Abstract
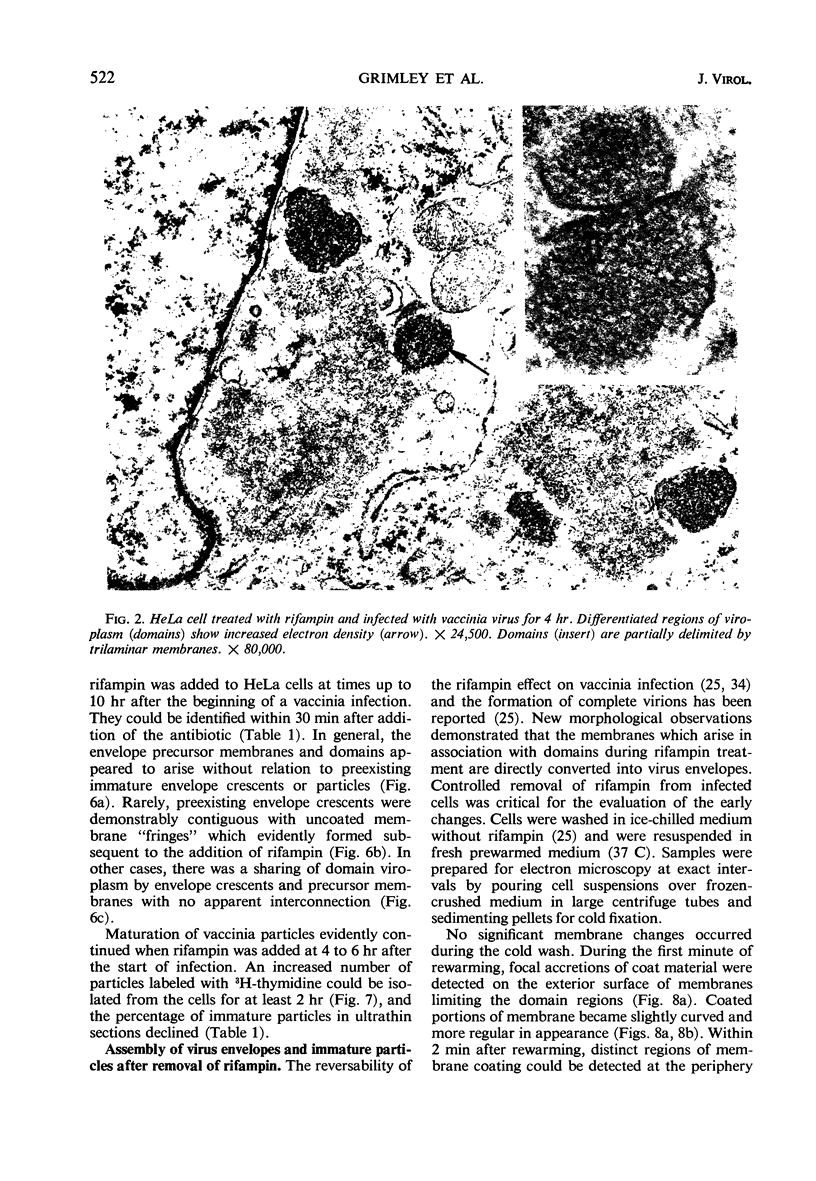
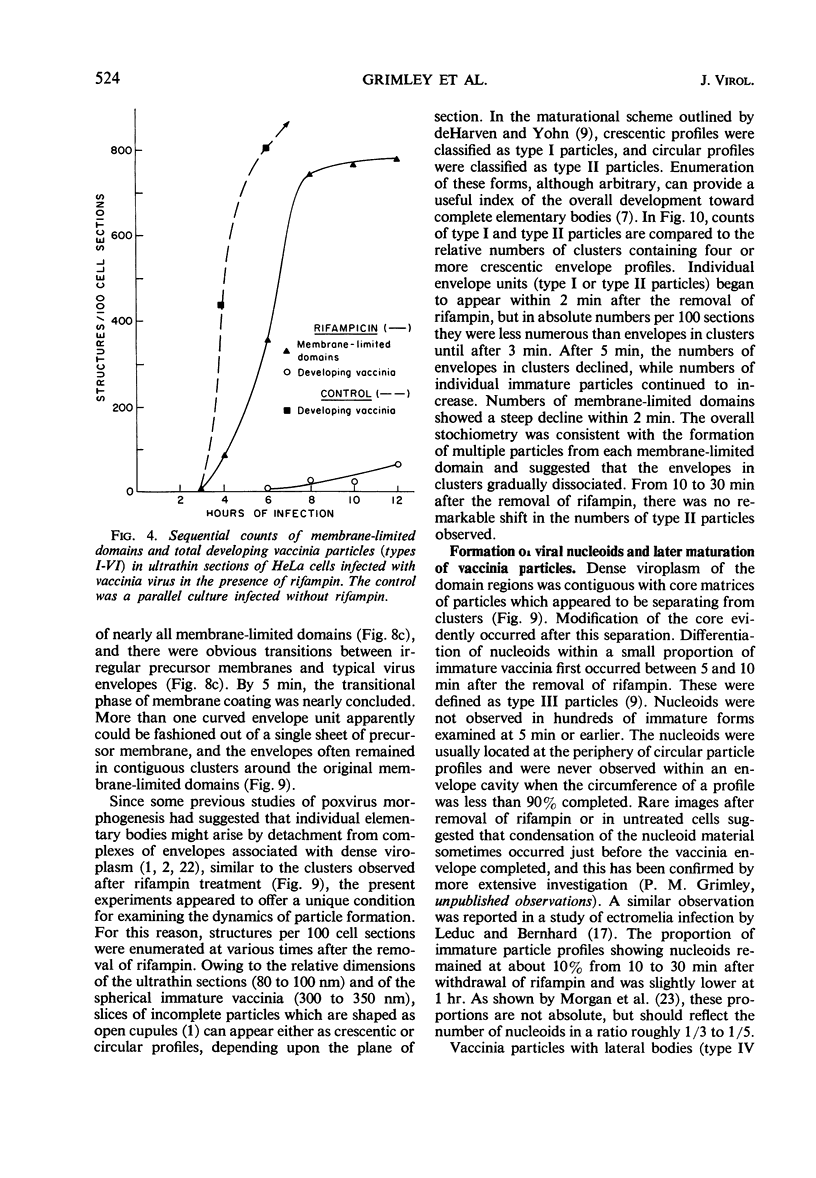
Assembly of vaccinia virus envelopes and immature vaccinia particles was interrupted in HeLa cells treated with rifampin (rifampicin). The primary action of rifampin on vaccinia morphogenesis appeared to occur during the stage of envelope formation. When envelopes and immature particles were already present, maturation could continue, even in the presence of rifampin. It was demonstrated that the trilaminar membranes of irregular contour which accumulate in the presence of rifampin are precursors of virus envelopes. When rifampin was removed under controlled conditions, synchronous transitions were observed as the precursor membranes rapidly converted into uniformly curved envelope units with a 10- to 12-nm coat on the convex surface. These experiments provided an opportunity to examine the sequence of some early events in vaccinia morphogenesis. Initially, nascent envelopes remained in clusters around dense viroplasm. Large numbers of single immature particles appeared within 10 min. Nucleation of immature particles was the first evidence of core differentiation and began within 5 to 10 min. Development of lateral bodies and modeling of the biconcave cores was observed within 30 min, and structurally mature virions were present by 2 hr after the removal of rifampin. High resolution autoradiography showed that viral deoxyribonucleic acid, which labeled with 3H-thymidine during rifampin treatment, was incorporated by the mature vaccinia which formed after rifampin was removed. Concentration of the viral deoxyribonucleic acid in core material evidently occurred after envelope assembly, probably coincident with nucleoid formation. Cytoplasmic crystalloid bodies accumulated during rifampin treatment; they appeared morphologically identical to vaccinia nucleoids and were heavily labeled by 3H-thymidine.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVAKYAN A. A., BYCKOVSKY A. F. ONTOGENESIS OF HUMAN SMALLPOX VIRUS. J Cell Biol. 1965 Mar;24:337–347. doi: 10.1083/jcb.24.3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BANFIELD W. G., BRINDLEY D. C. An electron microscopic study of the epidermal lesion of molluscum contagiosum. Ann N Y Acad Sci. 1959 Jul 21;81:145–163. doi: 10.1111/j.1749-6632.1959.tb49303.x. [DOI] [PubMed] [Google Scholar]
- Ben-Ishai Z., Heller E., Goldblum N., Becker Y. Rifampicin and poxvirus replication. Nature. 1969 Oct 4;224(5214):29–32. doi: 10.1038/224029a0. [DOI] [PubMed] [Google Scholar]
- Caro L. G. A common source of difficulty in high-resolution radioautography. J Cell Biol. 1969 Jun;41(3):918–919. doi: 10.1083/jcb.41.3.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers R. W., Bowling M. C., Grimley P. M. Glutaraldehyde fixation in routine histopathology. Arch Pathol. 1968 Jan;85(1):18–30. [PubMed] [Google Scholar]
- Costanzo F., Fiume L., La Placa M., Mannini-Palenzona A., Novello F., Stirpe F. Ribonucleic acid polymerase induced by vaccinia virus: lack of inhibition by rifampicin and alpha-amanitin. J Virol. 1970 Feb;5(2):266–269. doi: 10.1128/jvi.5.2.266-269.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALES S., SIMINOVITCH L. The development of vaccinia virus in Earle's L strain cells as examined by electron microscopy. J Biophys Biochem Cytol. 1961 Aug;10:475–503. doi: 10.1083/jcb.10.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dales S., Mosbach E. H. Vaccinia as a model for membrane biogenesis. Virology. 1968 Aug;35(4):564–583. doi: 10.1016/0042-6822(68)90286-9. [DOI] [PubMed] [Google Scholar]
- De Harven E., Yohn D. S. The fine structure of the Yaba monkey tumor poxvirus. Cancer Res. 1966 May;26(5):995–1008. [PubMed] [Google Scholar]
- EASTERBROOK K. B. Interference with the maturation of vaccinia virus by isatin beta-thiosemicarbazone. Virology. 1962 Jun;17:245–251. doi: 10.1016/0042-6822(62)90114-9. [DOI] [PubMed] [Google Scholar]
- Grimley P. M., Berezesky I. K., Friedman R. M. Cytoplasmic structures associated with an arbovirus infection: loci of viral ribonucleic acid synthesis. J Virol. 1968 Nov;2(11):1326–1338. doi: 10.1128/jvi.2.11.1326-1338.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller E., Argaman M., Levy H., Goldblum N. Selective inhibition of vaccinia virus by the antibiotic rifampicin. Nature. 1969 Apr 19;222(5190):273–274. doi: 10.1038/222273a0. [DOI] [PubMed] [Google Scholar]
- KAJIOKA R., SIMINOVITCH L., DALES S. THE CYCLE OF MULTIPLICATION OF VACCINIA VIRUS IN EARLE'S STRAIN L CELLS. II. INITIATION OF DNA SYNTHESIS AND MORPHOGENESIS. Virology. 1964 Nov;24:295–309. doi: 10.1016/0042-6822(64)90168-0. [DOI] [PubMed] [Google Scholar]
- Katz E., Grimley P., Moss B. Reversal of anti-viral effects of rifampicin. Nature. 1970 Sep 5;227(5262):1050–1051. doi: 10.1038/2271050a0. [DOI] [PubMed] [Google Scholar]
- Katz E., Moss B. Formation of a vaccinia virus structural polypeptide from a higher molecular weight precursor: inhibition by rifampicin. Proc Natl Acad Sci U S A. 1970 Jul;66(3):677–684. doi: 10.1073/pnas.66.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopriwa B. M. The influence of development on the number and appearance of silver grains in electron microscopic radioautography. J Histochem Cytochem. 1967 Sep;15(9):501–515. doi: 10.1177/15.9.501. [DOI] [PubMed] [Google Scholar]
- LEDUC E. H., BERNHARD W. Electron microscope study of mouse liver infected by ectromelia virus. J Ultrastruct Res. 1962 Jun;6:466–488. doi: 10.1016/s0022-5320(62)80003-3. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Permanganate; a new fixative for electron microscopy. J Biophys Biochem Cytol. 1956 Nov 25;2(6):799–802. doi: 10.1083/jcb.2.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORGAN C., ELLISON S. A., ROSE H. M., MOORE D. H. Serial sections of vaccinia virus examined at one stage of development in the electron microscope. Exp Cell Res. 1955 Dec;9(3):572–578. doi: 10.1016/0014-4827(55)90086-0. [DOI] [PubMed] [Google Scholar]
- MORGAN C., ELLISON S. A., ROSE H. M., MOORE D. H. Structure and development of viruses observed in the electron microscope. II. Vaccinia and fowl pox viruses. J Exp Med. 1954 Sep 1;100(3):301–310. doi: 10.1084/jem.100.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi N., Pasqualucci C. R., Ballotta R., Sensi P. Rifampicin: a new orally active rifamycin. Chemotherapy. 1966;11(5):285–292. doi: 10.1159/000220462. [DOI] [PubMed] [Google Scholar]
- Mcauslan B. R. Rifampicin inhibition of vaccinia replication. Biochem Biophys Res Commun. 1969 Oct 8;37(2):289–295. doi: 10.1016/0006-291x(69)90733-5. [DOI] [PubMed] [Google Scholar]
- Moss B., Katz E., Rosenblum E. N. Vaccinia virus directed RNA and protein synthesis in the presence of rifampicin. Biochem Biophys Res Commun. 1969 Aug 22;36(5):858–865. doi: 10.1016/0006-291x(69)90688-3. [DOI] [PubMed] [Google Scholar]
- Moss B., Rosenblum E. N., Katz E., Grimley P. M. Rifampicin: a specific inhibitor of vaccinia virus assembly. Nature. 1969 Dec 27;224(5226):1280–1284. doi: 10.1038/2241280a0. [DOI] [PubMed] [Google Scholar]
- Nagaya A., Pogo B. G., Dales S. Biogenesis of vaccinia: separation of early stages from maturation by means of rifampicin. Virology. 1970 Apr;40(4):1039–1051. doi: 10.1016/0042-6822(70)90150-9. [DOI] [PubMed] [Google Scholar]
- Rosenkranz H. S., Rose H. M., Morgan C., Hsu K. C. The effect of hydroxyurea on virus development. II. Vaccinia virus. Virology. 1966 Apr;28(4):510–519. doi: 10.1016/0042-6822(66)90235-2. [DOI] [PubMed] [Google Scholar]
- Salpeter M. M., Bachmann L., Salpeter E. E. Resolution in electron microscope radioautography. J Cell Biol. 1969 Apr;41(1):1–32. doi: 10.1083/jcb.41.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer R. Morphogénèse et ultrastructure du virus fibromateux de Shope. Pathol Microbiol (Basel) 1968;31(3):129–146. [PubMed] [Google Scholar]
- Scherrer R. Viral and host deoxyribonucleic acid synthesis in Shope fibroma virus-infected cells as studied by means of high-resolution autoradiography. J Virol. 1968 Dec;2(12):1418–1428. doi: 10.1128/jvi.2.12.1418-1428.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sippel A., Hartmann G. Mode of action of rafamycin on the RNA polymerase reaction. Biochim Biophys Acta. 1968 Mar 18;157(1):218–219. doi: 10.1016/0005-2787(68)90286-4. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Engelman D. M. Current models for the structure of biological membranes. J Cell Biol. 1969 Sep;42(3):613–646. doi: 10.1083/jcb.42.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subak-Sharpe J. H., Timbury M. C., Williams J. F. Rifampicin inhibits the growth of some mammalian viruses. Nature. 1969 Apr 26;222(5191):341–345. doi: 10.1038/222341a0. [DOI] [PubMed] [Google Scholar]
- Terzakis J. A. Uranyl acetate, a stain and a fixative. J Ultrastruct Res. 1968 Jan;22(1):168–184. doi: 10.1016/s0022-5320(68)90055-5. [DOI] [PubMed] [Google Scholar]
- Vanha-Perttula T., Grimley P. M. Loss of proteins and other macromolecules during preparation of cell cultures for high resolution autoradiography. Quantitation by a micromethod. J Histochem Cytochem. 1970 Aug;18(8):565–573. doi: 10.1177/18.8.565. [DOI] [PubMed] [Google Scholar]
- di Mauro E., Synder L., Marino P., Lamberti A., Coppo A., Tocchini-Valentini G. P. Rifampicin sensitivity of the components of DNA-dependent RNA polymerase. Nature. 1969 May 10;222(5193):533–537. doi: 10.1038/222533a0. [DOI] [PubMed] [Google Scholar]