Abstract
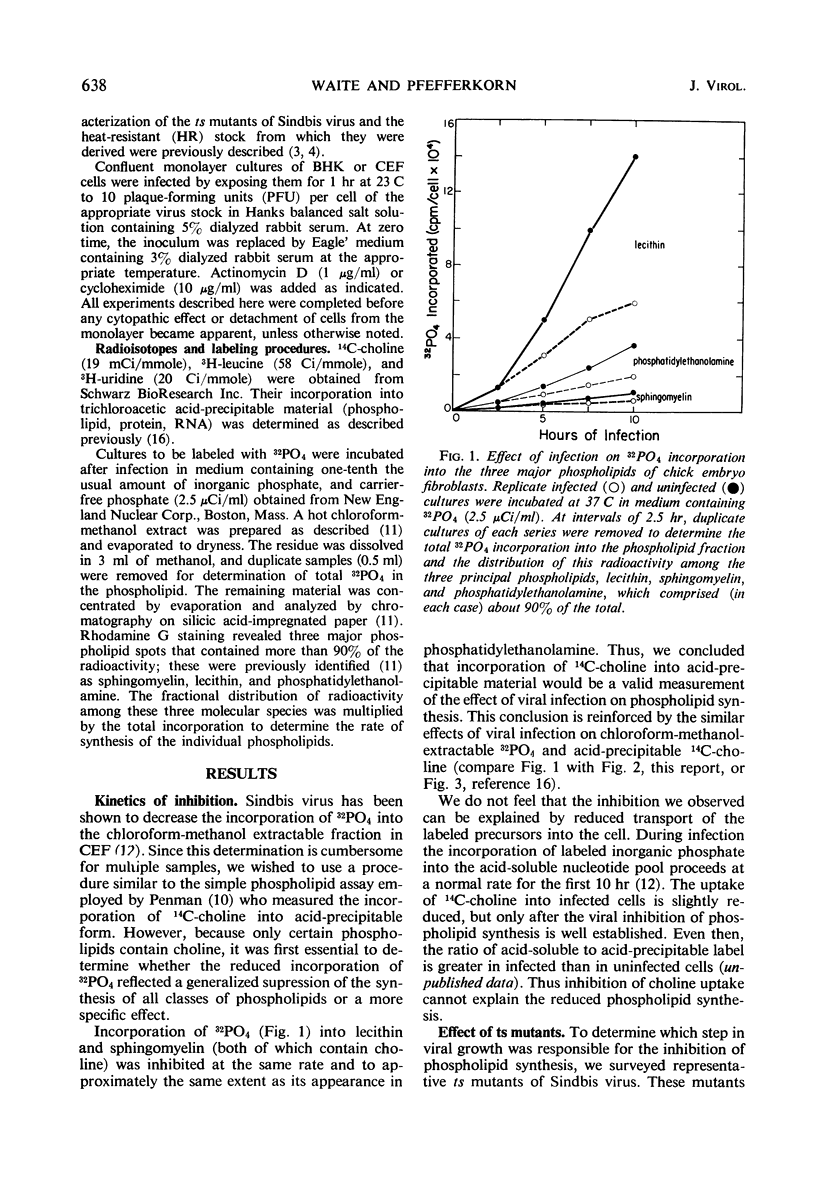
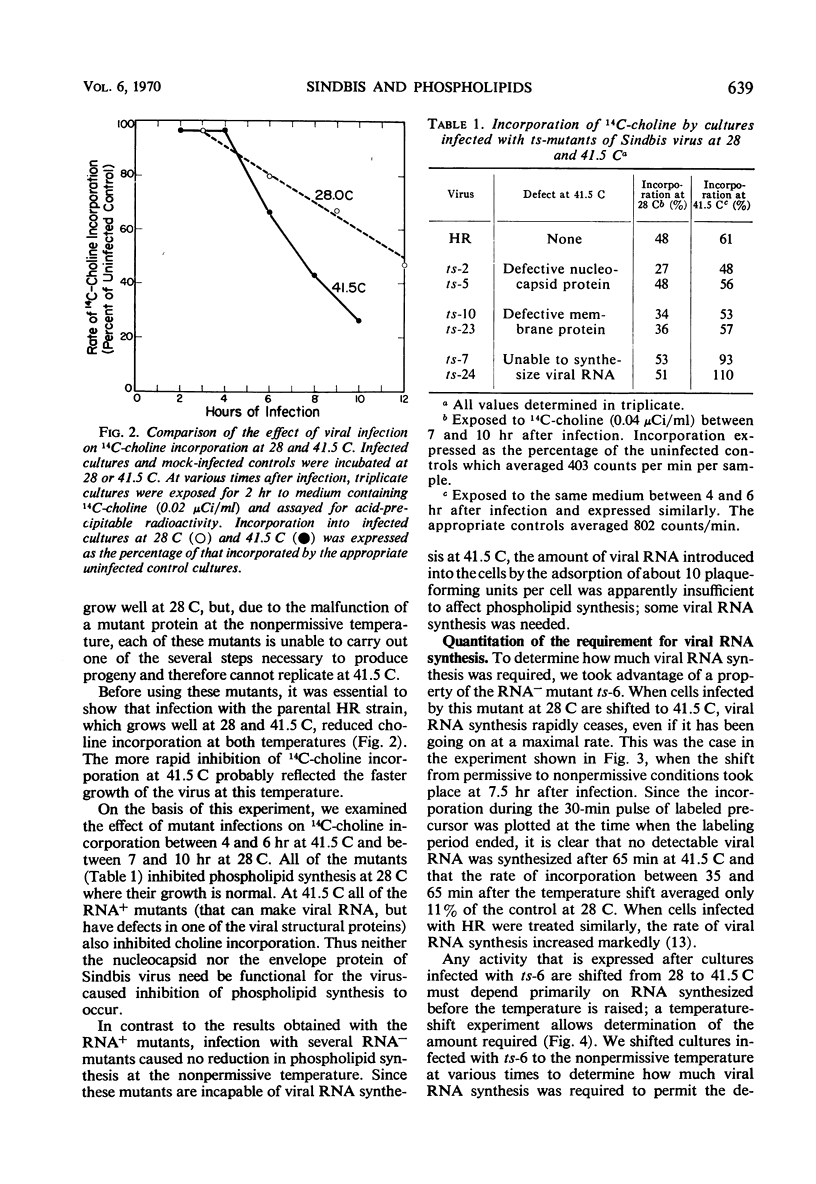
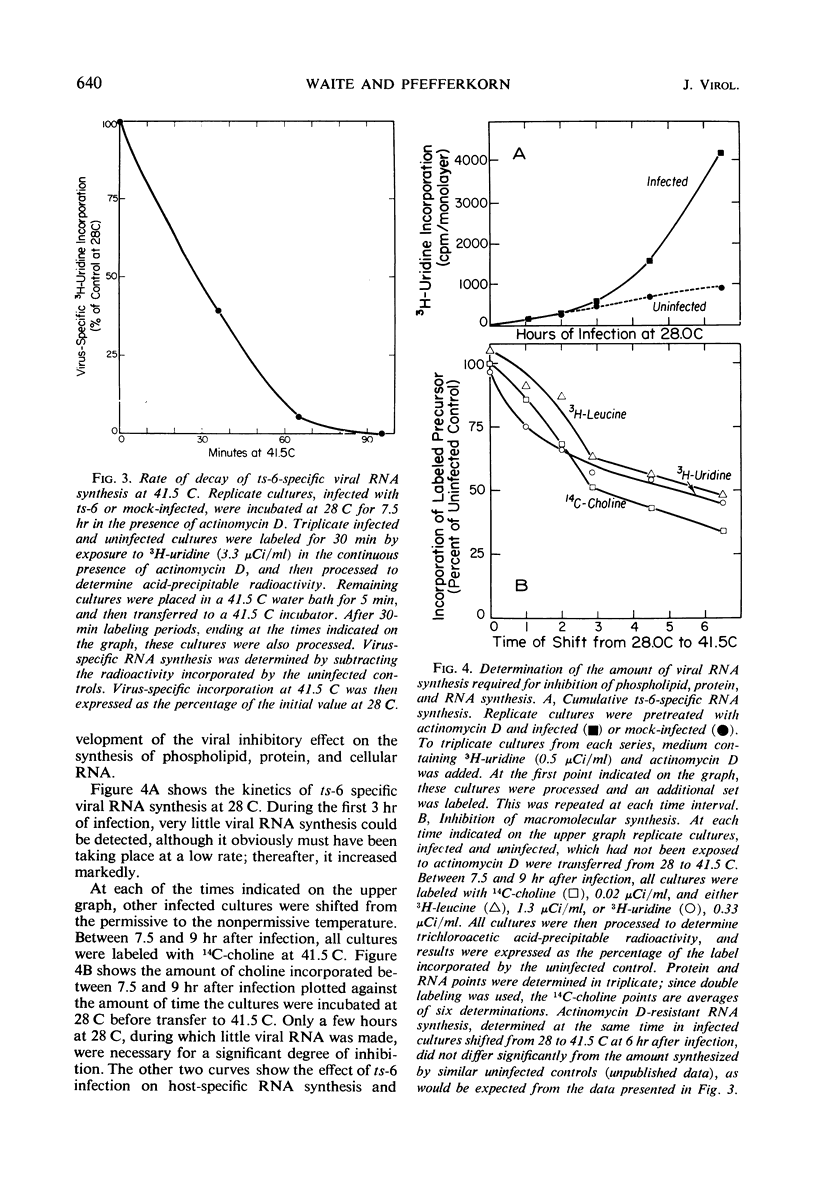
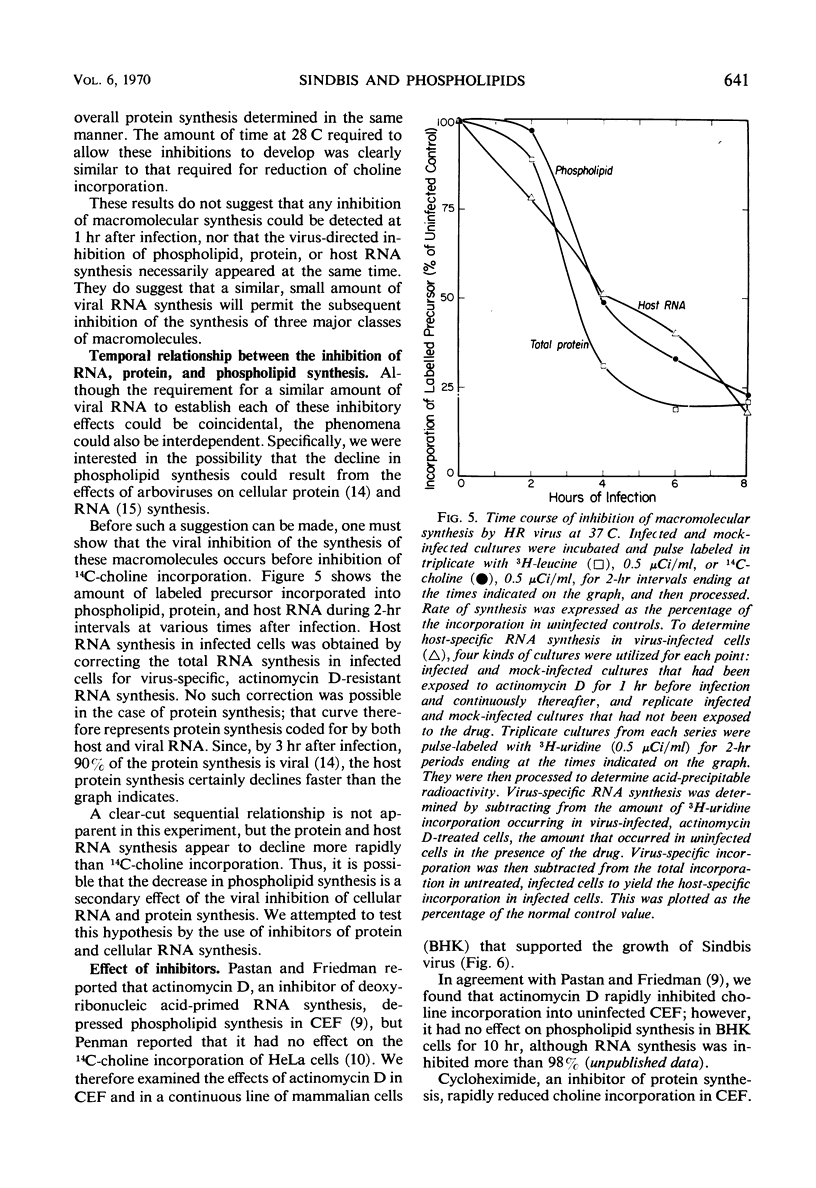
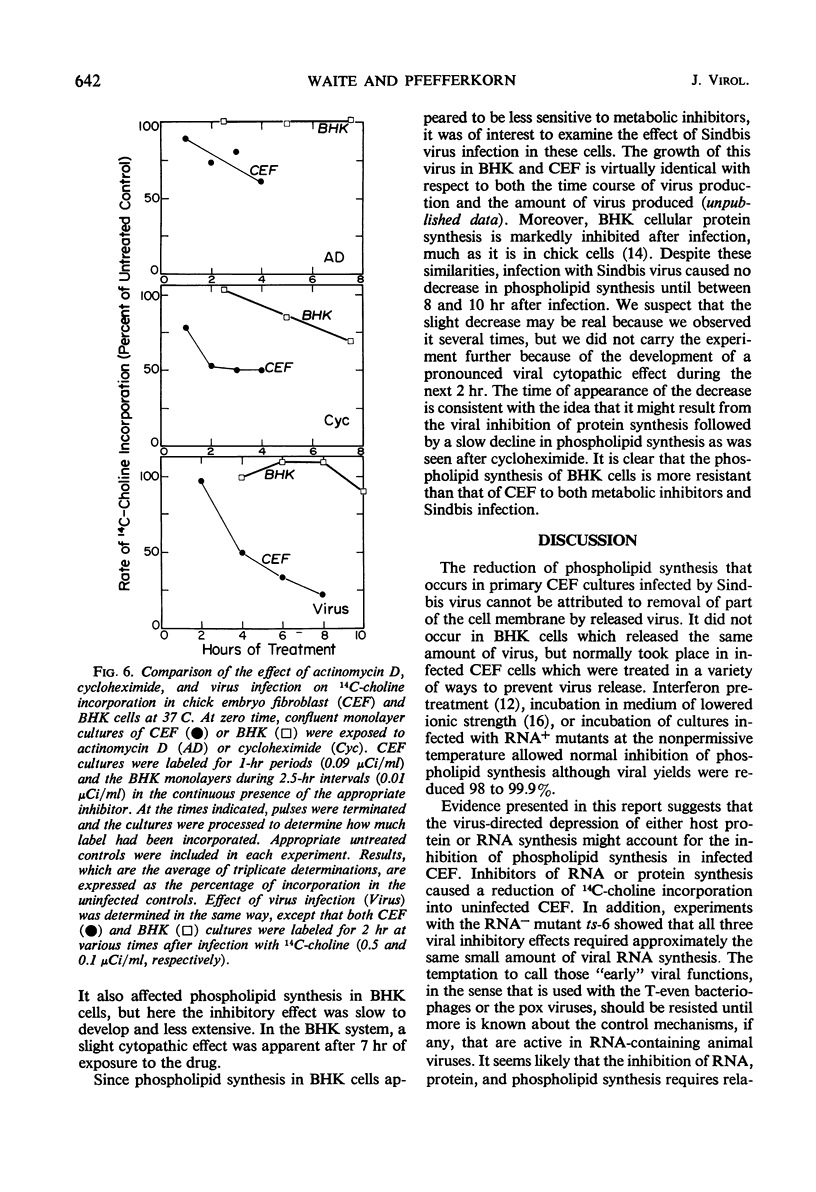
We investigated the metabolic requirements for the decrease in phospholipid synthesis previously observed by Pfefferkorn and Hunter in primary cultures of chick embryo fibroblasts infected with Sindbis virus. The incorporation of 32PO4 into all classes of phospholipids was found to decline at the same rate and to the same extent; thus, incorporation of 14C-choline into acid-precipitable form provided a convenient measure of phospholipid synthesis that was used in subsequent experiments. Experiments with temperature-sensitive mutants suggested that some viral ribonucleic acid (RNA) synthesis was essential for the inhibition of choline incorporation, but that functional viral structural proteins were not required. The reduction in phospholipid synthesis was probably a secondary effect of infection resulting from viral inhibition of the cellular RNA and protein synthesis. All three inhibitory effects required about the same amount of viral RNA synthesis; the inhibition of host RNA and protein synthesis began sooner than the decline in phospholipid synthesis; and both actinomycin D and cycloheximide inhibited 14C-choline incorporation in uninfected cells. In contrast, incorporation of 14C-choline into BHK-21 cells was not decreased by 10 hr of exposure to actinomycin D and declined only slowly after cycloheximide treatment. Growth of Sindbis virus in BHK cells did not cause the marked stimulation of phospholipid synthesis seen in picornavirus infections of other mammalian cells; however, inhibition was seen only late in infection.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acheson N. H., Tamm I. Replication of Semliki Forest virus: an electron microscopic study. Virology. 1967 May;32(1):128–143. doi: 10.1016/0042-6822(67)90261-9. [DOI] [PubMed] [Google Scholar]
- Amako K., Dales S. Cytopathology of Mengovirus infection. II. Proliferation of membranous cisternae. Virology. 1967 Jun;32(2):201–215. doi: 10.1016/0042-6822(67)90270-x. [DOI] [PubMed] [Google Scholar]
- Burge B. W., Pfefferkorn E. R. Functional defects of temperature-sensitive mutants of Sindbis virus. J Mol Biol. 1968 Jul 14;35(1):193–205. doi: 10.1016/s0022-2836(68)80047-6. [DOI] [PubMed] [Google Scholar]
- Burge B. W., Pfefferkorn E. R. Isolation and characterization of conditional-lethal mutants of Sindbis virus. Virology. 1966 Oct;30(2):204–213. doi: 10.1016/0042-6822(66)90096-1. [DOI] [PubMed] [Google Scholar]
- CORNATZER W. E., SANDSTROM W., FISCHER R. G. The effect of poliomyelitis virus type I (Mahoney strain) on the phospholipid metabolism of the HeLa cell. Biochim Biophys Acta. 1961 May 13;49:414–415. doi: 10.1016/0006-3002(61)90151-2. [DOI] [PubMed] [Google Scholar]
- FRANKLIN R. M., BALTIMORE D. Patterns of macromolecular synthesis in normal and virus-infected mammalian cells. Cold Spring Harb Symp Quant Biol. 1962;27:175–198. doi: 10.1101/sqb.1962.027.001.019. [DOI] [PubMed] [Google Scholar]
- Grimley P. M., Berezesky I. K., Friedman R. M. Cytoplasmic structures associated with an arbovirus infection: loci of viral ribonucleic acid synthesis. J Virol. 1968 Nov;2(11):1326–1338. doi: 10.1128/jvi.2.11.1326-1338.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORGAN C., HOWE C., ROSE H. M. Structure and development of viruses as observed in the electron microscope. V. Western equine encephalomyelitis virus. J Exp Med. 1961 Jan 1;113:219–234. doi: 10.1084/jem.113.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PENMAN S. STIMULATION OF THE INCORPORATION OF CHOLINE IN POLIOVIRUS-INFECTED CELLS. Virology. 1965 Jan;25:149–152. doi: 10.1016/0042-6822(65)90263-1. [DOI] [PubMed] [Google Scholar]
- PFEFFERKORN E. R., HUNTER H. S. PURIFICATION AND PARTIAL CHEMICAL ANALYSIS OF SINDBIS VIRUS. Virology. 1963 Jul;20:433–445. doi: 10.1016/0042-6822(63)90092-8. [DOI] [PubMed] [Google Scholar]
- PFEFFERKORN E. R., HUNTER H. S. THE SOURCE OF THE RIBONUCLEIC ACID AND PHOSPHOLIPID OF SINDBIS VIRUS. Virology. 1963 Jul;20:446–456. doi: 10.1016/0042-6822(63)90093-x. [DOI] [PubMed] [Google Scholar]
- Pastan I., Friedman R. M. Actinomycin D: inhibition of phospholipid synthesis in chick embryo cells. Science. 1968 Apr 19;160(3825):316–317. doi: 10.1126/science.160.3825.316. [DOI] [PubMed] [Google Scholar]
- Scheele C. M., Pfefferkorn E. R. Inhibition of interjacent ribonucleic acid (26S) synthesis in cells infected by Sindbis virus. J Virol. 1969 Aug;4(2):117–122. doi: 10.1128/jvi.4.2.117-122.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Burge B. W., Darnell J. E. Sindbis virus infection of chick and hamster cells: synthesis of virus-specific proteins. Virology. 1969 Mar;37(3):367–376. doi: 10.1016/0042-6822(69)90220-7. [DOI] [PubMed] [Google Scholar]
- TAYLOR J. STUDIES ON THE MECHANISM OF ACTION OF INTERFERON. I. INTERFERON ACTION AND RNA SYNTHESIS IN CHICK EMBRYO FIBROBLASTS INFECTED WITH SEMLIKI FOREST VIRUS. Virology. 1965 Mar;25:340–349. doi: 10.1016/0042-6822(65)90053-x. [DOI] [PubMed] [Google Scholar]
- Waite M. R., Pfefferkorn E. R. Inhibition of Sindbis virus production by media of low ionic strength: intracellular events and requirements for reversal. J Virol. 1970 Jan;5(1):60–71. doi: 10.1128/jvi.5.1.60-71.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]


