Abstract
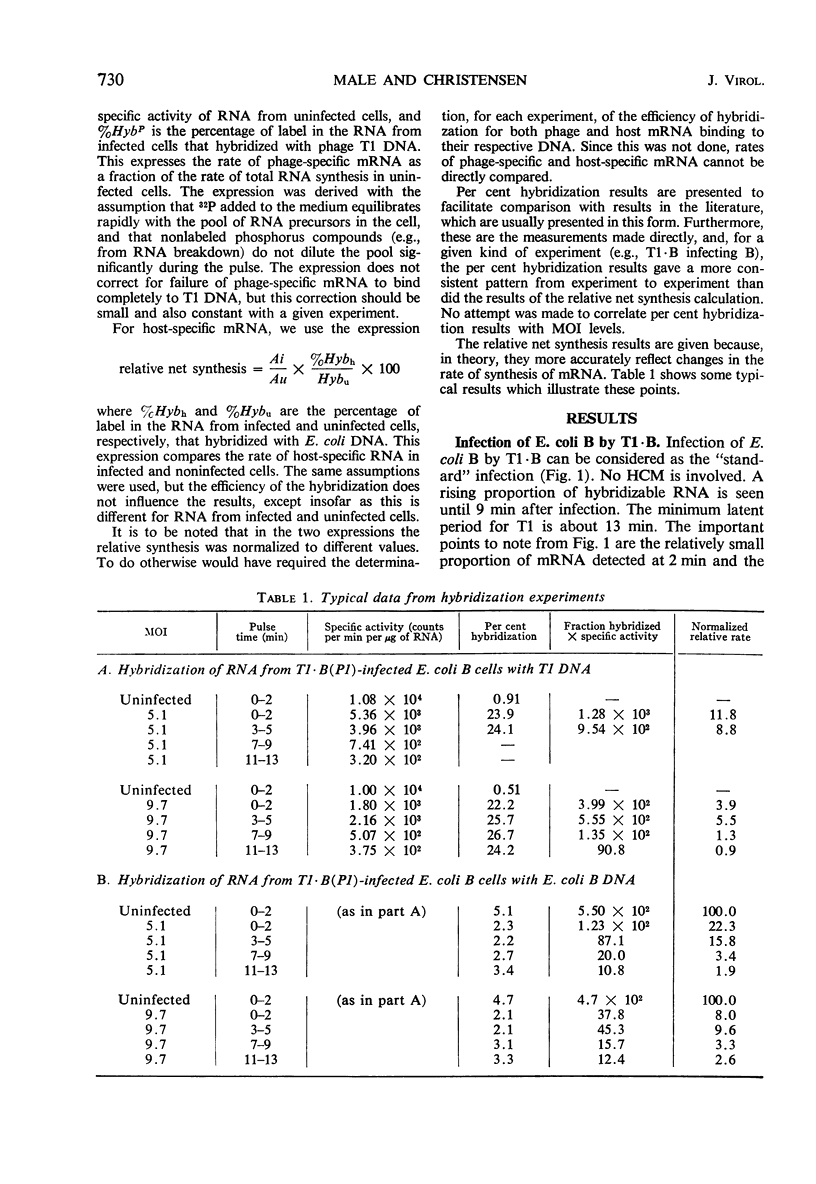
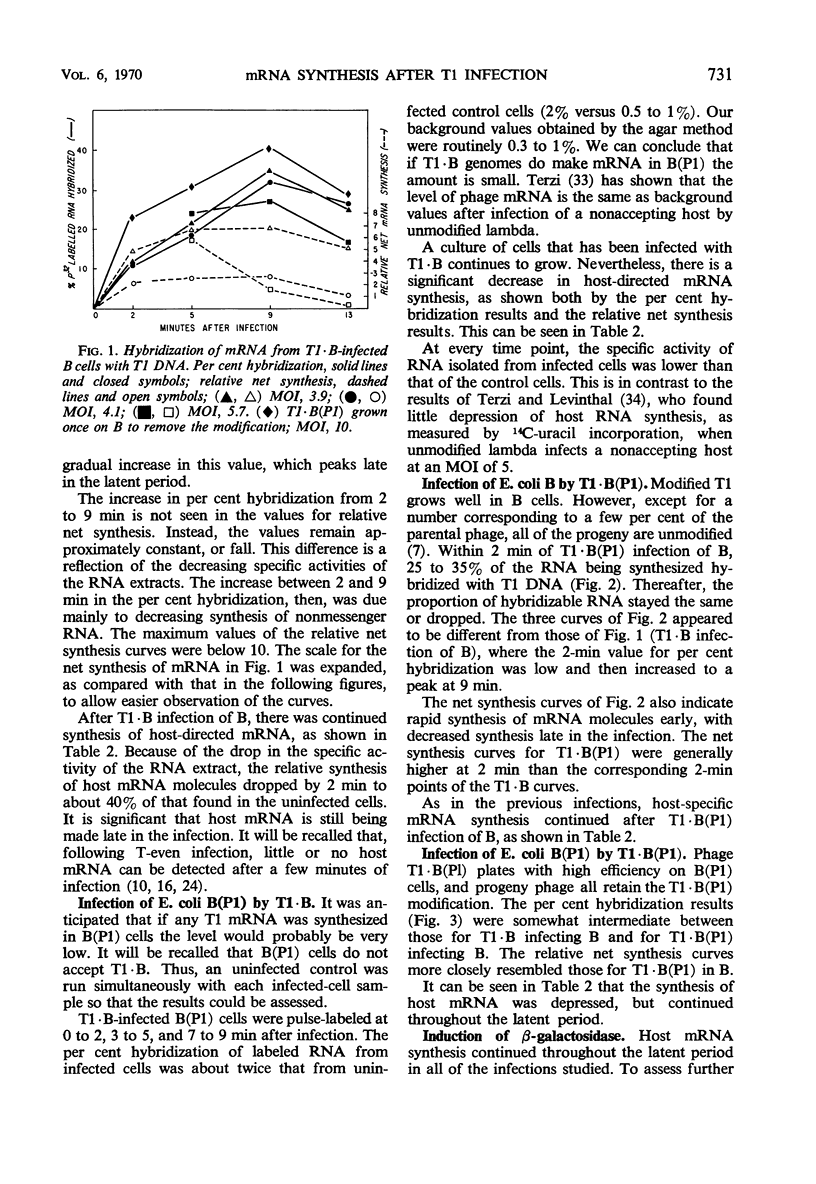
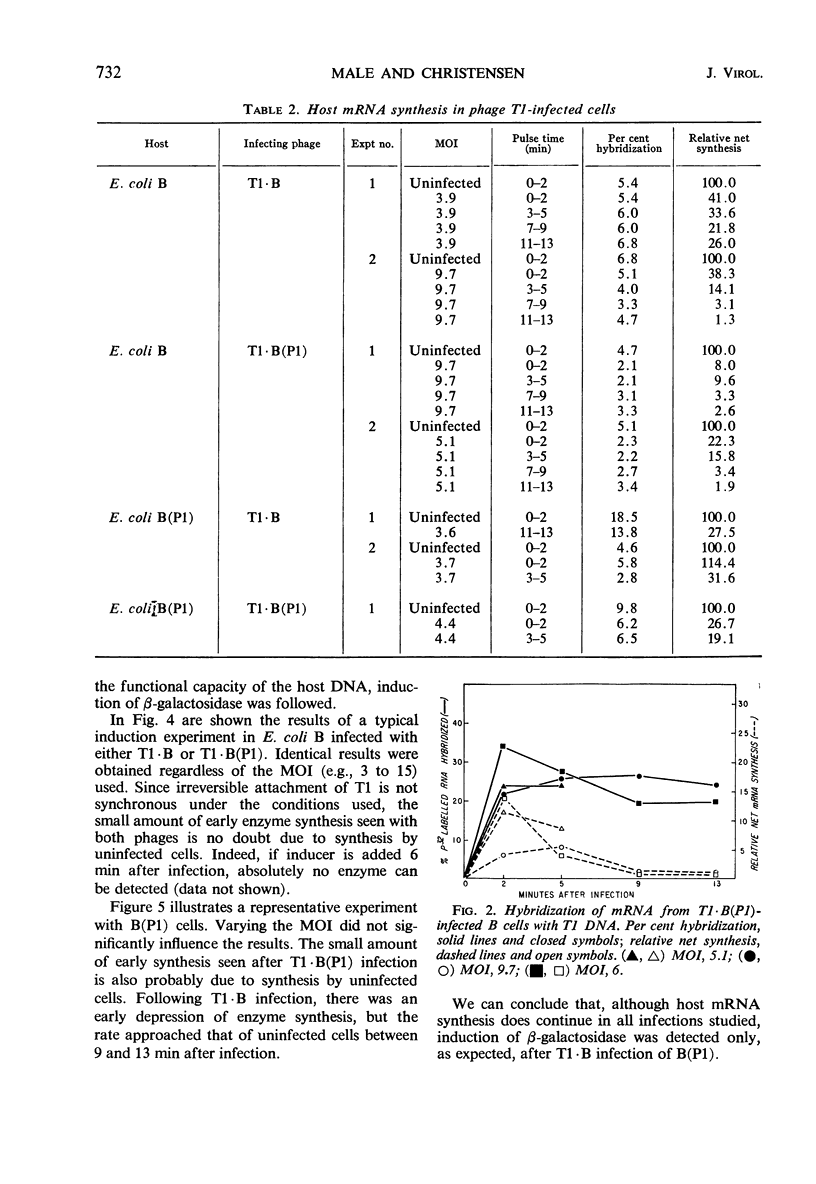
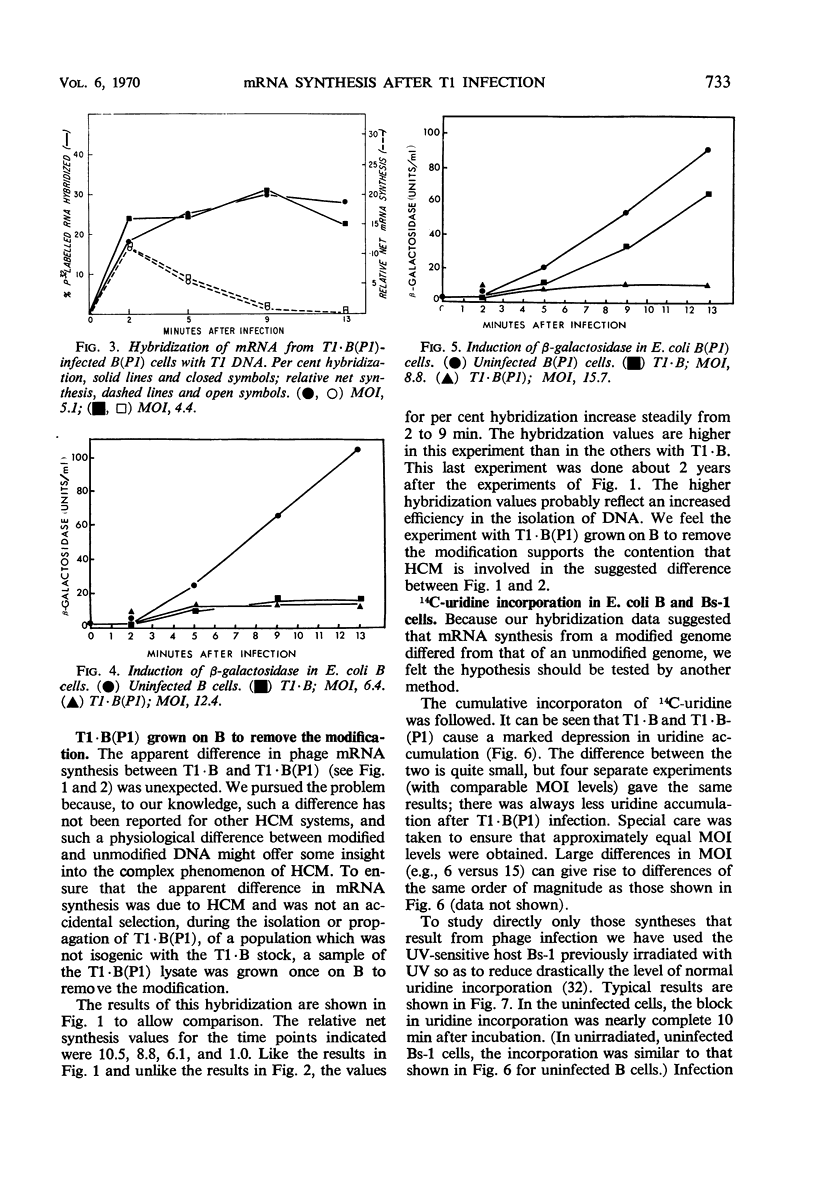
Synthesis of host-specific and phage-specific messenger ribonucleic acid (mRNA) was studied in bacteria infected by unmodified (T1 · B) or modified [T1 · B(P1)] bacteriophage T1. In a “standard” infection of Escherichia coli B by T1 · B (no host-controlled modification involved), the rate and amount of T1 mRNA synthesis was intermediate between those values reported for infections by a virulent phage such as T4 or a temperate phage such as lambda. The initial rate of mRNA synthesis was slightly increased after T1 · B(P1) infection of E. coli B in comparison with T1 · B infection of the same host. Little or no phage mRNA synthesis could be detected in T1 · B infection of E. coli B(P1). Phage mRNA synthesis in T1 · B(P1)-infected E. coli B(P1) cells was approximately the same in amount as that seen in T1 · B(P1) infection of E. coli B. Synthesis of host-specific mRNA continued throughout the latent period in all infections studied. However, the enzyme β-galactosidase could not be induced, except after T1 · B infection of E. coli B(P1). In an attempt to understand the apparent differences in mRNA synthesis after infection of E. coli B by phages T1 · B or T1 · B(P1), the effect of altered T1 deoxyribonucleic acid (DNA) methylation on mRNA synthesis was studied. Methyl-deficient T1 DNA, made in cells infected with ultraviolet-irradiated phage T3, inhibited 14C-uridine incorporation more strongly than normal T1. One passage of methyl-deficient T1 through E. coli B restored uracil incorporation rates to those seen with ordinary T1. This suggests that methylation of T1 DNA can influence the rate of phage mRNA synthesis. However, attempts to relate the difference in mRNA synthesis seen between T1 · B and T1 · B(P1) in E. coli B to the activity of the P1 modification gene were not conclusive.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arber W. Host-controlled modification of bacteriophage. Annu Rev Microbiol. 1965;19:365–378. doi: 10.1146/annurev.mi.19.100165.002053. [DOI] [PubMed] [Google Scholar]
- BOLTON E. T., McCARTHY B. J. A general method for the isolation of RNA complementary to DNA. Proc Natl Acad Sci U S A. 1962 Aug;48:1390–1397. doi: 10.1073/pnas.48.8.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresler S. E., Kiselev N. A., Manjakov V. F., Mosevitsky M. I., Timkovsky A. L. Isolation and physicochemical investigation of T1 bacteriophage DNA. Virology. 1967 Sep;33(1):1–9. doi: 10.1016/0042-6822(67)90087-6. [DOI] [PubMed] [Google Scholar]
- Brody E. N., Mackal R. P., Evans E. A., Jr Properties of infectious T1 deoxyribonucleic acid. J Virol. 1967 Feb;1(1):76–85. doi: 10.1128/jvi.1.1.76-85.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARO L. G. THE MOLECULAR WEIGHT OF LAMBDA DNA. Virology. 1965 Feb;25:226–236. doi: 10.1016/0042-6822(65)90201-1. [DOI] [PubMed] [Google Scholar]
- CHRISTENSEN J. R. FURTHER STUDIES OF HOST-CONTROLLED MODIFICATION OF BACTERIOPHAGES P2 AND T1. Virology. 1964 Nov;24:270–277. doi: 10.1016/0042-6822(64)90166-7. [DOI] [PubMed] [Google Scholar]
- DREXLER H., CHRISTENSEN J. R. Genetic crosses between restricted and unrestricted phage T1 in lysogenic and non-lysogenic hosts. Virology. 1961 Jan;13:31–39. doi: 10.1016/0042-6822(61)90028-9. [DOI] [PubMed] [Google Scholar]
- DUNN D. B., SMITH J. D. The occurrence of 6-methylaminopurine in deoxyribonucleic acids. Biochem J. 1958 Apr;68(4):627–636. doi: 10.1042/bj0680627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furth J. J., Pizer L. I. Deoxyribonucleic acid-dependent ribonucleic acid synthesis in Escherichia coli infected with bacteriophage T2. J Mol Biol. 1966 Jan;15(1):124–135. doi: 10.1016/s0022-2836(66)80214-0. [DOI] [PubMed] [Google Scholar]
- Gefter M., Hausmann R., Gold M., Hurwitz J. The enzymatic methylation of ribonucleic acid and deoxyribonucleic acid. X. Bacteriophage T3-induced S-adenosylmethionine cleavage. J Biol Chem. 1966 May 10;241(9):1995–2006. [PubMed] [Google Scholar]
- Grasso R. J., Paigen K. Loss of host-controlled restriction and modification of phage lambda in Escherichia coli K12 previously infected with UV-irradiated coli-phage dT3. Virology. 1969 May;38(1):191–194. doi: 10.1016/0042-6822(69)90145-7. [DOI] [PubMed] [Google Scholar]
- Hirsch-Kauffmann M., Sauerbier W. Inhibition of modification and restriction for phages lambda and T-1 by co-infecting T3. Mol Gen Genet. 1968;102(2):89–94. doi: 10.1007/BF01789134. [DOI] [PubMed] [Google Scholar]
- Howes W. V. Protein synthesis in Escherichia coli: a phage-mediated interruption of translation. Biochim Biophys Acta. 1965 Aug 10;103(4):711–713. doi: 10.1016/0005-2787(65)90095-x. [DOI] [PubMed] [Google Scholar]
- KLEIN A., SAUERBIER W. ROLE OF METHYLATION IN HOST CONTROLLED MODIFICATION OF PHAGE T1. Biochem Biophys Res Commun. 1965 Feb 3;18:440–445. doi: 10.1016/0006-291x(65)90728-x. [DOI] [PubMed] [Google Scholar]
- Kaiser A. D. On the internal structure of bacteriophage lambda. J Gen Physiol. 1966 Jul;49(6):171–178. doi: 10.1085/jgp.49.6.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennell D. Inhibition of host protein synthesis during infection of Escherichia coli by bacteriophage T4. I. Continued synthesis of host ribonucleic acid. J Virol. 1968 Nov;2(11):1262–1271. doi: 10.1128/jvi.2.11.1262-1271.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubinski H., Opara-Kubinska Z., Szybalski W. Patterns of interaction between polyribonucleotides and individual DNA strands derived from several vertebrates, bacteria and bacteriophages. J Mol Biol. 1966 Sep;20(2):313–329. doi: 10.1016/0022-2836(66)90067-2. [DOI] [PubMed] [Google Scholar]
- Kühnlein U., Linn S., Arber W. Host specificity of DNA produced by Escherichia coli. XI. In vitro modification of phage fd replicative form. Proc Natl Acad Sci U S A. 1969 Jun;63(2):556–562. doi: 10.1073/pnas.63.2.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEDERBERG S. Suppression of the multiplication of heterologous bacteriophages in lysogenic bacteria. Virology. 1957 Jun;3(3):496–513. doi: 10.1016/0042-6822(57)90006-5. [DOI] [PubMed] [Google Scholar]
- MCCARTHY B. J., BOLTON E. T. INTERACTION OF COMPLEMENTARY RNA AND DNA. J Mol Biol. 1964 Feb;8:184–200. doi: 10.1016/s0022-2836(64)80128-5. [DOI] [PubMed] [Google Scholar]
- MCCARTHY B. J., HOYER B. H. IDENTITY OF DNA AND DIVERSITY OF MESSENGER RNA MOLECULES IN NORMAL MOUSE TISSUES. Proc Natl Acad Sci U S A. 1964 Oct;52:915–922. doi: 10.1073/pnas.52.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M., Witten C., Mantei N., Echols H. Inhibition of host nucleic acid synthesis by bacteriophage T4: effect of chloramphenicol at various multiplicities of infection. J Mol Biol. 1966 May;17(1):273–278. doi: 10.1016/s0022-2836(66)80107-9. [DOI] [PubMed] [Google Scholar]
- REVEL H. R., LURIA S. E., ROTMAN B. Biosynthesis of B-D-galactosidase controlled by phage-carried genes. I. Induced beta-D-galactosidase biosynthesis after transduction of gene z-plus by phage. Proc Natl Acad Sci U S A. 1961 Dec 15;47:1956–1967. doi: 10.1073/pnas.47.12.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLY W. S., ECHOLS H., ADLER J. CONTROL OF VIRAL MESSENGER RNA AFTER LAMBDA PHAGE INFECTION AND INDUCTION. Proc Natl Acad Sci U S A. 1965 Feb;53:378–385. doi: 10.1073/pnas.53.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STENT G. S., FUERST C. R. Inactivation of bacteriophages by decay of incorporated radioactive phosphorus. J Gen Physiol. 1955 Mar 20;38(4):441–458. doi: 10.1085/jgp.38.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUEOKA N., CHENG T. Y. Fractionation of nucleic acids with the methylated albumin column. J Mol Biol. 1962 Mar;4:161–172. doi: 10.1016/s0022-2836(62)80048-5. [DOI] [PubMed] [Google Scholar]
- Skalka A., Butler B., Echols H. Genetic control of transcription during development of phage gamma. Proc Natl Acad Sci U S A. 1967 Aug;58(2):576–583. doi: 10.1073/pnas.58.2.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson P. A., Setlow R. B. Effects of ultraviolet radiation on macromolecular synthesis in Escherichia coli. J Mol Biol. 1966 Jan;15(1):201–219. doi: 10.1016/s0022-2836(66)80221-8. [DOI] [PubMed] [Google Scholar]
- Terzi M., Levinthal C. Effects of lambda-phage infection on bacterial synthesis. J Mol Biol. 1967 Jun 28;26(3):525–535. doi: 10.1016/0022-2836(67)90320-8. [DOI] [PubMed] [Google Scholar]
- Terzi M. Physiology of bacteriophage lambda in the restrictive host. J Mol Biol. 1968 May 28;34(1):165–173. doi: 10.1016/0022-2836(68)90242-8. [DOI] [PubMed] [Google Scholar]
- Thomas C. A., Jr The arrangement of information in DNA molecules. J Gen Physiol. 1966 Jul;49(6):143–169. doi: 10.1085/jgp.49.6.143. [DOI] [PMC free article] [PubMed] [Google Scholar]


