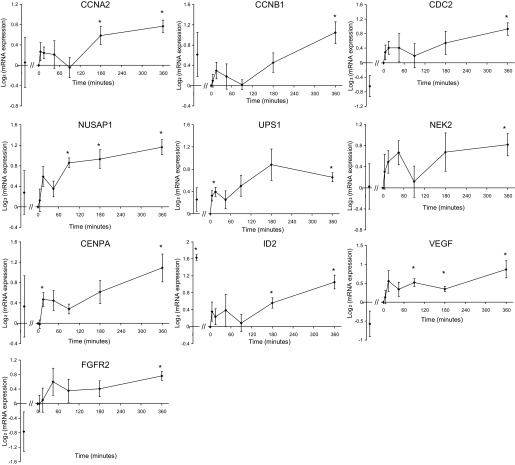
Three cyclin-related genes, cyclins A2 (CCNA2) and B1 (CCNB1) and cyclin-dependent kinase 1 (CDC2), were significantly overexpressed at 6 h. The temporal expression patterns of the three genes are highly similar, as shown in Fig. 4. The coordinated regulation of CDC2 and cyclin B indicates that endothelial cells may enter mitosis after exposure to elevated frequency. Recent studies have associated increases in cyclin A and B1 gene expression with endothelial proliferation (29, 40).
Fig. 4.
Temporal gene expression of selected genes. All expression values are normalized to time 0 (the pre-step-up controls). In each panel, the first data point represents the expression level in static cultured endothelial cells obtained from the control set experiments. *P < 0.05, one-sample t-test against zero without false discovery controls. Error bars represent standard error of the mean. See Table 2 for definitions of abbreviations.
Other mitosis-related genes upregulated by the elevated shear frequency include nucleolar and spindle-associated protein 1 (NUSAP1), a regulator in the formation of microtubule bundle during mitosis (32, 33); ubiquitin-specific protease 1, a positive regulator of DNA repair during G2 phase (27); and NIMA (never in mitosis A)-related kinase 2 (NEK2), a regulator of nuclear envelope breakdown and centromere separation at the onset of mitosis (31). Centromere protein A, a centromere binding gene (38a), was also overexpressed under elevated shear frequency after 15 min. These genes were all found to be overexpressed during the G2/M transition in animal cells (8, 31).
As might be expected from the IPA results, these cell cycle-related genes have highly similar temporal profiles, as shown in Fig. 4. Immediately after the step-up of shear frequency, these genes appear to undergo a moderate adaptive response, as evidenced by their overexpression around 15 to 45 min. The expression values then diminish toward the baseline level. From 90 min up to 6 h, the expression of these genes gradually increases. This highly coordinated expression profile suggests that these genes may be involved in a particular pathway that can sense the shear stress frequency and respond by regulating endothelial turnover.
Several genes involved in angiogenesis and atherogenesis are sensitive to increased shear frequency.
Inhibitor of DNA binding 2 (ID2), an angiogenic gene promoting endothelial cell growth and migration (2, 7, 25), was upregulated by the frequency step-up. Importantly, ID2 was one of the three genes that were also upregulated by the magnitude step-up, suggesting it may be an important regulator of endothelial response to shear stress alteration. Lasorella et al. (21) demonstrated that overexpression of ID2 can induce the expression of VEGF. Indeed, the expression level of VEGF was increased significantly in this study (Fig. 4). Fibroblast growth factor receptor 2, a potent angiogenesis signal transduction gene (3, 10), was also upregulated by the elevated shear frequency.
In addition to the differentially regulated genes identified using SAM, we also examined the expression of an a priori set of genes that are believed to participate in atherogenesis and are known to respond to shear stress. The list of genes was generated from the literature and can be found in Zhang and Friedman (41). Only six of these genes were sensitive to the elevation in shear frequency with a t-test P value < 0.05 (no FDR control was applied): ICAM-1, c-jun, caveolin-1, BMP4, HMOX1, and KLF4. Overall, an acute increase in shear frequency evidently has a small effect on the regulation of these atherosclerosis-related genes.
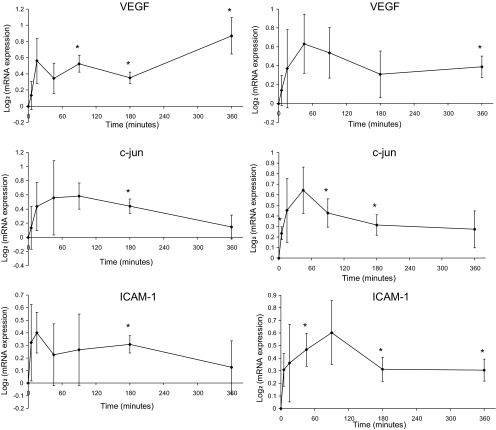
We further performed real-time quantitative-PCR to measure the expression levels of VEGF, c-jun, and ICAM-1 at each time point, as shown in Fig. 5. The results were consistent with the microarray measurements.
Fig. 5.
Temporal gene expression of VEGF, c-jun, and ICAM1 measured by microarray (left) and confirmed by RT-PCR (right); n = 4 for each time point. Expression values were normalized by the pre-step-up control levels. Error bars represent standard error of the mean. *P < 0.05, one-way t-test against zero.
The global gene expression profile gradually responds to the elevated shear frequency.
Although a moderate early adaptive response was found for the cell-cycle regulation genes in Fig. 4, the response of the global transcriptional profile to an increase in frequency is gradual. Scatter plots and hierarchical clustering were used to compare the global expression profiles at different time points as described in Zhang and Friedman (41). Unlike the magnitude step-up study, where at 45 min endothelial cells were found to have distinct gene expression patterns, the global transcriptional profiles at different time points are similar to each other in the present study. The means and variances of the expression values of all genes were also calculated for each time point. The means are all close to zero (log-scale), and the variances at each time point are similar. The temporal similarity of the transcriptional profiles was further supported by principal component analysis.
For gene ontology analysis, a gene list was obtained for each time point using a one-sample t-test P-value cutoff of 0.05 as described in Zhang and Friedman (41). The numbers of genes used in the GeneGo analysis were 326, 381, 372, 418, 506, and 499 at 5, 15, 45, 90, 180, and 360 min, respectively. Consistent with the results described above, pathway and gene ontology analysis suggested that VEGF signaling and activation pathways are sensitive to the frequency step-up. Other identified pathways include the lipid metabolism and focal adhesion kinase pathways. As expected, the cell-cycle G1-S transition and mitosis processes ranked highly in the cellular processes GO analysis.
DISCUSSION
Endothelial permeability.
In contrast to the rapid increase in endothelial permeability observed after an acute increase in shear stress magnitude (41), this study revealed a more modest response of endothelial permeability to the increase in shear stress frequency. Although an early adaptive response was absent, there was a clear and statistically significant increase in endothelial permeability from 50 min to 6 h after exposure to a higher shear frequency. The observed increase in permeability was relatively small (1.2-fold increase at 6 h); however, it is possible that endothelial permeability could eventually increase to a significantly higher level after longer exposure to the elevated shear frequency. The dependence of endothelial permeability on the frequency of chronically applied shear stress is still unknown in vivo and in vitro. The gradual increase in permeability in response to higher shear frequency may be a result of increased endothelial mitosis, as suggested by our microarray studies, since mitosis creates leakage sites for transendothelial biomolecular transport (18, 26, 36). The lack of a rapid increase in permeability immediately after the frequency step-up suggests that the increase in shear stress frequency was unable to induce sufficient cytoskeletal reorganization and junctional remodeling activity in contrast to shear magnitude step-ups. This is consistent with Himburg et al. (15), who found no effect of shear stress frequency on endothelial cell alignment or the expression and localization of several structural proteins, including actin, tubulin, and vascular endothelial-cadherin.
The measured permeability values of porcine aortic endothelial cells in our study are consistent with previous in vitro studies (19, 39). However, it is worth noting that the permeability to albumin of cultured cells is usually higher than the estimated values of in vivo arterial permeability (28, 34). This may be due to several structural factors in addition to differences in the resistance of the endothelial layer per se. Notwithstanding, much has been learned about vascular endothelial permeability from well-controlled in vitro studies. The near-immediate increase in endothelial permeability in response to an acute increase in shear, seen in our previous in vitro study (41), is consistent with the results of our earlier in vivo experiments (12, 14).
Endothelial gene expression.
Endothelial transcriptional activities are much less sensitive to increases in shear frequency than in shear magnitude, as indicated by the fewer identified genes (37 vs. 86) and the more gradual alteration of global transcriptional profiles. Only three genes, ID2, serine/threonine kinase 38 like, and SCH SH2-domain binding protein 1, were identified as sensitive to both magnitude and frequency step-ups. Furthermore, the triphasic adaptive response (induction period, early adaptation, and late remodeling) of the endothelial transcriptome seen in the magnitude step-up study was not observed in this study. Instead, the endothelial response to an increase in frequency is gradual and without a distinct adaptation process. Therefore, endothelial cells have distinctly different mechanotransduction mechanisms for shear step-ups in magnitude and frequency.
The acute increase in shear frequency upregulates a set of cell-cycle regulating and angiogenic genes. The upregulation of CCNA2, CCNB1, and CDC2 has been associated with endothelial proliferation (29, 40). The overexpression of the mitosis-related genes, NUSAP1, NEK2, and centromere protein A, indicates that the frequency increase may prime the endothelial cells for the G2/M transition (8, 31). Together with the upregulation of VEGF, ID2, and fibroblast growth factor receptor 2, increased shear frequency seems to signal for endothelial proliferation.
The effects of chronically applied shear frequency on endothelial gene expression has been studied by Himburg et al. (15) using the same microarray platform. We reanalyzed their data with the bioinformatic methods used in this study and determined that no gene was significantly differentially expressed when comparing endothelial cells exposed to 15 ± 15 dyn/cm2 at 1 and 2 Hz for 24 h. Therefore, the genes found in this study appear to be specific to the transient adaptive response. These identified genes, especially those previously unrecognized as shear sensitive, merit further investigation as potential regulators in atherogenesis.
Overall, we found in this study that the endothelial adaptive response to increased frequency is distinctly different than the response to increased shear magnitude, suggesting the involvement of different endothelial mechanotransduction mechanisms. However, consistent with the previous study (41), our data support the hypothesis that the endothelial phenotype during an adaptive response to a change in the shear environment is different than that of fully adapted endothelial cells. Further investigation should be encouraged to understand how the adaptation processes influences the development of atherosclerosis.
Implications for exercise.
Although the focus of this study is to understand the fundamental mechanobiology of endothelial adaptation, our results may shed some light on the effects of physical exercise on endothelial cells. Proposed 20 years ago by Laughlin and McAllister (22), it is well accepted that shear stress is a primary signal in mediating exercise-induced vascular adaptation and thus is a key player in the prevention of endothelial dysfunction and cardiovascular diseases (23). Physical exercise increases heart rate and stroke volume, leading to an increase in both the magnitude and the frequency of local shear stress. Thus this study, together with our previous study (41), may serve as an in vitro exercise model for endothelial cells, with the early adaptive response (15 to 90 min) being particularly relevant to daily exercise of typical duration. The increase in shear magnitude predominantly promotes the expression of a set of anti-inflammatory and antioxidative genes (41), whereas the increase in shear frequency may promote cell proliferation and prime the cells for angiogenesis. Although the combined effect of simultaneous changes in shear magnitude and frequency deserves further investigation, it is reasonable to speculate that during exercise, increased shear frequency facilitates the wound healing process to repair damaged or dysfunctional endothelial cells, while at the same time, the increased shear magnitude suppresses inflammation and oxidative stress.
The experiments reported here and in Zhang and Friedman (41), in which the cells were initially adapted to physiological levels of time-varying shear, are more representative of the in vivo situation than if the altered hemodynamic environment were imposed on static cells. This is evident from the differences in gene expression summarized in Fig. 2. Similarly, it is likely that the regulatory pathways governing adaptation depend on the initial state of the cells.
One important component of exercise physiology is flow-mediated dilation (FMD), which can be regarded as a vessel-level adaptive response. FMD increases blood vessel diameter, which moderates the increase in shear that would otherwise accompany an exercise-induced increase in flow. Notwithstanding FMD, endothelial cells in the larger arteries experience increases in both shear magnitude and frequency under exercise conditions (30). Tinken et al. (37) showed that handgrip exercise caused a 60% increase in brachial artery shear rate. Taylor et al. (35) showed that bicycle exercise led to 70 and 300% increases in shear stress in the supraceliac aorta and infrarenal aorta, respectively. In earlier work (14), we also found that unilateral femoral arteriovenous shunting in swine induced an increase in shear in the ipsilateral distal iliac artery, notwithstanding the dilation of the vessel. Endothelial cell phenotypes in vivo are likely to be regulated by the combined effect of adaptation to magnitude and frequency. The present work and that reported in Zhang and Friedman (41) demonstrate the separate endothelial responses to increases in shear frequency and magnitude. In principle, this should permit the overall effect of exercise-induced transients on gene expression to be predicted, although it is recognized that interactions between changes in shear and frequency have not yet been examined.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant HL-050442 and American Heart Association Grant 09PRE2080238 (to J. Zhang).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.Z. conception and design of research; J.Z. performed experiments; J.Z. analyzed data; J.Z. and M.H.F. interpreted results of experiments; J.Z. prepared figures; J.Z. drafted manuscript; J.Z. and M.H.F. edited and revised manuscript; J.Z. and M.H.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. Jeff LaMack, Heather Himburg, and Charles S. Wallace for help and Duke Microarray Facility for technical services.
Present address of J. Zhang: W. L. Gore & Associates, zhangji@wlgore.com.
REFERENCES
- 1.Bao X, Lu C, Frangos JA. Temporal gradient in shear but not steady shear stress induces PDGF-A and MCP-1 expression in endothelial cells: role of NO, NF kappa B, and egr-1. Arterioscler Thromb Vasc Biol 19: 996–1003, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Benezra R. Role of Id proteins in embryonic and tumor angiogenesis. Trends Cardiovasc Med 11: 237–241, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Bikfalvi A, Klein S, Pintucci G, Rifkin DB. Biological roles of fibroblast growth factor-2. Endocr Rev 18: 26–45, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Chien S. Effects of disturbed flow on endothelial cells. Ann Biomed Eng 36: 554–562, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chien S. Molecular basis of rheological modulation of endothelial functions: importance of stress direction. Biorheology 43: 95–116, 2006 [PubMed] [Google Scholar]
- 6.Colgan OC, Ferguson G, Collins NT, Murphy RP, Meade G, Cahill PA, Cummins PM. Regulation of bovine brain microvascular endothelial tight junction assembly and barrier function by laminar shear stress. Am J Physiol Heart Circ Physiol 292: H3190–H3197, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Coma S, Amin DN, Shimizu A, Lasorella A, Iavarone A, Klagsbrun M. Id2 promotes tumor cell migration and invasion through transcriptional repression of semaphorin 3F. Cancer Res 70: 3823–3832, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crawford DF, Piwnica-Worms H. The G(2) DNA damage checkpoint delays expression of genes encoding mitotic regulators. J Biol Chem 276: 37166–37177, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Cunningham KS, Gotlieb AI. The role of shear stress in the pathogenesis of atherosclerosis. Lab Invest 85: 9–23, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Friesel RE, Maciag T. Molecular mechanisms of angiogenesis: fibroblast growth factor signal transduction. FASEB J 9: 919–925, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Hahn C, Schwartz MA. The role of cellular adaptation to mechanical forces in atherosclerosis. Arterioscler Thromb Vasc Biol 28: 2101–2107, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hazel AL, Grzybowski DM, Friedman MH. Modeling the adaptive permeability response of porcine iliac arteries to acute changes in mural shear. Ann Biomed Eng 31: 412–419, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Helderman F, Segers D, de Crom R, Hierck BP, Poelmann RE, Evans PC, Krams R. Effect of shear stress on vascular inflammation and plaque development. Curr Opin Lipidol 18: 527–533, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Henderson JM, Aukerman JA, Clingan PA, Friedman MH. Effect of alterations in femoral artery flow on abdominal vessel hemodynamics in swine. Biorheology 36: 257–266, 1999 [PubMed] [Google Scholar]
- 15.Himburg HA, Dowd SE, Friedman MH. Frequency-dependent response of the vascular endothelium to pulsatile shear stress. Am J Physiol Heart Circ Physiol 293: H645–H653, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Himburg HA, Friedman MH. Correspondence of low mean shear and high harmonic content in the porcine iliac arteries. J Biomech Eng 128: 852–856, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Himburg HA, Grzybowski DM, Hazel AL, LaMack JA, Li XM, Friedman MH. Spatial comparison between wall shear stress measures and porcine arterial endothelial permeability. Am J Physiol Heart Circ Physiol 286: H1916–H1922, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Kennedy JH, Tedgui A. Normal and pathological aspects of mass transport across the vascular wall. Cardiovasc Surg 3: 611–615, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Kudo S, Tsuzaka M, Ikeda M, Tanishita K. Albumin permeability across endothelial monolayers under long-term shear stress. JSME International Journal Series C-Mechanical Systems Machine Elements and Manufacturing 48: 419–424, 2005 [Google Scholar]
- 20.LaMack JA, Friedman MH. Individual and combined effects of shear stress magnitude and spatial gradient on endothelial cell gene expression. Am J Physiol Heart Circ Physiol 293: H2853–H2859, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Lasorella A, Rothschild G, Yokota Y, Russell RG, Iavarone A. Id2 mediates tumor initiation, proliferation, and angiogenesis in Rb mutant mice. Mol Cell Biol 25: 3563–3574, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laughlin MH, McAllister RM. Exercise training-induced coronary vascular adaptation. J Appl Physiol 73: 2209–2225, 1992 [DOI] [PubMed] [Google Scholar]
- 23.Laughlin MH, Newcomer SC, Bender SB. Importance of hemodynamic forces as signals for exercise-induced changes in endothelial cell phenotype. J Appl Physiol 104: 588–600, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsumura ME, Lobe DR, McNamara CA. Contribution of the helix-loop-helix factor Id2 to regulation of vascular smooth muscle cell proliferation. J Biol Chem 277: 7293–7297, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Michel CC, Curry FE. Microvascular permeability. Physiol Rev 79: 703–761, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Oestergaard VH, Langevin F, Kuiken HJ, Pace P, Niedzwiedz W, Simpson LJ, Ohzeki M, Takata M, Sale JE, Patel KJ. Deubiquitination of FANCD2 is required for DNA crosslink repair. Mol Cell 28: 798–809, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogunrinade O, Kameya GT, Truskey GA. Effect of fluid shear stress on the permeability of the arterial endothelium. Ann Biomed Eng 30: 430–446, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Oviedo PJ, Hermenegildo C, Tarin JJ, Cano A. Raloxifene increases proliferation of human endothelial cells in association with increased gene expression of cyclins A and B1. Fertil Steril 88: 326–332, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. J Physiol 568: 357–369, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quarmby LM, Mahjoub MR. Caught Nek-ing: cilia and centrioles. J Cell Sci 118: 5161–5169, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Raemaekers T, Ribbeck K, Beaudouin J, Annaert W, Van Camp M, Stockmans I, Smets N, Bouillon R, Ellenberg J, Carmeliet G. NuSAP, a novel microtubule-associated protein involved in mitotic spindle organization. J Cell Biol 162: 1017–1029, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ribbeck K, Groen AC, Santarella R, Bohnsack MT, Raemaekers T, Kocher T, Gentzel M, Gorlich D, Wilm M, Carmeliet G, Mitchison TJ, Ellenberg J, Hoenger A, Mattaj IW. NuSAP, a mitotic RanGTP target that stabilizes and cross-links microtubules. Mol Biol Cell 17: 2646–2660, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tarbell JM. Shear stress and the endothelial transport barrier. Cardiovasc Res 87: 320–330, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor CA, Cheng CP, Espinosa LA, Tang BT, Parker D, Herfkens RJ. In vivo quantification of blood flow and wall shear stress in the human abdominal aorta during lower limb exercise. Ann Biomed Eng 30: 402–408, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Tedgui A. Endothelial permeability under physiological and pathological conditions. Prostaglandins Leukot Essent Fatty Acids 54: 27–29, 1996 [DOI] [PubMed] [Google Scholar]
- 37.Tinken TM, Thijssen DH, Hopkins N, Dawson EA, Cable NT, Green DJ. Shear stress mediates endothelial adaptations to exercise training in humans. Hypertension 55: 312–318, 2010 [DOI] [PubMed] [Google Scholar]
- 38.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA 98: 5116–5121, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38a.Valdivia MM, Hamdouch K, Ortiz M, Astola A. CENPA a genomic marker for centromere activity and human diseases. Curr Genomics 10: 326–335, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Warboys CM, Eric Berson R, Mann GE, Pearson JD, Weinberg PD. Acute and chronic exposure to shear stress have opposite effects on endothelial permeability to macromolecules. Am J Physiol Heart Circ Physiol 298: H1850–H1856, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xie L, Frank PG, Lisanti MP, Sowa G. Endothelial cells isolated from caveolin-2 knockout mice display higher proliferation rate and cell cycle progression relative to their wild-type counterparts. Am J Physiol Cell Physiol 298: C693–C701, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang J, Friedman MH. Adaptive response of vascular endothelial cells to an acute increase in shear stress magnitude. Am J Physiol Heart Circ Physiol 302: H983–H991, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]