Abstract
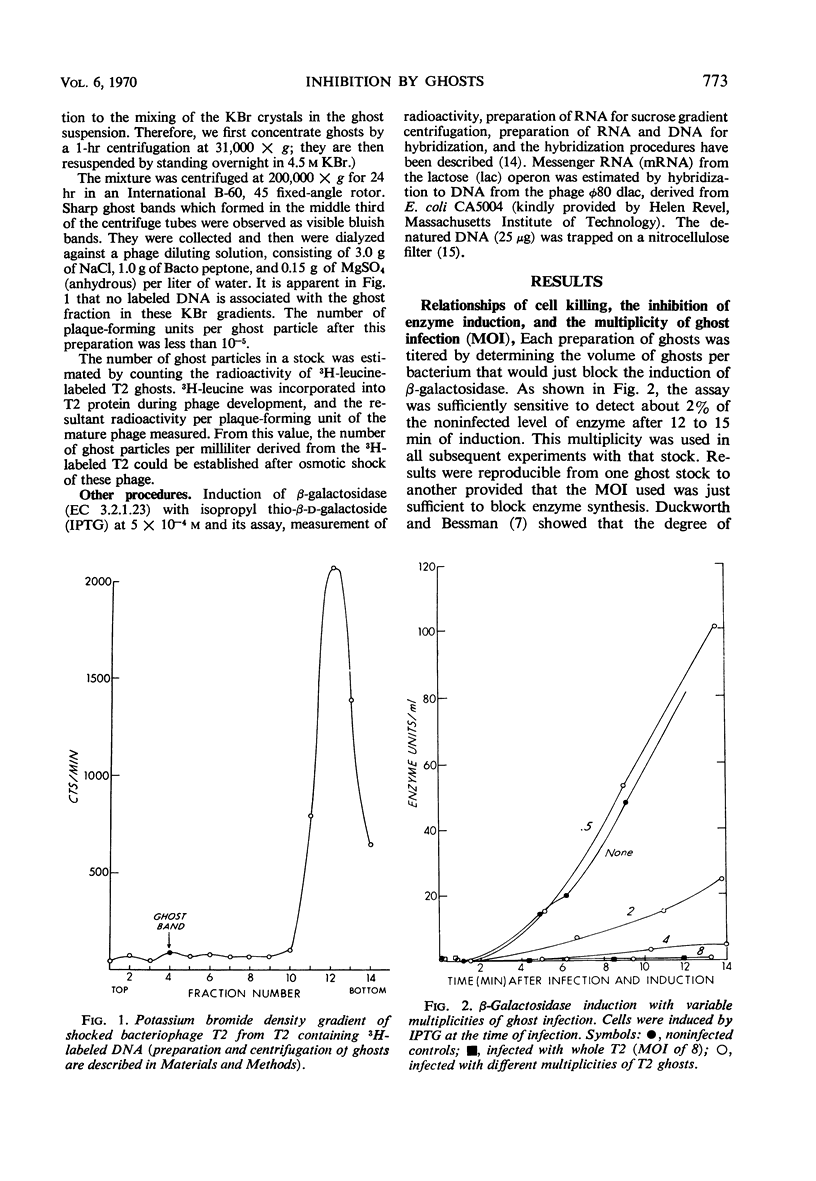
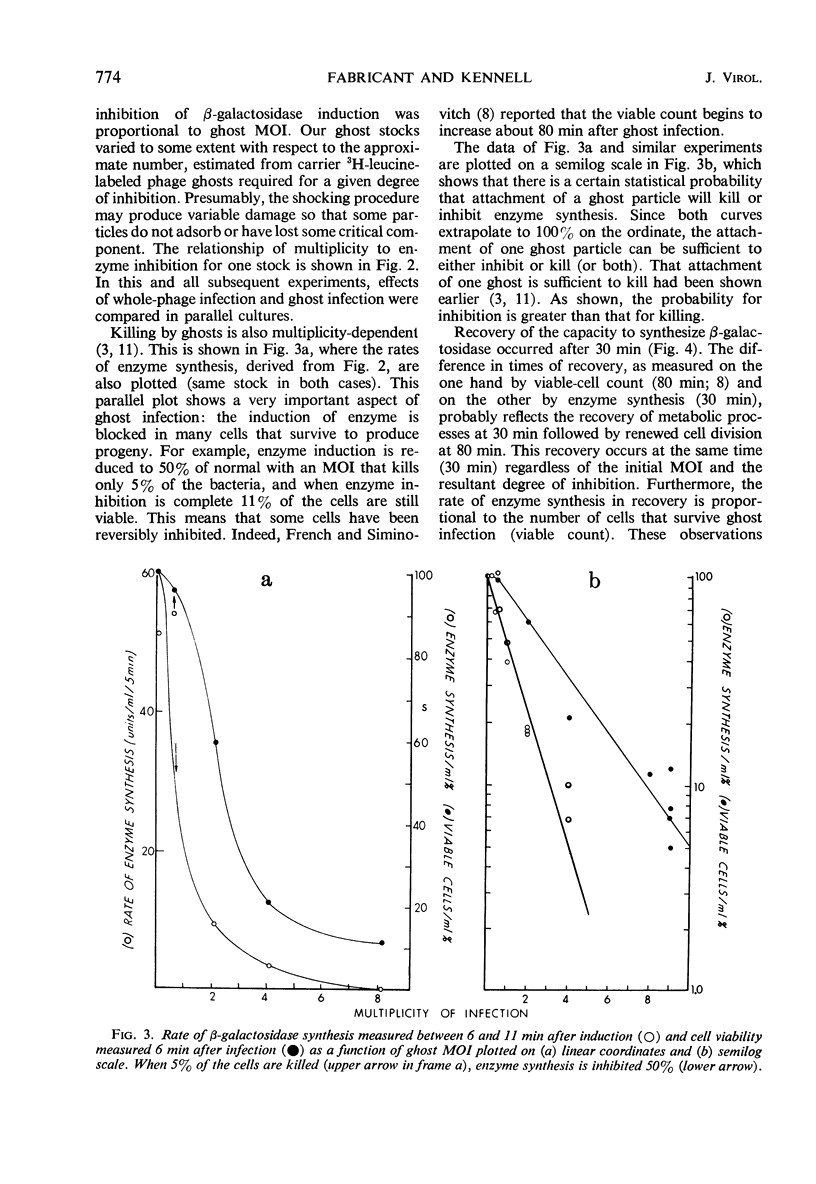
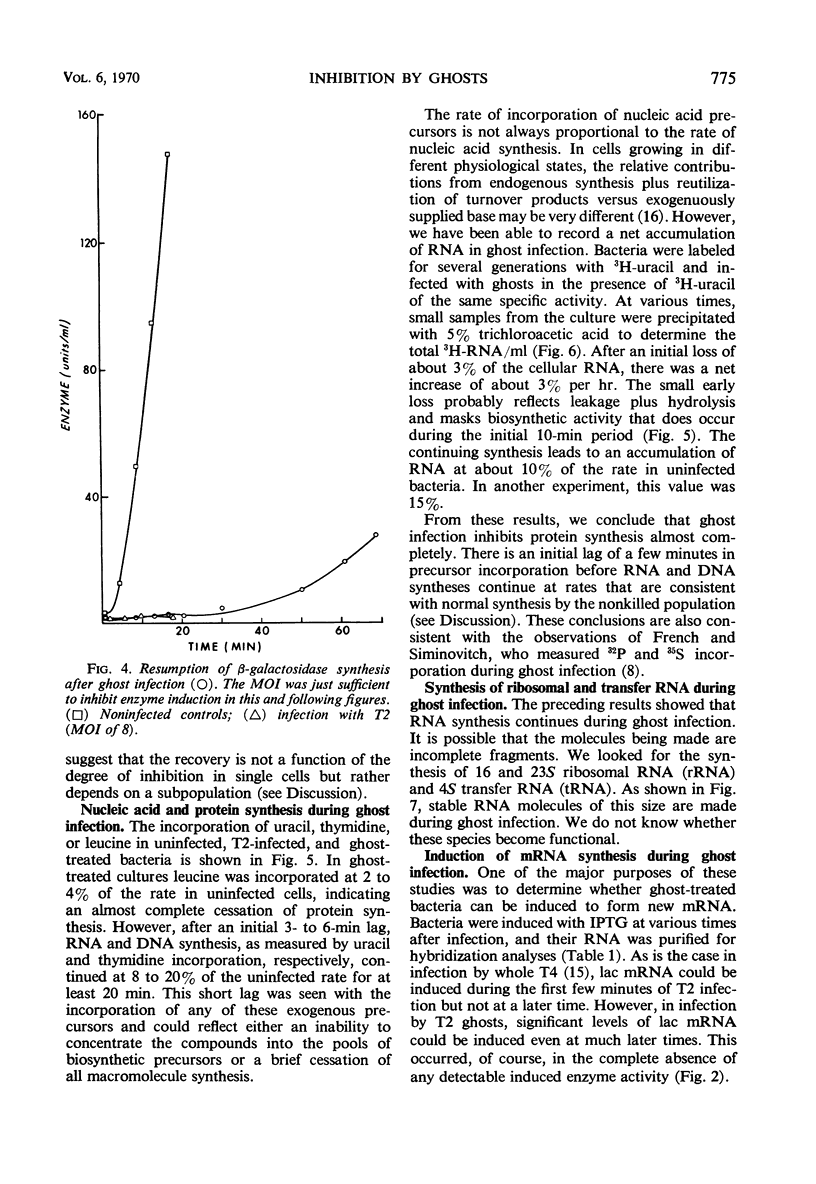
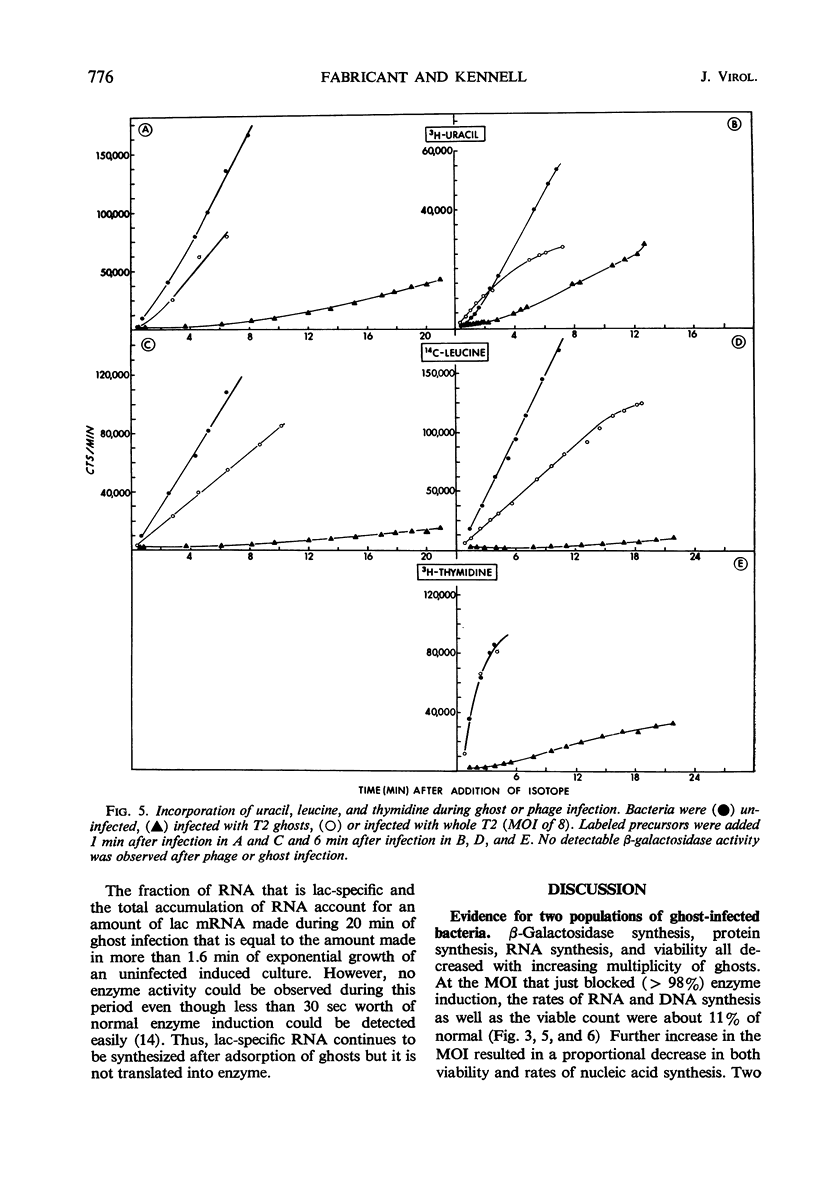
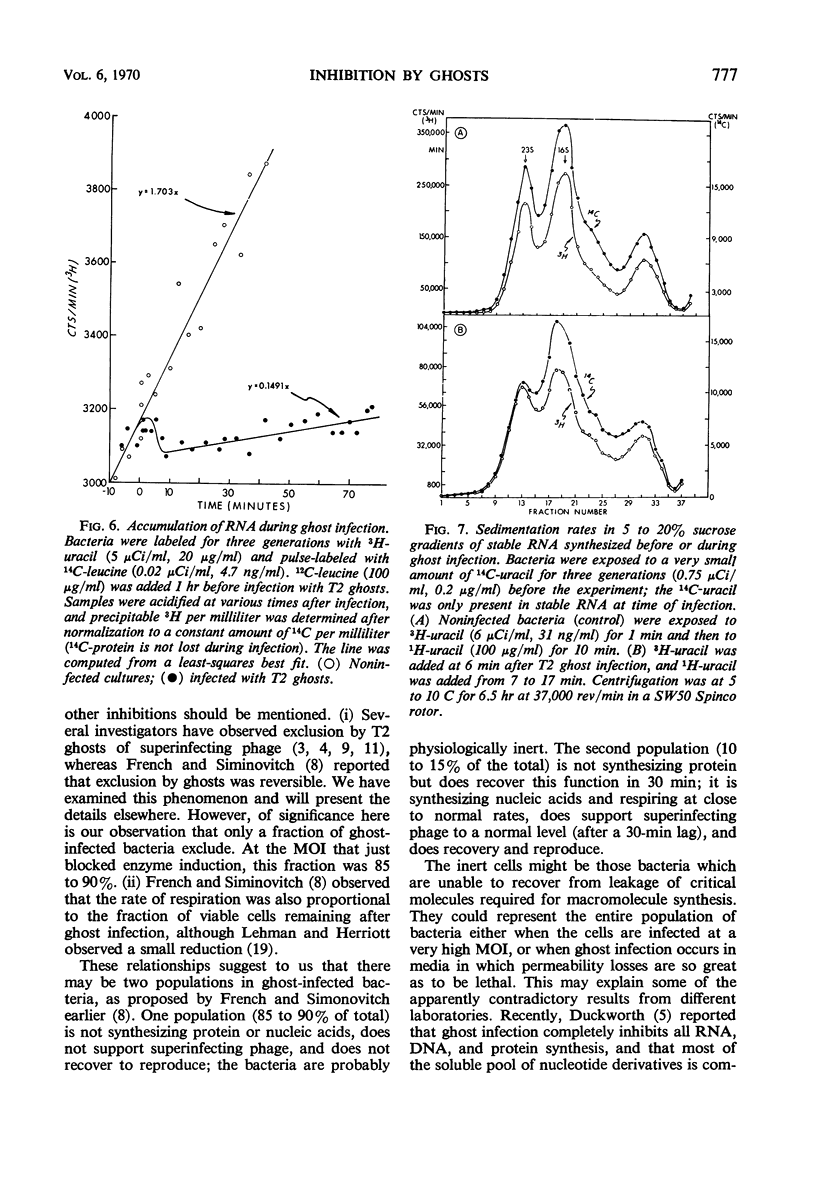
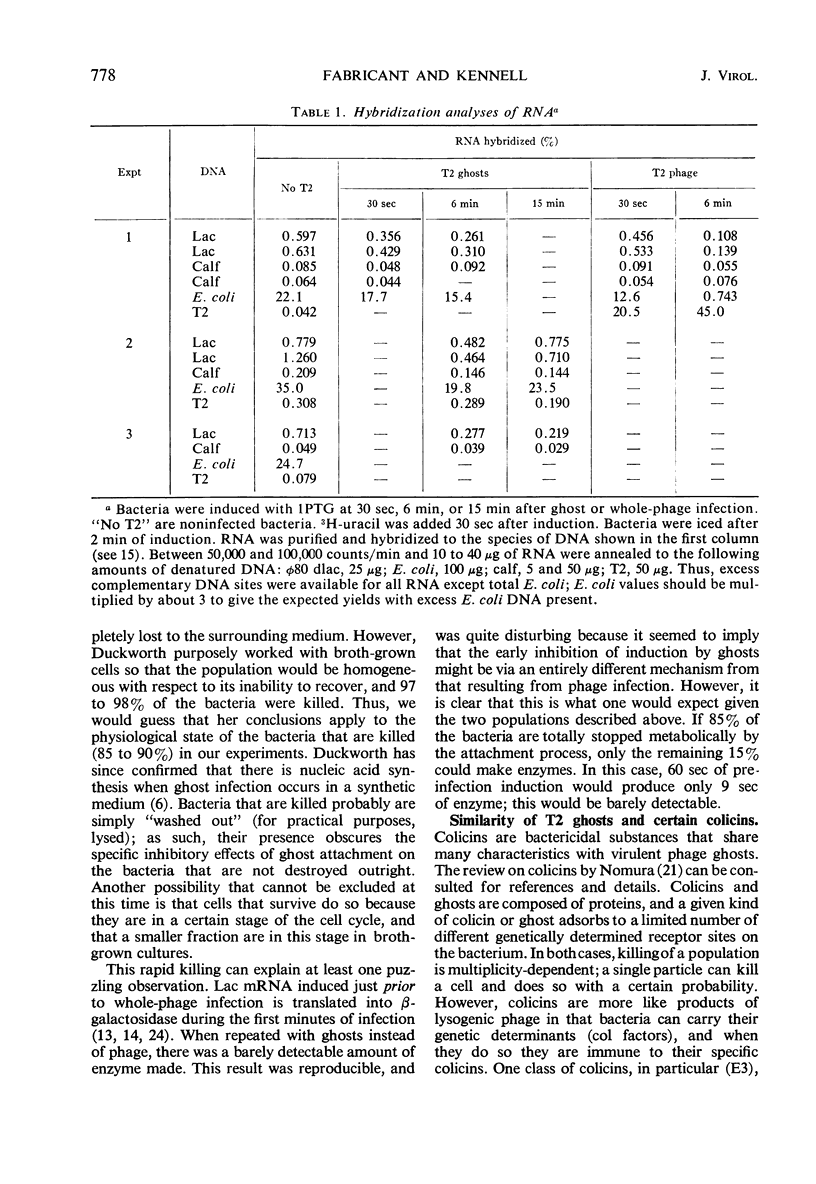
Deoxyribonucleic acid (DNA)-less T2 “ghosts” were prepared by osmotic shock and purified by KBr density gradient centrifugation. Escherichia coli B was treated with these ghosts in inorganic salts-glycerol medium to see which features of phage infection could be elicited by ghosts. At a multiplicity that was just sufficient to block induction of β-galactosidase (EC 3.2.1.23), 89% of the bacteria were killed and the rates of ribonucleic acid (RNA) and DNA synthesis were about 10 to 15% of normal. However, protein synthesis was almost completely blocked but resumed after 30 min. During this period, it was possible to induce messenger RNA (mRNA) from the lactose operon, although this mRNA could not be translated into active β-galactosidase. These results suggest to us that the viable cells surviving ghost infection synthesize nucleic acids at close to a normal rate but are temporarily blocked in protein synthesis. The continued formation of untranslated host mRNA mimics the pattern of bacterial synthesis just after whole-phage infection, and is consistent with the interpretation that the immediate block in the initiation of host translation by these viruses is due to their attachment.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENZER S. Induced synthesis of enzymes in bacteria analyzed at the cellular level. Biochim Biophys Acta. 1953 Jul;11(3):383–395. doi: 10.1016/0006-3002(53)90057-2. [DOI] [PubMed] [Google Scholar]
- BONIFAS V., KELLENBERGER E. Etude de l'action des membranes du bactériophage T2 sur Escherichia coli. Biochim Biophys Acta. 1955 Mar;16(3):330–338. doi: 10.1016/0006-3002(55)90234-1. [DOI] [PubMed] [Google Scholar]
- Duckworth D. H., Bessman M. J. Assay for the Killing Properties of T2 Bacteriophage and Their "Ghosts". J Bacteriol. 1965 Sep;90(3):724–728. doi: 10.1128/jb.90.3.724-728.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth D. H. Biological activity of bacteriophage ghosts and "take-over" of host functions by bacteriophage. Bacteriol Rev. 1970 Sep;34(3):344–363. doi: 10.1128/br.34.3.344-363.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth D. H. The metabolism of T4 phage ghost-infected cells. I. Macromolecular synthesis and ransport of nucleic acid and protein precursors. Virology. 1970 Mar;40(3):673–684. doi: 10.1016/0042-6822(70)90212-6. [DOI] [PubMed] [Google Scholar]
- FRENCH R. C., SIMINOVITCH L. The action of T2 bacteriophage ghosts on Escherichia coli B. Can J Microbiol. 1955 Dec;1(9):757–774. doi: 10.1139/m55-090. [DOI] [PubMed] [Google Scholar]
- GRAHAM A. F. The fate of the infecting phage particle. Ann Inst Pasteur (Paris) 1953 Jan;84(1):90–98. [PubMed] [Google Scholar]
- HERRIOTT R. M., BARLOW J. L. The protein coats or ghosts of coli phage T2. II. The biological functions. J Gen Physiol. 1957 Nov 20;41(2):307–331. doi: 10.1085/jgp.41.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERRIOTT R. M. Nucleic-acid-free T2 virus "ghosts" with specific biological action. J Bacteriol. 1951 Jun;61(6):752–754. doi: 10.1128/jb.61.6.752-754.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu W. T., Weiss S. B. Selective translation of T4 template RNA by ribosomes from T4-infected Escherichia coli. Proc Natl Acad Sci U S A. 1969 Sep;64(1):345–351. doi: 10.1073/pnas.64.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaempfer R. O., Magasanik B. Mechanism of beta-galactosidase induction in Escherichia coli. J Mol Biol. 1967 Aug 14;27(3):475–494. doi: 10.1016/0022-2836(67)90053-8. [DOI] [PubMed] [Google Scholar]
- Kennell D. Inhibition of host protein synthesis during infection of Escherichia coli by bacteriophage T4. I. Continued synthesis of host ribonucleic acid. J Virol. 1968 Nov;2(11):1262–1271. doi: 10.1128/jvi.2.11.1262-1271.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennell D. Inhibition of host protein synthesis during infection of Escherichia coli by bacteriophage T4. II. Induction of host messenger ribonucleic acid and its exclusion from polysomes. J Virol. 1970 Aug;6(2):208–217. doi: 10.1128/jvi.6.2.208-217.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennell D., Kotoulas A. Magnesium starvation of Aerobacter aerogenes. II. Rates of nucleic acid synthesis and methods for their measurement. J Bacteriol. 1967 Jan;93(1):345–356. doi: 10.1128/jb.93.1.345-356.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konisky J., Nomura M. Interaction of colicins with bacterial cells. II. Specific alteration of Escherichia coli ribosomes induced by colicin E3 in vivo. J Mol Biol. 1967 Jun 14;26(2):181–195. doi: 10.1016/0022-2836(67)90290-2. [DOI] [PubMed] [Google Scholar]
- LEHMAN I. R., HERRIOTT R. M. The protein coats or ghosts or coliphage T2. III. Metabolic studies of Escherichia coli B infected with T2 bacteriophage ghosts. J Gen Physiol. 1958 May 20;41(5):1067–1082. doi: 10.1085/jgp.41.5.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVIN A. P., BURTON K. Inhibition of enzyme formation following infection of Escherichia coli with phage T2r. J Gen Microbiol. 1961 Jun;25:307–314. doi: 10.1099/00221287-25-2-307. [DOI] [PubMed] [Google Scholar]
- Landy A., Spiegelman S. Exhaustive hybridization and its application to an analysis of the ribonucleic acid synthesized in T4-infected cells. Biochemistry. 1968 Feb;7(2):585–591. doi: 10.1021/bi00842a011. [DOI] [PubMed] [Google Scholar]
- Nomura M. Colicins and related bacteriocins. Annu Rev Microbiol. 1967;21:257–284. doi: 10.1146/annurev.mi.21.100167.001353. [DOI] [PubMed] [Google Scholar]
- Nomura M., Witten C., Mantei N., Echols H. Inhibition of host nucleic acid synthesis by bacteriophage T4: effect of chloramphenicol at various multiplicities of infection. J Mol Biol. 1966 May;17(1):273–278. doi: 10.1016/s0022-2836(66)80107-9. [DOI] [PubMed] [Google Scholar]
- Rouvière J., Wyngaarden J., Cantoni J., Gros F., Kepes A. Effect of T4 infection on messenger RNA synthesis in Escherichia coli. Biochim Biophys Acta. 1968 Aug 23;166(1):94–114. doi: 10.1016/0005-2787(68)90494-2. [DOI] [PubMed] [Google Scholar]
- SHER I. H., MALLETTE M. F. The adaptive nature of the formation of lysine decarboxylase in Escherichia coli B. Arch Biochem Biophys. 1954 Oct;52(2):331–339. doi: 10.1016/0003-9861(54)90131-9. [DOI] [PubMed] [Google Scholar]
- SKOELD O., BUCHANAN J. M. INHIBITION OF DEOXYRIBONUCLEIC ACID-DIRECTED RIBONUCLEIC ACID POLYMERASE IN ESCHERICHIA COLI AFTER INFECTION WITH BACTERIOPHAGE T4. Proc Natl Acad Sci U S A. 1964 Apr;51:553–560. doi: 10.1073/pnas.51.4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp K., Green M. H. Ribonucleic acid synthesis in T2-infected Escherichia coli during "stringent" control. J Bacteriol. 1968 Jul;96(1):111–116. doi: 10.1128/jb.96.1.111-116.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver S., Levine E., Spielman P. M. Cation fluxes and permeability changes accompanying bacteriophage infection of Escherichia coli. J Virol. 1968 Aug;2(8):763–771. doi: 10.1128/jvi.2.8.763-771.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S., Weinberg F., So A. G., Davie E. W. RNA polymerase and the shut-off of host RNA and protein synthesis in T4 phage infection. Nature. 1970 Jan 3;225(5227):62–63. doi: 10.1038/225062a0. [DOI] [PubMed] [Google Scholar]
- Travers A. A. Bacteriophage sigma factor for RNA polymerase. Nature. 1969 Sep 13;223(5211):1107–1110. doi: 10.1038/2231107a0. [DOI] [PubMed] [Google Scholar]
- Warren R. J., Bose S. K. Bacteriophage-induced inhibition of host functions. I. Degradation of Escherichia coli deoxyribonucleic acid after T4 infection. J Virol. 1968 Apr;2(4):327–334. doi: 10.1128/jvi.2.4.327-334.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiberg J. S. Mutants of bacteriophage T4 unable to cause breakdown of host DNA. Proc Natl Acad Sci U S A. 1966 Mar;55(3):614–621. doi: 10.1073/pnas.55.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]


