Abstract
Background
Hepatosteatosis is associated with increased expression of tumor necrosis factor alpha (TNFα) and interleukin (IL)-12, major T helper (Th) 1 cytokines, and reduced hepatic NKT cell numbers. The relationship between lipid accumulation, cytokine expression, and hepatic NKT cells is not known. This study was conducted to assess the role of IL-12 in the development of hepatic steatosis and its potential impact on liver NKT cells.
Methods
Male C57Bl/6 wild type (Wt) and IL-12-deficient (IL12−/−) mice were fed a choline deficient diet (CDD) for 0, 10 or 20 weeks.
Findings
CDD led to marked hepatosteatosis, reduced hepatic but not splenic NKT cell numbers and function and increased hepatic expression of the Th1-type cytokines IL-12, interferon gamma (IFNγ) and TNFα in wt mice. Absence of IL-12 resulted in similar CDD-induced hepatosteatosis, but preserved hepatic NKT cells and significantly reduced hepatic IFNγ and TNFα expression. Treatment of CDD fed mice with lipopolysaccharide led to a significant increase in hepatic IL12 expression and Kupffer cell (KC)_depletion reduced liver IL-12 expression and restored NKT cells in CDD-induced fatty liver. Interestingly, KCs from CDD fed mice failed to produce increased quantities of IL12 upon activation in vitro when compared to similarly treated KCs from control fed mice suggesting that secondary factors in vivo promote heightened IL-12 production. Finally, human livers with severe steatosis showed a substantial decrease in NKT and NK cells.
Conclusions
Hepatosteatosis reduces the numbers of hepatic NKT cells in a KC and IL-12-dependent manner. Our results suggest a pivotal and multi-functional role of KC-derived IL-12 in the altered immune response in steatotic liver, a process which is likely active within human non-alcoholic fatty liver disease.
Keywords: liver, cytokines, innate immunity, lipid, T helper
Introduction
Over the last decade, the role of the liver as a major organ of the innate immune system with immunomodulatory functions has been increasingly recognized. The liver contains one of the largest populations of resident macrophages (Kupffer cells [KCs]), natural killer (NK) cells and natural killer T (NKT) cells 1. They all are important mediators in the innate immune system, though NKT cells with phenotypic markers of both NK cells and T cells could represent a link between innate and adaptive immunity 2. NKT cells have attracted a great deal of attention during the last years, given their role in a variety of immunological responses including cancer, microbial infection and autoimmunity 3-5. Most NKT cells recognize lipid antigens presented by the atypical major histocompatibility complex (MHC) class I-like molecule CD1d, expressed primarily on antigen-presenting cells including monocytes, macrophages, dendritic cells and B-cells 2, 6. Importantly, the functional properties attributed to NKT cells appear to be largely due to CD1d-restricted T cells 7. In addition, NKT cells are either CD4+ or CD4−CD8−, in contrast to typical CD8+ Class I restricted T cells. Most notably, NKT cells express an extremely limited T cell receptor repertoire, as their TCR is composed almost exclusively of Vα14/Jα281 paired with Vα8, Vα7, or Vα2 8, 9, that bind lipids, glycolipids, or highly hydrophobic peptides presented by CD1d molecules 6, 10. CD1d-restricted T cells demonstrate potent production of both T-helper (Th)-1 associated cytokines like Interleukin (IL)-12 and Interferon (IFN) γ, and Th2 associated cytokines like IL-4 and IL-10 11 and, therefore, are suitable to affect/control the local environment in an either pro-inflammatory or anti-inflammatory manner.
Previously, we reported that hepatosteatosis induced by feeding choline deficient diet (CDD) for 6 weeks_was associated with a substantial reduction in resident NKT cell numbers, concomitant with increased Th1 cytokine production (i.e. tumor necrosis factor (TNF)-α, IL-12 and IFNγ) but unchanged levels of Th2 cytokines (i.e. IL-4 and IL-10) 12. Similar observations have been made in different models of obesity. Leptin-deficient ob/ob mice 13, insulin-resistant fa/fa Zucker rats 14 and diet-induced obesity 12, 15 in rodents have shown that hepatosteatosis is associated with changes in local cytokine patterns, resembling a state of chronic hepatic inflammation with changes in hepatic lymphocyte subpopulations 12, 15, 16. Together these results suggest that the presence of fat in the liver alters the hepatic immune system, which likely contributes to their increased susceptibility to secondary insults. We recently showed that the predominance of Th1 cytokines was even more pronounced after T cell activation in steatotic liver, associated with elevated signal transducer and activator of transcription (STAT) 4 and T-box transcription factor expressed in T cells (T-bet), crucial transcription factors for Th1 commitment 12.
IL-12 was initially termed natural killer cell stimulatory factor because of its ability to stimulate NK cells 17, but it also was found to stimulate T-regulatory cells and T cells 18. IL-12 plays an essential role in the protective immune responses against intracellular pathogens by directing the development of Th1 reactions 19, 20. Different studies suggest a significant involvement of IL-12 in the process of hepatosteatosis as it influences the local Th1/Th2 balance and NKT cell activation and regulation, but the exact role of IL-12 in diet-induced pathogenesis of hepatosteatosis has not been explored 21, 22.
The aim of the present study was to evaluate the role of IL-12 in hepatosteatosis and its impact on hepatic resident NKT cells and further to determine whether humans suffering from hepatosteatosis show a similar reduction in hepatic NKT cells. To this end, using a choline-deficient diet (CDD) model of hepatosteatosis, we have identified the importance of IL-12 production by Kupffer cells in the reduction of hepatic NKT cells and translated this observation of reduced NKT cell numbers to human liver samples with hepatosteatosis.
Materials and Methods
Animals and Treatment
All animals received humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals”. The conduct of the study was approved by the University Institutional Animal Care and Use Committee. Male wild-type (wt) C57BL/6 mice and IL-12 p40-deficient mice were obtained from the Jackson Laboratories (Bar Harbor, ME). Mice received a choline-deficient-diet (CDD; Dyets, Bethlehem, PA) for 0, 10 or 20 weeks which results in hepatocellular lipid accumulation. For lipopolysaccharide (LPS) studies, wild type mice fed CDD for 0 or 20 weeks were administered LPS (from E.coli, Sigma, St. Louis MO) at a concentration of 2.5mg/kg (or saline vehicle) by intraperitoneal injection 6 hours prior to sacrifice. For NKT cell activation, wt mice fed CDD for 0 or 10 weeks were administered alpha-galactosylceramide (αGal, Alexis Chemicals, Axxora, San Diego, CA) at a concentration of 200ng/g by intravenous injection 3 hours prior to sacrifice. All animals were housed in pathogen-free barrier facilities accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. All animals had free access to food and water. After 0, 10 or 20 weeks of feeding mice were sacrificed, serum and tissue collected, and samples stored at −80°C until used.
Depletion and detection of macrophages
Multilamellar liposomes containing clodronate (a gift obtained from Roche Diagnostics GmbH, Mannheim, Germany) were prepared as described 24. Mice were inoculated intravenously with 100 μ l of 0.5 mg/ml liposome encapsulated clodronate or PBS as control suspended in saline. Mice, after 10 weeks on CDD, received clodronate liposomes (or PBS liposomes as control) for the following 3 weeks; mice after 20 weeks on CDD received clodronate liposomes (or PBS liposomes as control) for the following 5 weeks every 4-5 days. During clodronate administration, mice were fed CDD continuously. Macrophages were stained immunohistochemically with F4/80 as previously described 23. Positive cells were counted from 10 high powered fields from each animal.
KC isolation and in vitro activation
KCs were enriched from livers of 10 week CSD or CDD fed mice. The liver was perfused with collagenase (0.6mg/ml Collagenase D, Roche Applied Sciences, Indianapolis, IN) followed by filtration through a 70μm nylon filter. Macrophages were enriched by density gradient centrifugation on Histogenz (0.288g/14ml, Sigma, St. Louis, MO). Cells at the interface were plated at 1×105 cells/well on a 48 well tissue culture plate in RPMI 1640 plus 10% fetal calf serum and antibiotics. Following 16hrs, the media was changed and cells treated with either saline or lipopolysaccharide (10μg/ml) for 3 hours. Cells were then collected, RNA isolated, and IL-12 gene expression analyzed by real time PCR.
Human liver samples and NKT cell staining
Human liver tissue with different degrees of hepatic steatosis was obtained from subjects undergoing routine diagnostic liver biopsy under a protocol approved by the UNC Institutional Review Board or the University of Heidelberg Review Board and subjects gave written informed consent. Samples presenting with fibrosis, cirrhosis, or significant inflammatory cell infiltrate indicating hepatitis were excluded. Samples were divided into three groups, those showing lipid accumulation in less than 5% of hepatocytes (n=5), those presenting with steatosis in 6-32% of hepatocytes (n=4), and those showing lipid accumulation in greater than 33% of hepatocytes per field (n=3). For staining, formalin-fixed, paraffin embedded sections were stained for NKT cells according to a modified protocol by Harada K et al. 25. In short, rehydrated samples were blocked with 10% goat serum, incubated with anti-CD57 (1:200, BD) over night at 4°C, followed by anti-mouse IgG linked to alkaline phosphatase (1:100, 1h, Sigma). Liquid permanent red (DAKO) stained subsequently CD57 positive cells red. For T-cell staining, slides were incubated over night at 4°C with mouse-anti CD3 (Novacastra) follwed by anti-mouse IgG HRP (1:200, 1h, Amersham). For color development by HRP, the TMB Substrate Kit (Vector Labs) was used, which stained CD3 positive cells blue and for alkaline phosphatase, liquid permanent red (DAKO) was used which stained CD57+ cells red. Double positive stained cells (purple) were identified as NKT cells.
Statistics
Data are presented as mean ± SEM. The statistical significance of difference between CDD-fed mice and control groups was determined by comparison of the mean using the independent samples t-test. A p value of < 0.05 was selected as the level of significance. Statistical analyses were performed using SPSS 11.0 software (SPSS Inc., Chicago, IL).
Results
CDD-induced hepatosteatosis reduces hepatic NKT cell number and function
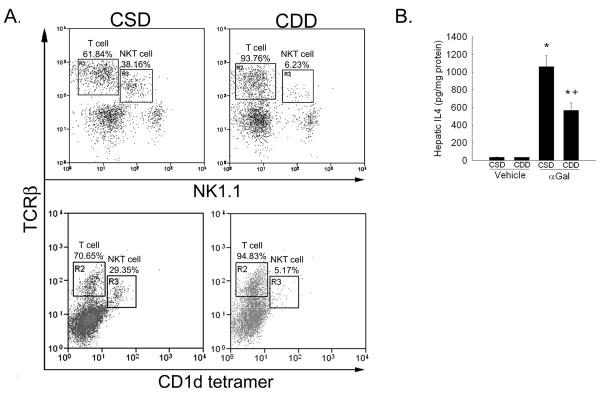
Previous studies have shown reduced numbers of NKT cells in the presence of liver lipid accumulation 1. To determine if CDD-induced hepatosteatosis could significantly alter hepatic NKT cell numbers and/or function, wt mice were fed CDD or CSD as control for 10 weeks followed by administration of either αGal or vehicle. As shown in Figure 1a, feeding wt mice CDD for 10 weeks led to a reduction hepatic NKT cells as assessed by percentages of TCRβ and NK1.1 double positive cells and by TCRβ and CD1d-tetramer/PBS57, a reduction which was associated with significantly reduced IL4 production when compared to αGal-treated CSD controls (Figure 1b). Together, these data confirm the reduction not only in number but also function of hepatic NKT cells.
Figure 1. Hepatic NKT cell number and function are reduced within the steatotic liver.
A. Hepatic mononuclear cells were analyzed by flow cytometry for T cell receptor beta (TCRβ ) and NK1.1 expression or TCRβ and binding to CD1d-tetramer loaded with PBS57 in mice fed choline sufficient diet (CSD) or choline deficient diet (CDD) for 10 weeks. Representative dot plots presented. B. Hepatic IL4 protein expression was measured by ELISA 3 hours following i.v. alpha galactosylceramide (αGal, 200ng/g) administration to 10 week CSD fed or CDD fed mice. n= 6-9 mice per group. *p<0.05 vs. CSD fed vehicle treated mice, +p<0.05 vs. CSD fed, αGal treated mice.
CDD-induced hepatosteatosis is associated with a significant increase in hepatic IL-12
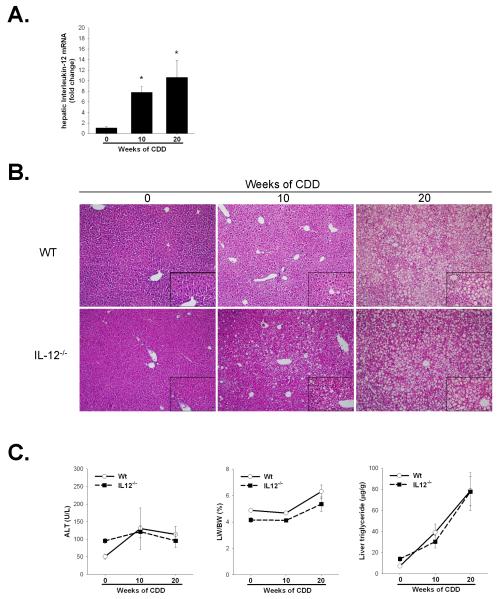
Hepatosteatosis is associated with increased Th1 cytokine expression 12, 15. In our previous study we reported, following 6 weeks of CDD feeding which induces mild hepatic lipid accumulation, a strong increase in hepatic Th1 (IFNγ, IL12) cytokine production both in control treated and concanavalin A treated CDD fed mice 12. Here we wished to better understand the effects of severe steatosis, a setting where hepatic NKT cell numbers and function are significantly reduced, on hepatic IL12 expression. Hepatic IL-12 mRNA-levels were significantly elevated following 10wks of CDD feeding compared to 0 week feeding (1.09 ± 0.17 fold change in control vs. 7.8 ± 1.09 fold change after 10 weeks CDD, p<0.05) an elevation which was sustained through 20wks of feeding (10.6 ± 2.8 fold change, p<0.05) (Figure 2a).
Figure 2. Increased IL12 production does not influence the progression of hepatosteatosis following long-term CDD feeding.
A. C57BL/6 mice were fed CDD for 0, 10 or 20 weeks. Hepatic IL-12 expression was determined by quantitative real time PCR of whole liver tissue while serum IL-12 was measured by ELISA. Values are means ± SEM,. B. H&E stained liver histology after 0, 10 and 20 weeks of CDD in C57BL/6 (Wt) and IL-12−/− mice. Representative photomicrographs are presented at 100x magnification with 400x inserts (bottom right of each image). C. Serum alanine aminotransferase, liver weight to body weight ratio (LW/BW) and hepatic triglycerides were measured following either 0, 10 or 20 weeks of CDD feeding. No significant differences were observed between Wt and IL-12−/− mice in any of these parameters. Values are means ± SEM, *p<0.05, n=4-8 animals per group.
Deficiency in IL12 does not affect the progression of CDD-induced hepatosteatosis
To study the potential implications of IL-12 in hepatosteatosis, IL-12-deficient mice were fed in addition to Wt mice for 0, 10 and 20 weeks with CDD. As shown in Figure 2b, microvesicular steatosis developed in both wt and IL12−/− mice following 10 weeks of CDD feeding which progressed in both to macrovesicular lipid accumulation following 20 weeks of feeding. This increased lipid accumulation occurred in the absence of significant hepatocellular injury as measured by serum alanine aminotransferase levels (Figure 2c). Liver weight to body weigh ratio was increased following 20 weeks of CDD feeding in both C57Bl/6 and IL-12−/− mice when compared to 0 weeks of feeding, Figure 2C. Finally, measurement of hepatic triglycerides confirmed the histopathological findings demonstrating similar levels of hepatic triglycerides after 10 and 20 weeks of CDD feeding in wt and IL12−/− mice (Figure 2c). Together, it is clear that IL12 does not significantly alter the progression of hepatosteatosis following long-term feeding of the choline deficient diet.
IL-12 promotes the induction of Th1-associated cytokines in CDD-induced hepatosteatosis
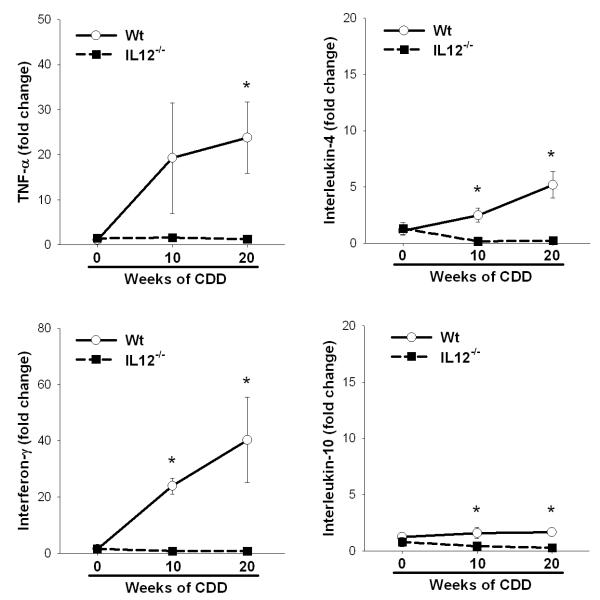
In addition to increased levels of IL-12, other key Th1-associated cytokines like TNFα and IFNγ are elevated in different models of hepatosteatosis, whereas Th2-associated cytokines are not affected 12, 15. Chronic CDD resulted in a significant increase in TNFα and IFNγ mRNA in Wt mice following 10 or 20 weeks of feeding, where only mild changes were observed in IL-4 or IL-10 expression. Surprisingly, the absence of IL-12 abrogated the steatosis-induced increases in Th1-associated cytokines, although mice deficient in IL-12 present with a comparable amount of hepatic fat accumulation (Figure 3).
Figure 3. IL-12 promotes Th1 cytokine expression within the steatotic liver.
Hepatic Th1 (TNFα and IFNγ) and Th2 (IL-4 or IL-10) cytokine gene expression was measured by real time PCR in wt and IL12−/− mice fed CDD for 0, 10 or 20 weeks. Values are means ± SEM, *p<0.05; n = 4-8 animals per group.
IL-12 is involved in CDD-induced hepatic NKT cell depletion
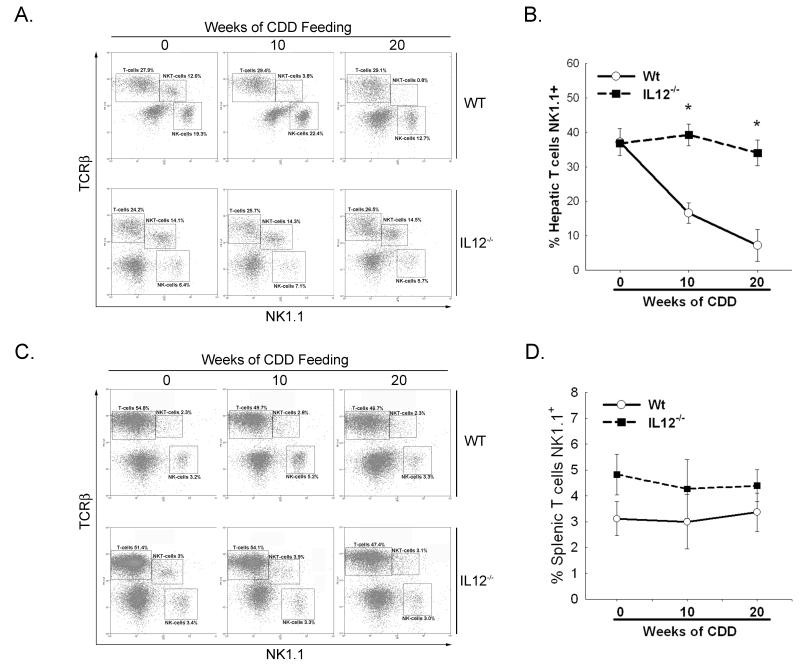
In the current study of CDD-induced hepatosteatosis, we observed a severity-dependent reduction in the hepatic NKT cell population in Wt mice. After 10wks of diet nearly 70% and after 20wks up to 98% of the resident NKT cell population was depleted (Figure 4a and b), a finding which was confined to the liver as splenic NKT cell populations were not affected by CDD feeding (Figure 4c and d). This significant reduction in liver NKT cells was abrogated in IL-12-deficient mice. After 10wks and 20wks of CDD, no changes in hepatic NKT cell numbers were observed when compared to 0wk CDD mice (Figure 4). Together, these results suggest that not IL-12 itself, but a combination of IL-12 in conjunction with changes in the fatty hepatic microenvironment are likely responsible for the depletion of hepatic NKT cells.
Figure 4. IL-12 is involved in steatosis-associated hepatic NKT cell depletion.
C57BL/6 (Wt) and Interleukin (IL)-12−/− mice were fed CDD for 0, 10 or 20 weeks and total liver mononuclear cells were analyzed by flow cytometry. Hepatic mononuclear cells (A) or splenocytes (C) were stained with anti-mouse T cell receptor β (TCR) and natural killer (NK) 1.1 antibodies. T cells, NKT cells and NK cells are outlined in boxes. Values represent percent of total liver mononuclear cells analyzed. B and D. Quantitation of hepatic (B) or splenic (D) NKT cells expressed as percent of total hepatic CD3+ cells expressing NK1.1. Figure is representative for 3-6 different experiments. Results are representative of 3-6 animals per group.
Hepatic macrophages contribute to IL-12 production
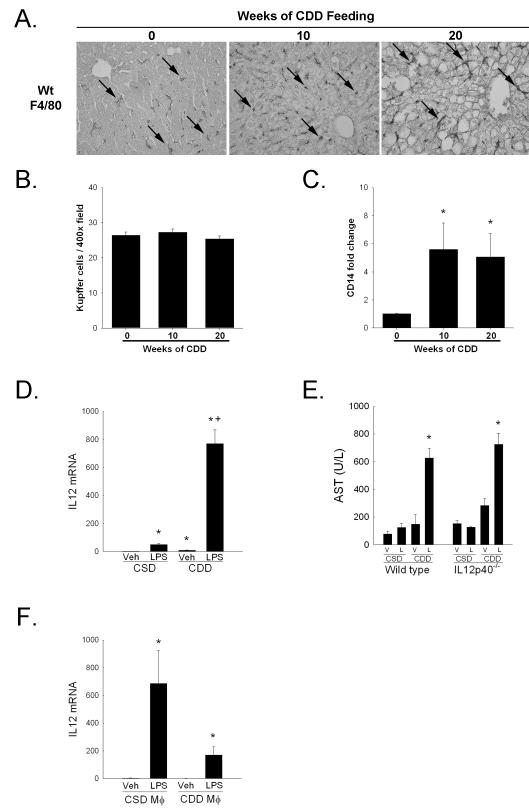
To evaluate the potential source of IL-12 in hepatosteatosis, we investigated the role of hepatic macrophages in fatty liver. Previous studies reported increased numbers of macrophages in fatty tissue 26, but the reports concerning fatty liver are inconsistent. Evaluation of macrophage numbers by immunohistochemistry with F4/80 did not reveal any increase in their numbers following 10 or 20 weeks of CDD feeding when compared to 0 week CDD mice (Figure 5 A and B). However, steatosis did alter the morphology of the KCs present with the prominent enlargement in cell size and an elongated, spindled shape. Consistent with the potential activation of KCs was a significant increase in hepatic CD14 gene expression in wt mice with hepatosteatosis (Figure 5C). To evaluate the influence of hepatosteatosis on KC function, mice fed a choline deficient diet or control diet for 20 weeks were administered lipopolysaccharide (2.5mg/kg) by intraperitoneal injection. Six hours following LPS injection, there was a large and significant increase in hepatic IL12 gene expression when compared to LPS-treated CSD-fed mice (Figure 5D). This increase in IL12 correlated with significantly increased serum aspartate aminotransferase levels in CDD fed but not CSD fed mice, injury which occurred independent of IL12 (Figure 5E). Interestingly, in vitro analysis of hepatic macrophage function did not reveal a significant increase in IL12 expression 3 hours following LPS stimulation in KCs from CDD fed mice when compared to CSD fed hepatic macrophages (Figure 5F) suggesting that secondary factors present within the steatotic liver prime macrophages for cytokine release. In sum, these data demonstrate the enhanced responsiveness of hepatic macrophages to endotoxin within the steatotic liver, a process which likely involves multiple factors for their activation.
Figure 5. Kupffer cells are activated within the steatotic liver.
A. Liver sections from wild type mice fed CDD for 0, 10 or 20 weeks were stained with F4/80. Representative 400x photomicrographs presented. B. Quantitation of F4/80 positive cells per 400x field. Ten fields per section from each mouse were analyzed. C. Hepatic CD14 gene expression was measured using real time PCR. D. Hepatic IL12 gene expression 6 hours following LPS injection. E. Serum aspartate aminotransferase levels 6 hours following lipopolysaccharide (LPS, 2.5mg/kg) injection in wt or IL12−/− mice fed choline sufficient diet (CSD) or CDD diet for 20 weeks. F. IL12 expression 3 hours following treatment with either saline or LPS (10μg/ml) in isolated hepatic macrophage (Mφ) from wt mice fed either a choline sufficient diet or choline deficient diet for 10 weeks. Representative data from two independent experiments shown. Values are mean ± SEM, *p<0.05 versus respective control, +p<0.05 versus LPS-treated choline sufficient diet fed mice; n = 4-8 animals per group.
KCs contribute to NKT cell loss within the steatotic liver
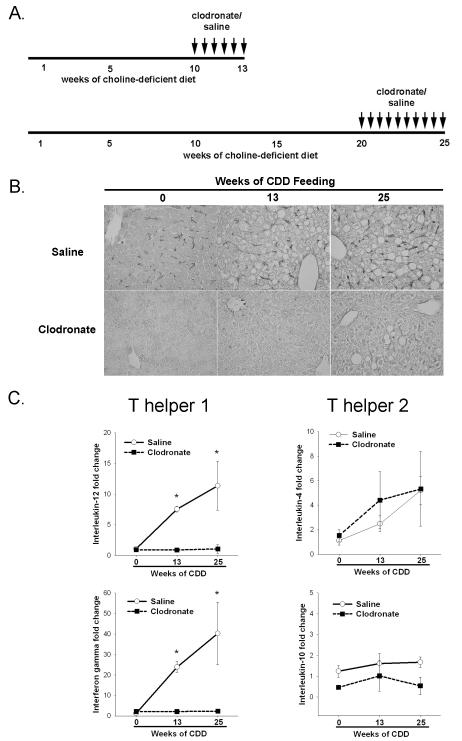
In order to study the functional role of macrophages in hepatosteatosis and specifically their impact on resident NKT cells numbers, mice with established fatty liver received clodronate by intravenous injection according to the time-table presented in Figure 6A. Clodronate is engulfed selectively by macrophages and induces apoptosis. The control group with fatty liver received saline (Figure 6B), whereas macrophage depletion was achieved effectively by clodronate treatment. The absence of macrophages after clodronate treatment was confirmed using immunohistochemistry (Figure 6B) and did not result in any changes in liver injury at either the 13 or 25 week time-point when compared to PBS encapsulated liposome treated CDD fed controls (data not shown) though loss of macrophages did show a trend toward a reduction in hepatic lipid accumulation (32.40±8.17 vs. 17.89±4.62 for CDD-fed mice treated with PBS liposomes vs. CDD-fed mice treated with clodronate encapsulated liposomes, p=0.104).
Figure 6. Clodronate depletes Kupffer cells and ameliorates increased Th1 associated cytokine expression in steatotic liver tissue.
A. Dosing regimen for macrophage depletion studies. Mice with established hepatosteatosis after 10 weeks of CDD were injected every 4-5 days with clodronate for the following 3 weeks, mice after 20 weeks of CDD received clodronate every 4-5 days for the following 5 weeks while continuously fed CDD. B. Liver sections stained with F4/80 antibody from wt mice administered vehicle or clodronate as described in A. Representative photomicrographs at 400x magnification presented. C. Hepatic Th1 and Th2 cytokine expression as assessed by real-time PCR in vehicle or clodronate treated animals. Values are mean ± SEM, *p<0.05. n=4-6 animals per group.
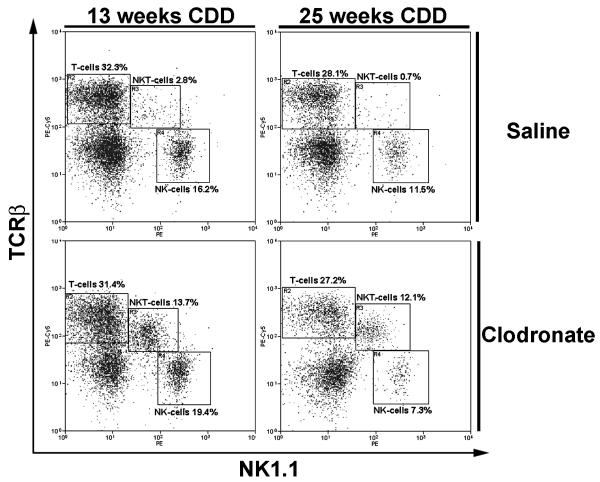
KCs play a major role in innate immunity and are a likely source for IL-12 in the liver 27. Therefore, we measured hepatic IL-12 and IFNγ expression as well as IL-4 and IL-10 gene expression using real-time PCR after clodronate (or vehicle) treatment in Wt mice (Figure 6C). KC depletion blunted steatosis-induced hepatic IL-12 and IFNγ gene expression and had no effect on Th2-associated cytokines. In addition, KC depletion led to a complete repopulation of hepatic NKT cells following 10 weeks of CDD (Figure 7). Even after 20 weeks of CDD with an almost complete loss of NKT cells in hepatosteatosis, 5 weeks of clodronate-treatment was sufficient to restore NKT cells. To eliminate possible non-specific effects of clodronate treatment, IL12–deficient mice fed CDD for 10 weeks were administered clodronate for 3 weeks. Hepatic NKT cell populations were not changed from untreated CDD fed mice (data not shown). These results suggest that KCs, either directly or indirectly, contribute to the production of IL-12 and continually deplete hepatic NKT cells within the steatotic liver.
Figure 7. Kupffer cells contribute to steatotosis-induced hepatic NKT cell depletion.
Hepatic mononuclear cells were isolated from vehicle or clodronate treated wild type mice fed choline deficient diet for indicated time periods and subpopulations of lymphocytes assessed by flow cytometry. Results are representative of 4-6 different experiments, p<0.01.
NKT cell populations are reduced in human non-alcoholic hepatosteatosis
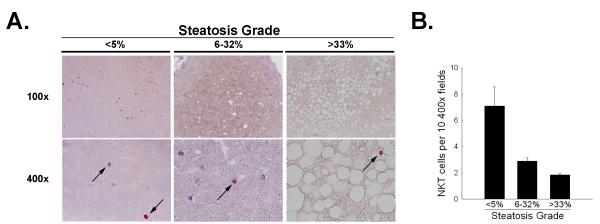
Recently, reduced peripheral NKT cell numbers in the blood of patients suffering from non alcoholic fatty liver disease have been reported 28, but no data exist regarding NKT-cell numbers in human steatotic livers. Human liver samples were obtained from the Liver Diseases Unit at the University of North Carolina and from the Department of Pathology at the University of Heidelberg from patients with different degrees of hepatosteatosis. Samples were selected based on the absence of severe inflammation, fibrosis or cirrhosis, to more clearly evaluate the influence of steatosis alone on hepatic NKT cells. As shown in Figure 8, liver sections from patients with steatosis present in 6-32% of hepatocytes had reduced numbers of NKT cells. With severe steatosis, hepatic NKT cells are even further reduced when compared to healthy control livers. Together, these data suggest that, as is seen in the rodent model of hepatosteatosis, non-alcoholic hepatosteatosis in humans results in a decrease in the numbers of hepatic NKT cells.
Figure 8. Hepatosteatosis is associated with decreased numbers of NKT and NK cells in humans.
Human liver biopsies were divided into three groups as described in methods. Slides were stained for CD3 and CD57, a marker for human NK cells. CD3 positive cells stained blue, NK positive cells present red. Double positive cells are termed NKT cells. A. Representative microphotographs of stained tissue are presented, NKT cells are pointed out by arrows. B. Quantitation of NKT cells were counted from at least 10 400x pictures of the tissue. Representative results for n = 3-5 per group. Values are means ± SEM.
Discussion
NKT cells represent a population of highly specialized lymphocytes involved in regulation of the immune system. As NKT are 20-25% of hepatic mononuclear cells and share features of both classical T cells and natural killer (NK) cells, they represent an important link between innate and adaptive immunity through production of both Th1 and Th2 associated cytokines 2. Findings from the current study confirm the ability of hepatocellular lipid accumulation to decrease the numbers and function of this important resident lymphocyte population both in mice and humans and experimentally define an IL-12 and KC-associated mechanism for their depletion. Together, these studies provide new and important information regarding the impact of hepatosteatosis on resident NKT cells.
Previous studies in fatty livers of obese, leptin-deficient ob/ob mice reported reduced NKT cell numbers within the liver 16, and this reduction was confirmed in a number of different models of diet-induced hepatosteatosis 12, 14, 15. Interestingly, either adoptive transfer of wild type NKT cells or activation of NKT cells with the naturally occurring glycolipid glucocerebroside in leptin deficient ob/ob mice led to changes in hepatic lipid content, specifically a movement from macrovesicular to microvesicular fat, and improvement in glucose tolerance suggesting that NKT cells may be inhibitors of hepatosteatosis 29, 30. However, our findings suggest that in CDD-induced hepatosteatosis, the restoration of NKT cells and suppression of Th1 cytokine response in IL12−/− mice is insufficient to affect hepatic triglyceride accumulation. The reasons for the lack of effect on lipid accumulation is not known with certainty. It is clear that other inflammatory cytokines, TNFα in particular, are capable of promoting non-alcoholic 31 and alcoholic steatosis 32 and studies from our own lab would suggest that TNFα is active and important for lipid accumulation in this model (unpublished observation). In the absence of IL12, TNFα levels are reduced to at or near control levels. It could be that their presence, even if at a very minimal level, is capable of promoting and sustaining lipid accumulation. Further study is required to better understand the critical factors for steatosis development. Nevertheless, the current study demonstrates the inability of IL12 alone to promote lipid accumulation. In sum, as the severity-dependent reduction of hepatic NKT cell numbers is restricted to the liver and peripheral NKT cells in spleen are not affected, the local hepatic environment seems to effectuate the loss of resident NKT cell numbers, a process which involves IL-12 production.
Over the time course of 20 weeks, CDD results in severe hepatosteatosis with absence of inflammation or necrosis. It is associated with a significant increase in hepatic IL-12 expression. IL-12 is known to stimulate NKT and other immune-competent T cells and NK cells to release IFNγ 33, but conflicting data exists concerning their viability after IL-12 exposure. Whereas Ito et al. 34, 35 found increased numbers of hepatic NKT cells after IL-12 stimulation, Takahashi et al. report the ability of IL-12 to reduce NKT cell-viability 36, and Matsui et al. also observed a reduction in NKT cell numbers by IL-12 through activation-induced cell death 37. In the current study, we demonstrate a significant increase in hepatic IL-12 expression and an IL-12-dependent loss of hepatic NKT cells within the steatotic liver. It is likely that multiple factors present within the steatotic liver sensitize NKT cells specifically to IL-12-associated depletion. Alternatively, IL12 could operate through other cytokines or factors as IL12-deficient mice show significant reductions in the expression of TNFα and IFNγ and the function of these mediators on NKT cell function has not been elucidated. In as much as IL12 promotes loss of NKT cells, it may also provide a supportive signal for hepatic NK cells. Originally described as an NK cell inducing factor 18, IL12 likely supports NK cell recruitment and or survival though the net effect this has on NKT cell function or hepatosteatosis development remains to be determined. Together, however, findings from the current study strongly implicate IL-12 as a key factor in the depletion of this important immunomodulatory cell population within the murine steatotic liver.
In addition to demonstrating the importance of IL-12 in the depletion of NKT cells from the steatotic liver, the current study has further investigated the potential source of IL-12 implicating KCs in this production. Upon activation by endotoxin, resident hepatic macrophages produce pro-inflammatory mediators including TNFα and IL-12 13, 38. In alcoholic liver disease, these mediators are associated with the promotion of liver injury and hepatocyte lipid accumulation 32. The impact of non-alcoholic steatosis on KC activation is not well understood. In leptin deficient mice which present with a fatty liver, hepatic macrophage cytokine production, including IL-12 production, is increased 13. Findings from the current study would further suggest that steatosis alone may induce significant KC activation. Using hepatic CD14 gene expression within the steatotic liver as an indirect measure of macrophage activation, we provide data showing a correlation between the severity of hepatosteatosis and the level of CD14 expression independent of alterations in hepatic macrophage numbers. In addition, upon stimulation with LPS in vivo, steatotic livers express significantly greater quantities of IL12 and show significantly enhanced hepatocellular injury though this injury occurs independently of IL-12 induction. Interestingly, KCs isolated from CDD fed wt mice did not express increased levels of IL12 either basally or when activated by LPS in vitro compared to comparably treated CSD fed wt mouse KCs suggesting that secondary factors present within the steatotic liver promote IL-12 production in the presence of LPS. Alternatively, KC-associated IL12 is not the only source of this important, NKT cell depleting cytokine. Previous studies in ischemia and reperfusion models have demonstrated the ability of hepatocytes to produce IL12 39. In the in vivo setting, changes in fat metabolism with unusually high levels of metabolites, increased oxidative stress with consequent lipid peroxidation could promote heightened KC responsiveness 40, 41 and the release of pro-inflammatory cytokines like TNFα or IL-12 by hepatocytes and lymphocytes 15, 42. Likewise, the potential impact of KCs on the development of hepatosteatosis in this model remains to be determined. It is clear that unlike loss of IL12, loss of hepatic macrophages leads to a substantial, though not significant, reduction in hepatic lipid accumulation following 10 and 20 weeks of CDD feeding indicating that other factors produced by KCs could potentially be important for hepatic lipid accumulation. Further study will be required to concretely identify these factors in the setting of severe hepatosteatosis. Data presented here do however implicate hepatic macrophages either directly or indirectly in the production of IL-12 as depletion of these cells reduced hepatic IL-12 expression, balanced the Th cytokine profile of the liver, and led to a reconstitution of NKT cells within the livers of mice with established hepatosteatosis. Together, these data provide additional new insights into the complexities and potential sources of IL-12 production within the steatotic liver.
Hepatosteatosis results in a shifted, Th1 predominated, cytokine response 12. The reasons for this shift likely involve increased oxidant production, reduced oxidant defense capacity, and/or modulation of intrahepatic immune cell populations or function. Data from the current study implicate IL-12 and/or loss of NKT cells in the promotion of a Th1-shifted response. IL-12 is known to induce T cells to produce large amounts of IFNγ and activate Th1 transcription factors including signal transducer and activator of transcription (STAT) 4 20. NKT cells may also balance the local cytokine response within the liver. Li and others reported an increased susceptibility of leptin deficient ob/ob mice to endotoxin-induced liver damage with an increase in the hepatic production of Th1 cytokines including IFNγ, a known mediator of endotoxin-induced liver damage 1. Loss of IL-12 reduced hepatic IFNγ expression in the current study, but also preserved the hepatic NKT cell populations, making it difficult to determine which of these are responsible for the disrupted Th balance in the steatotic liver. Nevertheless, findings from the current study demonstrate the importance of IL12 in the depletion of hepatic NKT cells in the setting of severe steatosis, a process which is associated with restoration of a balanced hepatic Th cytokine profile.
Utilizing biopsies from patients with mild to severe hepatosteatosis, we present intriguing evidence to suggest a similar correlation between the degree of steatosis and the numbers of hepatic NKT cells present in humans where NKT cell numbers decreased when hepatosteatosis was moderate to severe. Due to small sample size, it was not possible to determine if IL-12 was up-regulated in these samples as was observed experimentally though previous investigations have demonstrated increased pro-inflammatory cytokines within the human steatotic liver 43. Nevertheless, the findings presented here provide the first line of evidence in humans to suggest that hepatosteatosis may indeed be a key modulator of hepatic lymphocyte populations.
In summary, these results demonstrate a connection between hepatosteatosis-induced IL-12 production and the loss of resident NKT cells and implicate NKT cells in the suppression of Th1-type cytokine production within the steatotic liver. Moreover, we provide evidence for the first time that suggests a similar connection between fatty liver and loss hepatic NKT cells in humans. Several questions remain including the nature of the stimulus responsible for increasing KC-associated IL-12 expression and the clear mechanism for how IL-12 mediates its effects on hepatic NKT cell populations. Also, it is of great interest what other effects a loss of NKT cells might have on the steatotic liver. It is well appreciated that both NKT cells and NK cells play a significant role in tumor cell surveillance and clearance and that disruption of NKT and/or NK cell function may predispose the liver to secondary pathologies including carcinogenesis. In conclusion, the current study provides important new mechanistic information regarding NKT cell depletion within the steatotic liver and identifies potential new therapeutic targets to preserve this critical resident immune cell population of the liver.
Supplementary Material
Acknowledgments
Grant Support: This work was supported by grants from the National Institutes for Alcohol Abuse and Alcoholism, AA016563 to Ian N Hines and AA014243 to Richard A Rippe and by the ABMRF/The Foundation for Alcohol Research (to Ian N Hines).
Abbreviations
- αGal
(alpha galactosylceramide)
- CDD
(choline deficient diet)
- CSD
(choline sufficient diet)
- DAB
(diaminobenzidine)
- ECL
(enhanced chemiluminescence)
- ELISA
(enzyme linked immunosorbent assay)
- H&E
(hematoxylin and eosin)
- HRP
(horse radish peroxidase)
- IFNγ
(interferon gamma)
- IL
(interleukin)
- KC
(Kupffer cell)
- LPS
(lipopolysaccharide)
- MHC
(major histocompatability complex)
- M-MLV
(murine moloney virus)
- NK
(natural killer)
- NKT
(natural killer T)
- PBS
(phosphate buffered saline)
- PCR
(polymerase chain reaction)
- PE
(phycoerythrin)
- RT
(reverse transcription)
- SDS-PAGE
(sodium dodecylsulfate polyacrylamide gel electrophoresis)
- SEM
(standard error of the mean)
- STAT
(signal transducer and activator of transcription)
- T-bet
(t box transcription factor expressed in T cells)
- TCR
(t cell receptor)
- Th
(T helper)
- TMB
(tetramethylbenzidine)
- TNFα
(tumor necrosis factor alpha)
- T-TBS
(tween-20 tris buffered saline)
- Wt
(wild type)
Footnotes
Publisher's Disclaimer: This PDF receipt will only be used as the basis for generating PubMed Central (PMC) documents. PMC documents will be made available for review after conversion (approx. 2-3 weeks time). Any corrections that need to be made will be done at that time. No materials will be released to PMC without the approval of an author. Only the PMC documents will appear on PubMed Central -- this PDF Receipt will not appear on PubMed Central.
Financial Disclosures: The authors have no financial disclosures to report.
Literature Cited
- 1.Li Z, Diehl AM. Innate immunity in the liver. Curr Opin Gastroenterol. 2003;19:565–571. doi: 10.1097/00001574-200311000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van KL. NKT cells: what’s in a name? Nat Rev Immunol. 2004;4:231–237. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 3.Gumperz JE. CD1d-restricted “NKT” cells and myeloid IL-12 production: an immunological crossroads leading to promotion or suppression of effective anti-tumor immune responses? J Leukoc Biol. 2004;76:307–313. doi: 10.1189/jlb.0104038. [DOI] [PubMed] [Google Scholar]
- 4.Skold M, Behar SM. Role of CD1d-restricted NKT cells in microbial immunity. Infect Immun. 2003;71:5447–5455. doi: 10.1128/IAI.71.10.5447-5455.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson SB, Delovitch TL. Janus-like role of regulatory iNKT cells in autoimmune disease and tumour immunity. Nat Rev Immunol. 2003;3:211–222. doi: 10.1038/nri1028. [DOI] [PubMed] [Google Scholar]
- 6.Porcelli SA, Modlin RL. The CD1 system: antigen-presenting molecules for T cell recognition of lipids and glycolipids. Annu Rev Immunol. 1999;17:297–329. doi: 10.1146/annurev.immunol.17.1.297. [DOI] [PubMed] [Google Scholar]
- 7.Bendelac A, Lantz O, Quimby ME, Yewdell JW, Bennink JR, Brutkiewicz RR. CD1 recognition by mouse NK1+ T lymphocytes. Science. 1995;268:863–865. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- 8.Taniguchi M, Koseki H, Tokuhisa T, Masuda K, Sato H, Kondo E, Kawano T, Cui J, Perkes A, Koyasu S, Makino Y. Essential requirement of an invariant V alpha 14 T cell antigen receptor expression in the development of natural killer T cells. Proc Natl Acad Sci U S A. 1996;93:11025–11028. doi: 10.1073/pnas.93.20.11025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lantz O, Bendelac A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4-8- T cells in mice and humans. J Exp Med. 1994;180:1097–1106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park SH, Bendelac A. CD1-restricted T-cell responses and microbial infection. Nature. 2000;406:788–792. doi: 10.1038/35021233. [DOI] [PubMed] [Google Scholar]
- 11.Lee PT, Benlagha K, Teyton L, Bendelac A. Distinct functional lineages of human V(alpha)24 natural killer T cells. J Exp Med. 2002;195:637–641. doi: 10.1084/jem.20011908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kremer M, Hines IN, Milton RJ, Wheeler MD. Favored T helper 1 response in a mouse model of hepatosteatosis is associated with enhanced T cell-mediated hepatitis. Hepatology. 2006;44:216–227. doi: 10.1002/hep.21221. [DOI] [PubMed] [Google Scholar]
- 13.Li Z, Lin H, Yang S, Diehl AM. Murine leptin deficiency alters Kupffer cell production of cytokines that regulate the innate immune system. Gastroenterology. 2002;123:1304–1310. doi: 10.1053/gast.2002.35997. [DOI] [PubMed] [Google Scholar]
- 14.Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Z, Soloski MJ, Diehl AM. Dietary factors alter hepatic innate immune system in mice with nonalcoholic fatty liver disease. Hepatology. 2005;42:880–885. doi: 10.1002/hep.20826. [DOI] [PubMed] [Google Scholar]
- 16.Guebre-Xabier M, Yang S, Lin HZ, Schwenk R, Krzych U, Diehl AM. Altered hepatic lymphocyte subpopulations in obesity-related murine fatty livers: potential mechanism for sensitization to liver damage. Hepatology. 2000;31:633–640. doi: 10.1002/hep.510310313. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi M, Fitz L, Ryan M, Hewick RM, Clark SC, Chan S, Loudon R, Sherman F, Perussia B, Trinchieri G. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989;170:827–845. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang BY, Kim E, Kim TS. Regulatory mechanisms and their therapeutic implications of interleukin-12 production in immune cells. Cell Signal. 2005;17:665–673. doi: 10.1016/j.cellsig.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 19.Hasko G, Szabo C. IL-12 as a therapeutic target for pharmacological modulation in immune-mediated and inflammatory diseases: regulation of T helper 1/T helper 2 responses. Br J Pharmacol. 1999;127:1295–1304. doi: 10.1038/sj.bjp.0702689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 21.Kaneda M, Kashiwamura S, Ueda H, Sawada K, Sugihara A, Terada N, Kimura-Shimmyo A, Fukuda Y, Shimoyama T, Okamura H. Inflammatory liver steatosis caused by IL-12 and IL-18. J Interferon Cytokine Res. 2003;23:155–162. doi: 10.1089/107999003321532493. [DOI] [PubMed] [Google Scholar]
- 22.Scott MJ, Hoth JJ, Gardner SA, Peyton JC, Cheadle WG. Genetic background influences natural killer cell activation during bacterial peritonitis in mice, and is interleukin 12 and interleukin 18 independent. Cytokine. 2004;28:124–136. doi: 10.1016/j.cyto.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Kremer M, Perry AW, Milton RJ, Rippe RA, Wheeler MD, Hines IN. Pivotal role of Smad3 in a mouse model of T cell-mediated hepatitis. Hepatology. 2007 doi: 10.1002/hep.21956. [DOI] [PubMed] [Google Scholar]
- 24.van RN, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 25.Harada K, Isse K, Tsuneyama K, Ohta H, Nakanuma Y. Accumulating CD57 + CD3 + natural killer T cells are related to intrahepatic bile duct lesions in primary biliary cirrhosis. Liver Int. 2003;23:94–100. doi: 10.1034/j.1600-0676.2003.00807.x. [DOI] [PubMed] [Google Scholar]
- 26.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bilzer M, Roggel F, Gerbes AL. Role of Kupffer cells in host defense and liver disease. Liver Int. 2006;26:1175–1186. doi: 10.1111/j.1478-3231.2006.01342.x. [DOI] [PubMed] [Google Scholar]
- 28.Xu CF, Yu CH, Li YM, Xu L, Du J, Shen Z. Association of the frequency of peripheral natural killer T cells with nonalcoholic fatty liver disease. World J Gastroenterol. 2007;13:4504–4508. doi: 10.3748/wjg.v13.i33.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Margalit M, Shalev Z, Pappo O, Sklair-Levy M, Alper R, Gomori M, Engelhardt D, Rabbani E, Ilan Y. Glucocerebroside ameliorates the metabolic syndrome in OB/OB mice. J Pharmacol Exp Ther. 2006;319:105–110. doi: 10.1124/jpet.106.104950. [DOI] [PubMed] [Google Scholar]
- 30.Elinav E, Pappo O, Sklair-Levy M, Margalit M, Shibolet O, Gomori M, Alper R, Thalenfeld B, Engelhardt D, Rabbani E, Ilan Y. Adoptive transfer of regulatory NKT lymphocytes ameliorates non-alcoholic steatohepatitis and glucose intolerance in ob/ob mice and is associated with intrahepatic CD8 trapping. J Pathol. 2006;209:121–128. doi: 10.1002/path.1950. [DOI] [PubMed] [Google Scholar]
- 31.Diehl AM, Li ZP, Lin HZ, Yang SQ. Cytokines and the pathogenesis of non-alcoholic steatohepatitis. Gut. 2005;54:303–306. doi: 10.1136/gut.2003.024935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yin M, Wheeler MD, Kono H, Bradford BU, Gallucci RM, Luster MI, Thurman RG. Essential role of tumor necrosis factor alpha in alcohol-induced liver injury in mice. Gastroenterology. 1999;117:942–952. doi: 10.1016/s0016-5085(99)70354-9. [DOI] [PubMed] [Google Scholar]
- 33.Ito H, Ando K, Nakayama T, Taniguchi M, Ezaki T, Saito K, Takemura M, Sekikawa K, Imawari M, Seishima M, Moriwaki H. Role of Valpha 14 NKT cells in the development of impaired liver regeneration in vivo. Hepatology. 2003;38:1116–1124. doi: 10.1053/jhep.2003.50471. [DOI] [PubMed] [Google Scholar]
- 34.Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsushita T, Ando K, Kimura K, Ohnishi H, Imawari M, Muto Y, Moriwaki H. IL-12 induces specific cytotoxicity against regenerating hepatocytes in vivo. Int Immunol. 1999;11:657–665. doi: 10.1093/intimm/11.5.657. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi M, Ogasawara K, Takeda K, Hashimoto W, Sakihara H, Kumagai K, Anzai R, Satoh M, Seki S. LPS induces NK1.1+ alpha beta T cells with potent cytotoxicity in the liver of mice via production of IL-12 from Kupffer cells. J Immunol. 1996;156:2436–2442. [PubMed] [Google Scholar]
- 37.Matsui K, Yoshimoto T, Tsutsui H, Hyodo Y, Hayashi N, Hiroishi K, Kawada N, Okamura H, Nakanishi K, Higashino K. Propionibacterium acnes treatment diminishes CD4+ NK1.1+ T cells but induces type I T cells in the liver by induction of IL-12 and IL-18 production from Kupffer cells. J Immunol. 1997;159:97–106. [PubMed] [Google Scholar]
- 38.Thakur V, Pritchard MT, McMullen MR, Nagy LE. Adiponectin normalizes LPS-stimulated TNF-alpha production by rat Kupffer cells after chronic ethanol feeding. Am J Physiol Gastrointest Liver Physiol. 2006;290:G998–1007. doi: 10.1152/ajpgi.00553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lentsch AB, Yoshidome H, Kato A, Warner RL, Cheadle WG, Ward PA, Edwards MJ. Requirement for interleukin-12 in the pathogenesis of warm hepatic ischemia/reperfusion injury in mice. Hepatology. 1999;30:1448–1453. doi: 10.1002/hep.510300615. [DOI] [PubMed] [Google Scholar]
- 40.Yoshida Y, Itoh N, Hayakawa M, Habuchi Y, Inoue R, Chen ZH, Cao J, Cynshi O, Niki E. Lipid peroxidation in mice fed a choline-deficient diet as evaluated by total hydroxyoctadecadienoic acid. Nutrition. 2006;22:303–311. doi: 10.1016/j.nut.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 41.Ikura Y, Ohsawa M, Suekane T, Fukushima H, Itabe H, Jomura H, Nishiguchi S, Inoue T, Naruko T, Ehara S, Kawada N, Arakawa T, Ueda M. Localization of oxidized phosphatidylcholine in nonalcoholic fatty liver disease: impact on disease progression. Hepatology. 2006;43:506–514. doi: 10.1002/hep.21070. [DOI] [PubMed] [Google Scholar]
- 42.Tomita K, Tamiya G, Ando S, Ohsumi K, Chiyo T, Mizutani A, Kitamura N, Toda K, Kaneko T, Horie Y, Han JY, Kato S, Shimoda M, Oike Y, Tomizawa M, Makino S, Ohkura T, Saito H, Kumagai N, Nagata H, Ishii H, Hibi T. Tumour necrosis factor alpha signalling through activation of Kupffer cells plays an essential role in liver fibrosis of non-alcoholic steatohepatitis in mice. Gut. 2006;55:415–424. doi: 10.1136/gut.2005.071118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi S, Diehl AM. Role of inflammation in nonalcoholic steatohepatitis. Curr Opin Gastroenterol. 2005;21:702–707. doi: 10.1097/01.mog.0000182863.96421.47. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.