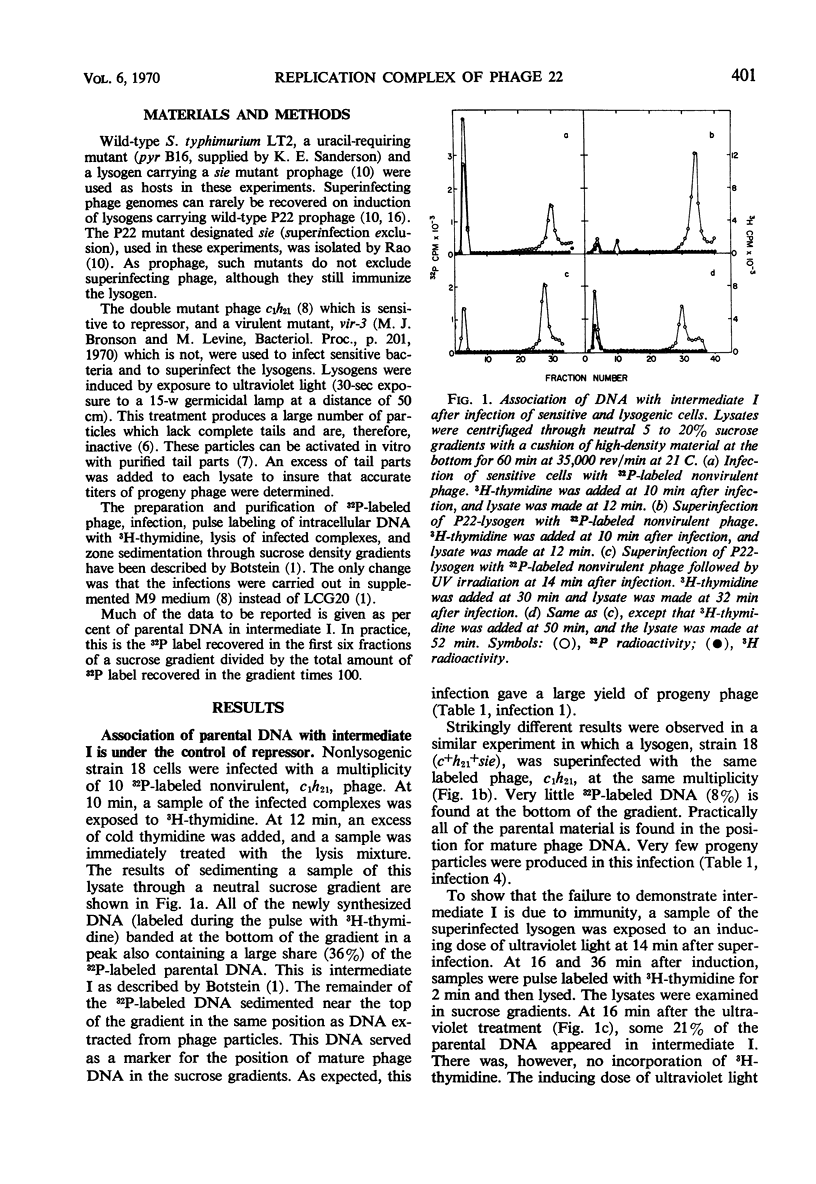
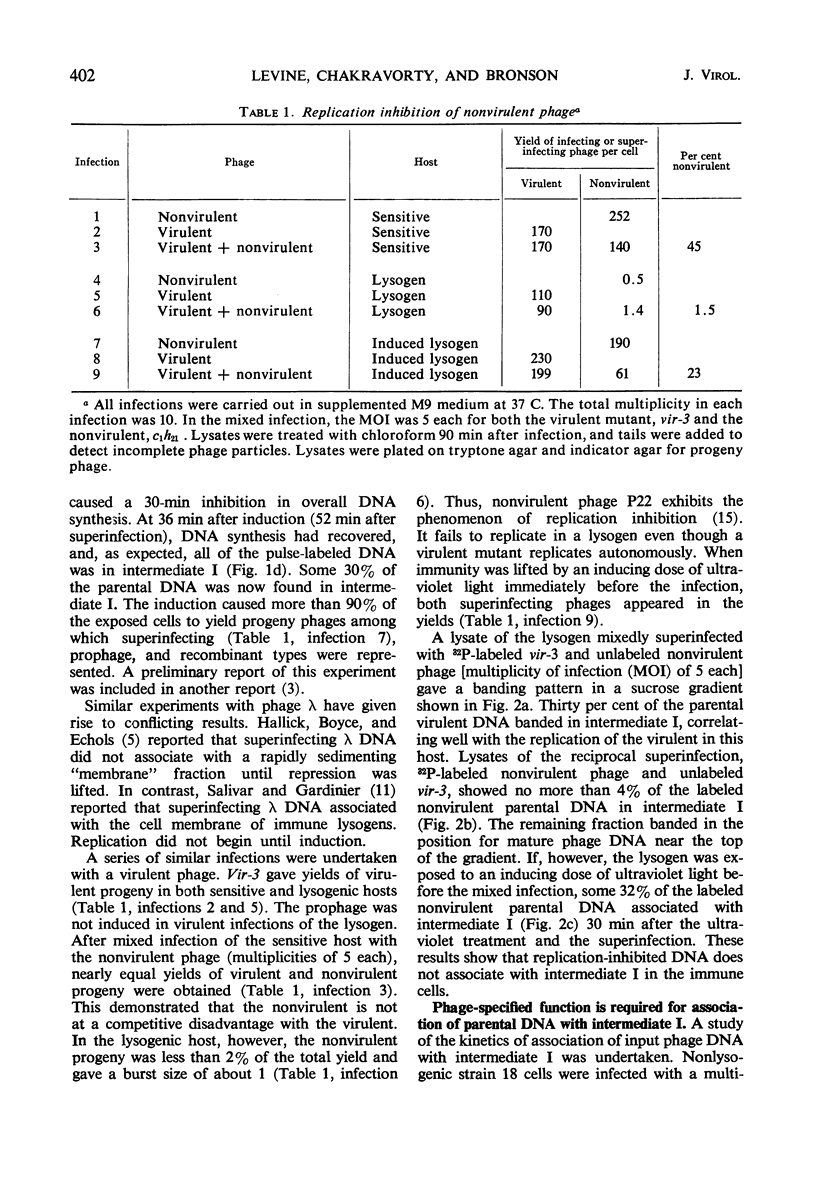
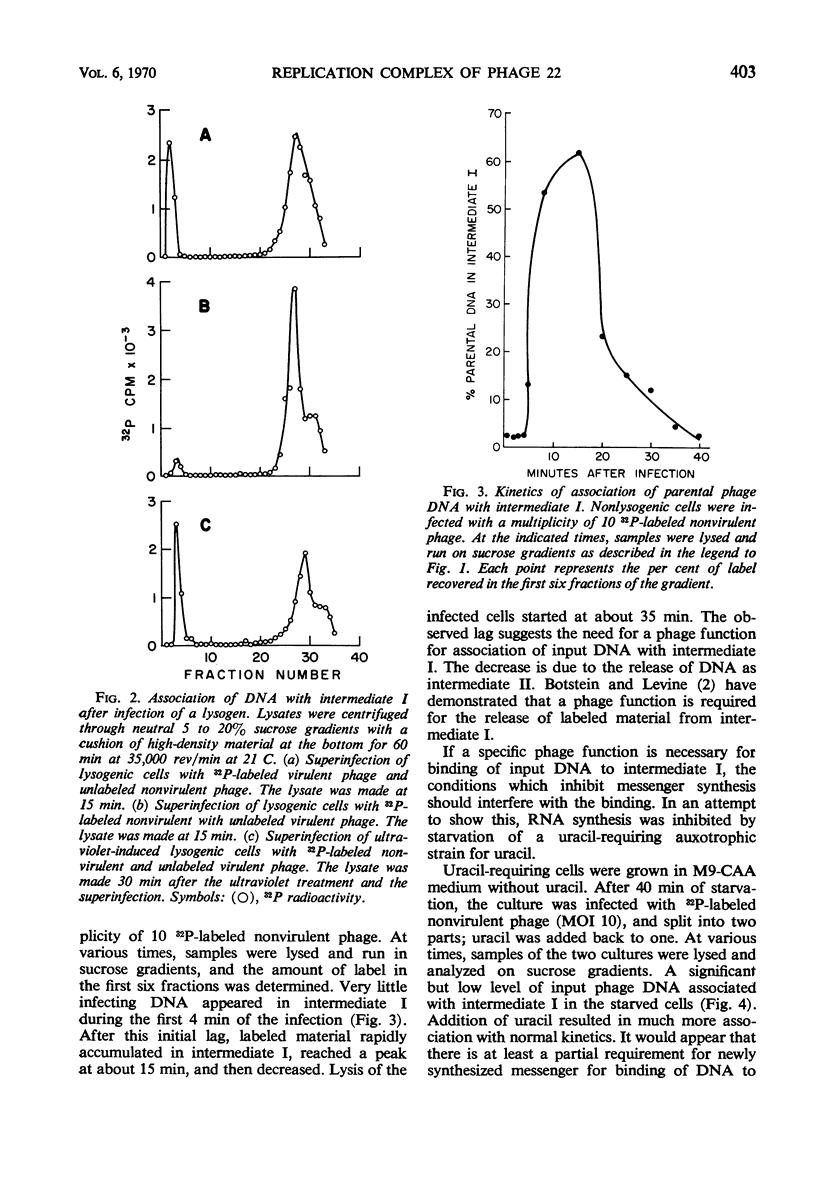
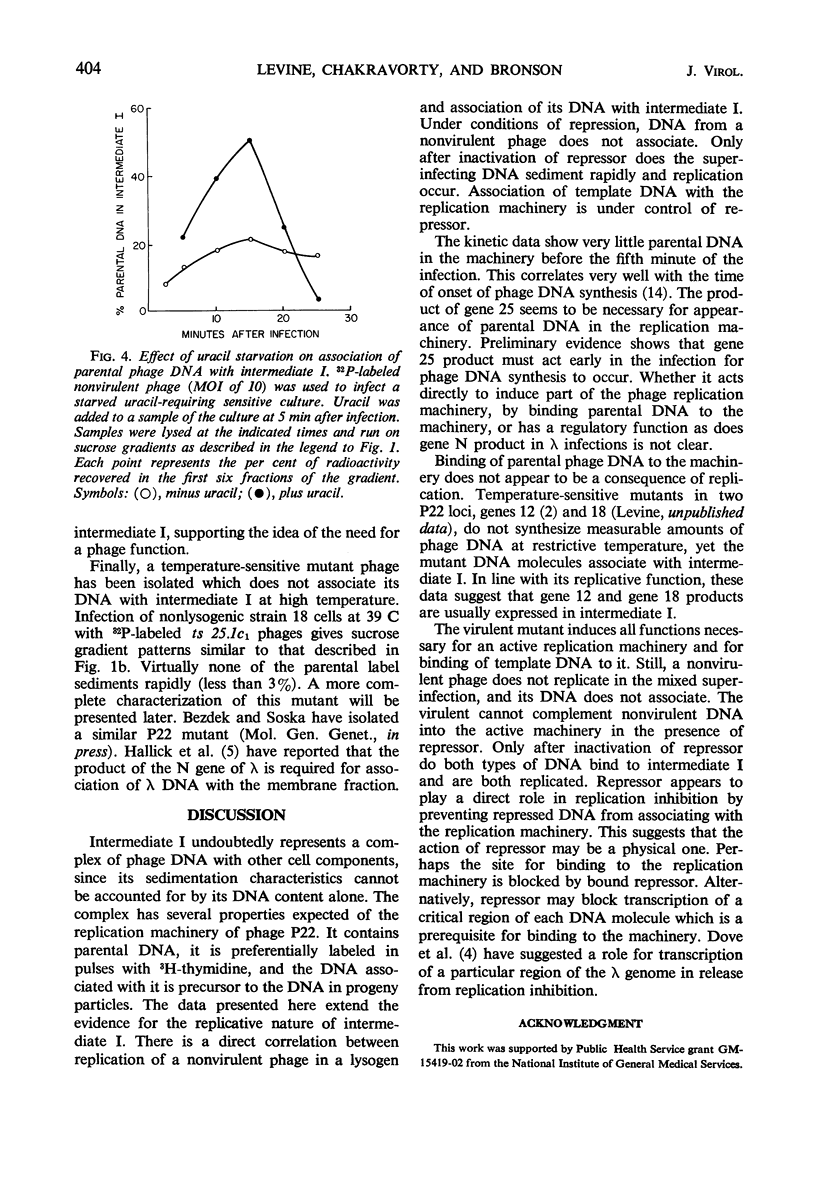
Abstract
A replication complex for the vegetative synthesis of the deoxyribonucleic acid (DNA) of the temperate phage P22 previously has been described. This complex is an association of parental phage DNA, most of the newly synthesized phage DNA made during pulses with 3H-thymidine, and other cell constituents, and has a sedimentation rate in neutral sucrose gradients of at least 1,000S. The complex is one of the intermediates, intermediate I, in the synthesis and maturation of phage P22 DNA after infection or induction. Evidence supporting the replicative nature of intermediate I is presented. Phage replication is repressed in lysogenic bacteria. On superinfection of P22 lysogens with nonvirulent phage, little association of the input phage DNA with a rapidly sedimenting fraction is demonstrable. However, after induction with ultraviolet light, the superinfecting parental phage DNA quickly acquires the rapid sedimentation rate characteristic of intermediate I; phage DNA synthesis follows; and progeny phages are produced. Infection with a virulent mutant of P22 produces progeny phages in lysogens. Its DNA associates with intermediate I. In mixed infection with the virulent phage, replication of nonvirulent phage P22 is still repressed, even though the virulent replicates normally. The nonvirulent input DNA does not associate with intermediate I. The repressor of the lysogenic cell prevents replication by interfering with the physical association of template material with intermediate I. A phage function is required for association of phage template with the replication machinery.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Botstein D., Levine M. Intermediates in the synthesis of phage P22 DNA. Cold Spring Harb Symp Quant Biol. 1968;33:659–667. doi: 10.1101/sqb.1968.033.01.075. [DOI] [PubMed] [Google Scholar]
- Botstein D., Levine M. Synthesis and maturation of phage P22 DNA. II. Properties of temperature-sensitive phage mutants defective in DNA metabolism. J Mol Biol. 1968 Jun 28;34(3):643–654. doi: 10.1016/0022-2836(68)90186-1. [DOI] [PubMed] [Google Scholar]
- Botstein D. Synthesis and maturation of phage P22 DNA. I. Identification of intermediates. J Mol Biol. 1968 Jun 28;34(3):621–641. doi: 10.1016/0022-2836(68)90185-x. [DOI] [PubMed] [Google Scholar]
- Hallick L., Boyce R. P., Echols H. Membrane association by bacteriophage lambda-DNA: possible direct role of regulator gene N. Nature. 1969 Sep 20;223(5212):1239–1242. doi: 10.1038/2231239a0. [DOI] [PubMed] [Google Scholar]
- Israel J. V., Anderson T. F., Levine M. in vitro MORPHOGENESIS OF PHAGE P22 FROM HEADS AND BASE-PLATE PARTS. Proc Natl Acad Sci U S A. 1967 Feb;57(2):284–291. doi: 10.1073/pnas.57.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel V. The production of inactive phage P22 particles following induction. Virology. 1967 Oct;33(2):317–322. doi: 10.1016/0042-6822(67)90150-x. [DOI] [PubMed] [Google Scholar]
- LEVINE M. Mutations in the temperate phage P22 and lysogeny in Salmonella. Virology. 1957 Feb;3(1):22–41. doi: 10.1016/0042-6822(57)90021-1. [DOI] [PubMed] [Google Scholar]
- LEVINE M., SMITH H. O. SEQUENTIAL GENE ACTION IN THE ESTABLISHMENT OF LYSOGENY. Science. 1964 Dec 18;146(3651):1581–1582. doi: 10.1126/science.146.3651.1581. [DOI] [PubMed] [Google Scholar]
- Rao R. N. Bacteriophage P22 controlled exclusion in Salmonella typhimurium. J Mol Biol. 1968 Aug 14;35(3):607–622. doi: 10.1016/s0022-2836(68)80017-8. [DOI] [PubMed] [Google Scholar]
- SMITH H. O., LEVINE M. THE SYNTHESIS OF PHAGE AND HOST DNA IN THE ESTABLISHMENT OF LYSOGENY. Virology. 1965 Apr;25:585–590. doi: 10.1016/0042-6822(65)90086-3. [DOI] [PubMed] [Google Scholar]
- Salivar W. O., Gardinier J. Replication of bacteriophage lambda DNA associated with the host cell membrane. Virology. 1970 May;41(1):38–51. doi: 10.1016/0042-6822(70)90052-8. [DOI] [PubMed] [Google Scholar]
- Smith H. O. Defective phage formation by lysogens of integration deficient phage P22 mutants. Virology. 1968 Feb;34(2):203–223. doi: 10.1016/0042-6822(68)90231-6. [DOI] [PubMed] [Google Scholar]
- Smith H. O., Levine M. Gene order in prophage P22. Virology. 1965 Oct;27(2):229–231. doi: 10.1016/0042-6822(65)90166-2. [DOI] [PubMed] [Google Scholar]
- THOMAS R., BERTANI L. E. ON THE CONTROL OF THE REPLICATION OF TEMPERATE BACTERIOPHAGES SUPERINFECTING IMMUNE HOSTS. Virology. 1964 Nov;24:241–253. doi: 10.1016/0042-6822(64)90163-1. [DOI] [PubMed] [Google Scholar]
- Walsh J., Meynell G. G. The isolation of non-excluding mutants of phage P22. J Gen Virol. 1967 Oct;1(4):581–582. doi: 10.1099/0022-1317-1-4-581. [DOI] [PubMed] [Google Scholar]


