Abstract
Background
Ginger root (Zingiber officinale) has been used traditionally for the treatment of gastrointestinal ailments such as motion sickness, dyspepsia and hyperemesis gravidarum, and is also reported to have chemopreventative activity in animal models. The gingerols are a group of structurally related polyphenolic compounds isolated from ginger and known to be the active constituents. Since Helicobacter pylori (HP) is the primary etiological agent associated with dyspepsia, peptic ulcer disease and the development of gastric and colon cancer, the anti-HP effects of ginger and its constituents were tested in vitro.
Materials and Methods
A methanol extract of the dried powdered ginger rhizome, fractions of the extract and the isolated constituents, 6-,8-, 10-gingerol and 6-shogoal, were tested against 19 strains of HP, including 5 CagA+ strains.
Results
The methanol extract of ginger rhizome inhibited the growth of all 19 strains in vitro with a minimum inhibitory concentration range of 6.25–50 µg/ml. One fraction of the crude extract, containing the gingerols, was active and inhibited the growth of all HP strains with an MIC range of 0.78 to 12.5 µg/ml and with significant activity against the CagA+ strains.
Conclusion
These data demonstrate that ginger root extracts containing the gingerols inhibit the growth of H. pylori CagA+ strains in vitro and this activity may contribute to its chemopreventative effects.
Keywords: Ginger, gingerols, chemoprevention, Helicobacter pylori, Zingiber officinale
In 1994, Helicobacter pylori (HP) was the first bacterium to be classified as a Group 1 carcinogen and a definite cause of gastric cancer in humans by the International Agency for Research on Cancer (1, 2). Since then, HP has been epidemiologically linked to adenocarcinoma of the distal stomach (3, 4) and recent studies have also found a positive association between HP infection and colorectal adenomas (5, 6). CagA is the strain-specific H. pylori gene that has been linked to the development of premalignant and malignant histological lesions (7). Thus, susceptibility of CagA+ HP strains is of note because, as compared to CagA-strains, infections caused by CagA+ strains significantly increase the risk of developing severe gastric inflammation, atrophic gastritis and noncardia gastric adenocarcinoma (7).
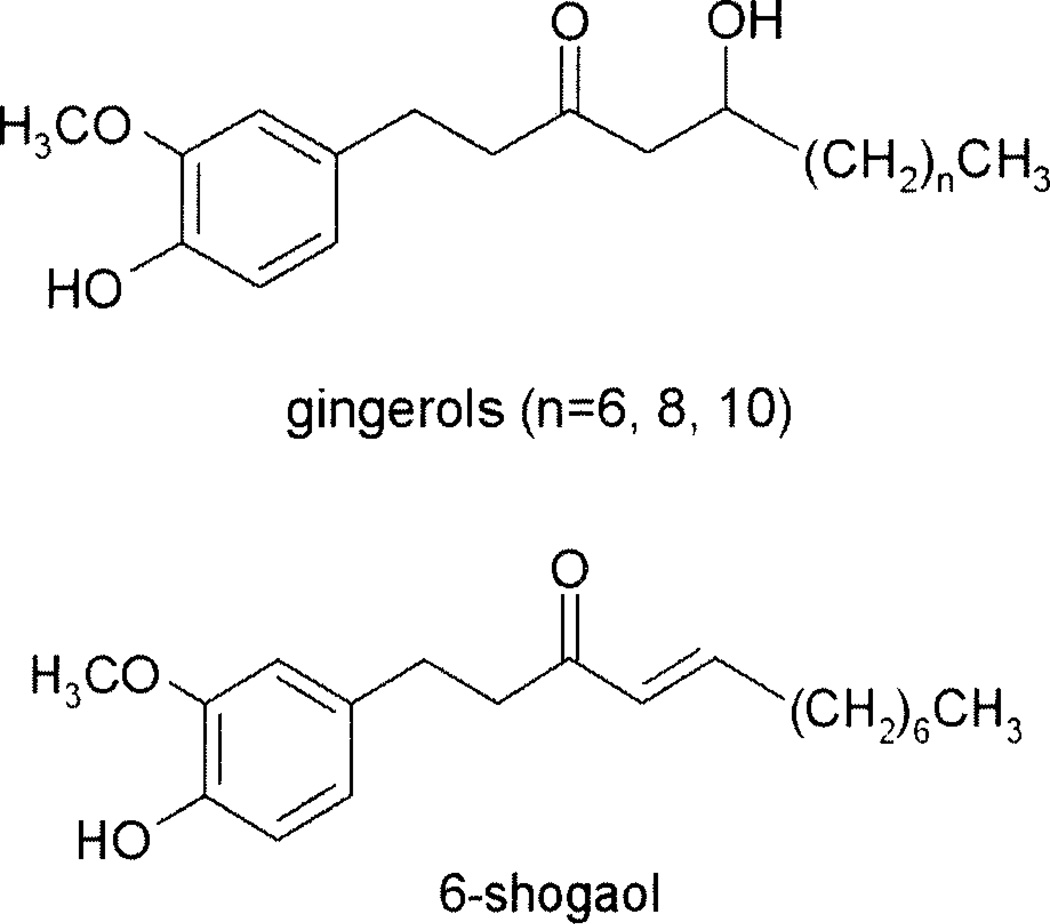
In view of the fact that cancer is a primary contributor to morbidity and mortality worldwide, it is no surprise that the identification of botanicals and plant-derived compounds having the capacity to interfere with carcinogenic processes has received considerable interest. For example, food plants, such as ginger (Zingiber officinale Roscoe, Zingiberaceae) have enjoyed worldwide popularity both as spices and as traditional medicines (8, 9). Ginger extracts have been used medicinally for the treatment of nausea and vomiting associated with motion sickness, postoperative nausea, hyperemesis gravidarum and for the treatment of dyspepsia and peptic ulcer disease (9–10). Furthermore, the gingerols, including [6]-gingerol (Figure 1), a phenolic compound present in ginger root, have been shown to have chemopreventive effects that are associated with their antioxidative and anti-inflammatory activities (11). However, the precise mechanism by which ginger and its chemical constituents exert their chemopreventative effects has not been fully elucidated. Thus, in light of the association between H. pylori and both gastric and colon cancers, we assessed the in vitro susceptibility of 19 strains of H. pylori (including 5 CagA+ strains) to a methanol extract of dried ginger rhizome and some isolated gingerols, as well as [6]-shogoal, a dehydration product of 6-gingerol.
Figure 1.
Structures of the gingerols and shogoal from ginger rhizome extract.
Materials and Methods
Dried ginger rhizomes were obtained from Frontier Natural. Products, Norway, Iowa, USA. The dried and milled whole rhizomes (1.05 kg) were extracted by maceration with MeOH three times (3 × 2 L) at room temperature, for 2 days each. After filtration and evaporation of the solvent under reduced pressure, the combined crude methanol extract (36.1 g) was subjected to column chromatography. The extract was chromatographed over a Silica gel column (7 × 68 cm, 230–400 mesh, 1 kg) and eluted with gradient mixtures of chloroform-MeOH, giving four fractions (F1–F6). the four fractions were evaluated in the HP assay, and the MIC (µg/ml) values of F1–F6 were > 100, 12.5, > 100, > 100, 100 and 100, respectively. Fraction 2 contained all of the activity and HPLC analysis indicated the presence of gingerols (6-, 8-, 10-) and 6-shogoal (Figure 1).
For gingerol isolation and identification, the crude methanol extract was extracted with 300 ml of methylene chloride for 5 minutes and then filtered through a plug of glass wool and centrifuged for 30 minutes at 10,000 rpm at 4°C, as previously described (12). The aqueous layer was removed and the methylene chloridc layer was filtered and dried using a stream of nitrogen at room temperature. The crude residue was dissolved initially in methylene chloride and then diluted with methylene chloride/hexane (50:50). The compounds in this solution were fractionated by normal phase HPLC as previously described (12). The compounds 6-, 8-, 10-gingerol and 6-shoagol were compared with standards obtained from ChromaDex, Santa Ana, CA, USA.
Stock solutions of the crude methanol extract, fraction 4 and the individual compounds, containing 10 mg/ml of DMSO/water (1:1), were prepared. The extracts and pure compounds were stored at −20°C in sterile borosilicate glass vials. A 10 mg sample of each plant extract or pure compound was tested for antibacterial activity using the HP in vitro susceptibility assay. Susceptibility testing was performed using the agar dilution procedure according to the guidelines described by the National Committee for Clinical Laboratory Standards (13). the extracts were dissolved in methanol and sterile distilled water was used for further serial dilutions of the dissolved plant extracts. Final test concentrations consisted of 100, 50, 25, 12.5, 6.25, 3.125, l.56 and 0.78 µg/ml for each sample. One ml of each concentration was added to 19 ml of molten Mueller-Hinton agar (pH 7.3) supplemented with 10% sterile defibrinated horse blood. Growth control plates, consisting of 20 ml of agar medium, were included in each experiment. Petri plates incorporating minimal to maximum volumes of vehicle solvent were included as a growth control to ensure the viability of the organisms was not affected by the solvent used to dissolve the plant extracts. For quality control and comparative analyses, the antibiotic amoxicillin was also tested with each batch of plant extracts.
A total of 14 clinical isolates, namely assession numbers: A2, A6, Ed, 002, 019A, 1022, 1050, 1058, 1060, 1080, 1153, 1175, 1452, 4126 and the ATCC 43504 (Rockville, MD, USA) possessing the CagA+ gene and expressing vacuolating cytotoxin of H. pylori, were used in the susceptibility testing. The other four CagA+ strains, assession numbers: M23-3, GTD7-13, G1-1 (10) and SSI (Sydney Strain CagA+), were provided by Dr. Richard Peek, Division of Gastroenterology and Infectious Disease, Department of Pathology, Vanderbilt University School of Medicine, Nashville, TN, USA. The clinical strains were encoded to protect the identity of the patient from which they were obtained. Some of the isolates were obtained from the Microbiology Laboratory at the University of Illinois Medical Center (Chicago, IL, USA), Abbott Laboratories (Abbott Park, IL, USA), and Dr. D.Y. Graham (Houston, TX, USA). The isolates obtained from Abbott Laboratories include organisms obtained from patients in Richmond, VA; Charlottesville, VA; Nashville, TN, USA; and Southampton, England. Clinical isolates were obtained from different geographic regions to ensure that the organisms were genetically distinct. Gram stain appearance and a positive urease test confirmed the identification of each organism. The organisms were stored frozen at −70°C in skimmed milk plus 17% glycerol.
For susceptibility testing, the organisms were inoculated onto 5% sheep blood agar plates and incubated at 37°C in a 10%, CO2 atmosphere for 72 hours. The organisms were then sub-cultured once to ensure reliable growth. An inoculum of each isolate was prepared by suspending the organism in 4.5 ml of sterile Mueller-Hinton broth and adjusting the turbidity to that of a 2.0 McFarland Standard using a spectrophotometer at 625 nm. This density produces a suspension of approximately 1 × 108 CFU/ml of H. pylori. The organisms were inoculated onto agar plates containing consecutive dilutions of the plant extracts via a 32-prong inoculating device. The device delivers 8 µl per spot resulting in a final inoculum of approximately 1 × 106 CFU/spot. After the spots had dried, the plates were incubated at 37°C in 10% CO2 and examined for growth after 3 days. All procedures were performed in duplicate. The minimum inhibitory concentration (MIC), defined as the lowest concentration of the compound at which there was no visible growth or only a faint haze, was determined for each plant extract and pure compound.
Results
The crude methanol extract of ginger rhizome inhibited the growth of the 14 clinical isolates, 4 CagA+ strains, as well as the ATCC-43504 strain H. pylori strains with an MIC of 25.0 µg/ml (range 6.25 to 50.0 µg/ml). The MIC range for amoxicillin was 0.0039 to 0.25 µg/ml. Fraction 2 of the chloroform extract contained all of the activity and had overall MIC of 12.5 µg/ml in the 19 HP strains (range 0.78 to 50.0 µg/ml). The control drug amoxicillin had an MIC range of 0.0039 to 0.25 µg/ml. Since fraction 2 consisted of four major chemical constituents, 6-, 8-, 10-gingerol and 6-shoagol, in a concentration of 7.6%, 1%, 13.3% and 2.0%, respectively (as determined by quantitative HPLC), each of these compounds was individually tested for activity against the 19 HP strains. 6-Gingerol, (l-[4’-hydroxy-3’-methoxyphenyl]-5-hydroxy-3-decanone), was active against all 19 strains of HP with an MIC of 12.5 µg/ml (range 3.125 to 100.0 µg/ml). 8-Gingerol had an overall MIC of 12.5 µg/ml, with a range of 3.125–100.0 µg/ml. 10-Gingerol was the most active, with an overall MIC of 6.25 µg/ml, range 0.78 to 50.0 µg/ml. 6-Shogoal, the dehydration product of 6-gingerol, was active only in higher concentrations with an MIC of 25.0 µg/ml (range 12.5 to 100.0 µg/ml).
However, when the resultant MIC data was analyzed specifically for CagA+ HP strains, the results demonstrated that the active fraction F-2 showed superior activity against the 5 CagA+ strains than the clinical HP strains used, with an MIC of 1.56 µg/ml (range 0.78–12.5 µg/ml) (Table I). Complete (100%) growth inhibition of all 5 CagA+ strains was observed at a concentration of 6.25 µg/ml, and thus these data demonstrate that ginger extracts have superior activity against HP strains containing the CagA+ cassette. The gingerols and shogoal were active against all CagA+ strains (Table I). Interestingly, 6-shogoal, the dehydration product of 6-gingerol, was not as active as the gingerols in this assay, indicating that degradation of the compound results in lowered activity.
Table I.
Minimum inhibitory concentrations of ginger extracts and gingerols in 5 CagA+ strains of Helicobacter pylori.
| HP Strain 43504 |
M23-3 | GTD7-13 | G1-1 | SS1 | A T C C |
|---|---|---|---|---|---|
| Ginger root-MeOH | 6.25 | 25.0 | 6.25 | 6.25 | 25.0 |
| Methanol-F2 | 0.78 | 6.25 | 1.56 | 0.78 | 6.25 |
| 6-gingerol | 6.25 | 12.0 | 12.5 | 3.125 | 6.25 |
| 8-gingerol | 3.125 | 6.25 | 6.25 | 3.125 | 6.25 |
| 10-gingerol | 1.56 | 6.25 | 1.56 | 1.56 | 6.25 |
| 6-shogoal | 12.5 | 25.0 | 12.5 | 12.5 | 25.0 |
Discussion
For thousands of years ginger root has been used in traditional medicine to treat gastrointestinal disorders, including dyspepsia, peptic ulcer, motion sickness and inflammatory disorders (9). However, more recently, ginger extracts and the gingerols have been shown to have potential chemopreventative activities. Gingerol inhibits tumor promotion in mouse skin, inhibits neoplastic transformation and activation of AP-1 in mouse epidermal JB6 cells treated with epidermal growth factor, suppresses proliferation of human cancer cells through the induction of apoptosis and abrogates pulmonary metastasis in mice implanted with B16F10 melanoma cells (15–19). Furthermore, dietary administration of gingerol to rodents ameliorated azoxvmethane-induced intestinal tumorigenesis (20). However, very little is known about the mechanisms by which ginger or its chemical constituents exert these effects. Ginger has a number of biological activities that may indirectly explain its activity including antioxidant effects, anti-inflammatory activity, prevention of AP-1 activation which is required for neoplastic transformation of cells, inhibition of prostaglandin biosynthesis, as well as inhibition of the activity and expression of cyclooxygenase (9, 11, 19, 21). However, considering the well established use of ginger for the treatment of gastrointestinal ailments, and the strong association between H. pylori and gastric and colon cancer, we hypothesized that ginger may exert its chemopreventative effects by directly inhibiting the growth of H. pylori, particularly the CagA+ strains. The data presented in this investigation shows that ginger extracts and the gingerols inhibit the growth of 19 clinical strains of H. pylori in vitro, and the active fraction containing the gingerols and 6-shogoal was very effective in inhibiting the growth of H. pylori CagA+ strains. These data provide a direct mechanism of action for ginger and further support its role as a chemopreventative agent.
Acknowledgements
We gratefully acknowledge the generous gift of the HP CagA+ strains from Dr. Richard Peck and Dr. Dawn Israel, Department of Pathology, Vanderbilt University School of Medicine, Nashville, TN, USA. NCCAM/NIH grant AT00412-02 made this publication possible.
References
- 1.IARC monographs on the evaluation of carcinogenic risks to humans. Vol. 61. Lyon, France: International Agency for Research on Cancer; 1994. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, Schistomsomes, Liver Flukes and Helicobacter pylori. Infections with Helicobacter pylori; pp. 177–201. [Google Scholar]
- 2.Correa P. Bacterial infections as a cause of cancer. J Natl Cancer Inst. 2002;95:E3. doi: 10.1093/jnci/95.7.e3. [DOI] [PubMed] [Google Scholar]
- 3.Scheiman JM, Cutler AF. Helicobacter pylori and gastric and cancer. Am J Med. 1999;106:222–226. doi: 10.1016/s0002-9343(98)00393-3. [DOI] [PubMed] [Google Scholar]
- 4.Figueiredo C, Machado JC, Pharoah P. Helicobacter pylori and interleukin 1 genotyping: an opportunity to identify high-risk individuals for gastric carcinoma. J Natl Cancer Inst. 2002;94:1680–1687. doi: 10.1093/jnci/94.22.1680. [DOI] [PubMed] [Google Scholar]
- 5.Breuer-Katschinski B, Nemes K, Marr A, Rump B, Leiendecker B, Breuer N, Goebell H. Helicobacter pylori and the risk of colonic adenomas. Colorectal Adenoma Study Group. Digestion. 1999;60:210–215. doi: 10.1159/000007661. [DOI] [PubMed] [Google Scholar]
- 6.Shmuely H, Passaro D, Figer A, Niv Y, Pitlik S, Samra Z, Koren R, Yahav J. Relationship between Helicobacter pylori CagA status and colorectal cancer. Am J Gastroenterol. 2001;96:3406–3410. doi: 10.1111/j.1572-0241.2001.05342.x. [DOI] [PubMed] [Google Scholar]
- 7.Censini S, Lange C, Xiang Z. Cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease associated virulence factors. Proc Natl Acad Sci USA. 1996;93:14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bone K. Ginger Brit. J Phytother. 1997;4:110–120. [Google Scholar]
- 9.Farnsworth RF, Fong HHS, Mahady GB. WHO Monographs on Selected Medicinal Plants. Volume 1. Geneva, Switzerland: WHO Publications; 1999. Rhizoma Zingiberis. [Google Scholar]
- 10.Fischer-Rasmussen W. Ginger treatment of hyperemesis gravidarum. Eur J Obs Gyn Reprod Biol. 1991;38:19–24. doi: 10.1016/0028-2243(91)90202-v. [DOI] [PubMed] [Google Scholar]
- 11.Surh YJ. Anti-tumor promoting potential of selected spice ingredients with antioxidative and anti-inflammatory activities: a short review. Food Chem Toxicol. 2002;40:1091–1097. doi: 10.1016/s0278-6915(02)00037-6. [DOI] [PubMed] [Google Scholar]
- 12.Hiserodt RD, Franzblau SG, Rosen RT. Isolation of 6-, 8- and 10-gingerol from ginger rhizome by HPLC and preliminary evaluation of inhibition of Mycobacterium avium and Mycobacterium tuberculosis. J Agrie Food Chem. 1998;46:2504–2508. [Google Scholar]
- 13.NCCLS. NCCLS document M100-S9. Wayne, PA: National Committee for Clinical Laboratory Standards; 1999. Performance Standards for Antimicrobial Susceptibility Testing: Ninth Informational Supplement. [Google Scholar]
- 14.Katiyar SK, Agarwal R, Mukhtar H. Inhibition of tumor promotion in SENCAR mouse skin by ethanol extract of Zingiber officinale rhizome. Cancer Res. 1996;56:1023–1030. [PubMed] [Google Scholar]
- 15.Lee E, Surh YJ. Induction of apoptosis in HL-60 cells by pungent vanilloids, [6]-gingerol, and [6]-paradol. Cancer Lett. 1998;134:163–168. doi: 10.1016/s0304-3835(98)00253-5. [DOI] [PubMed] [Google Scholar]
- 16.Lee E, Park KK, Lee JM, Chun KS, Kang JY, Lee SS, Surh YJ. Suppression of mouse skin tumor promotion and induction of apoptosis in HL-60 cells by Alpinia oxyphylla Miquel (Zingiberaceae) Carcinogenesis (Lond) 1998;19:1377–1381. doi: 10.1093/carcin/19.8.1377. [DOI] [PubMed] [Google Scholar]
- 17.Bode AM, Wei-Ya Ma, Surh YJ, Dong ZG. Inhibition of epidermal growth factor-induced cell transformation and activator protein 1 activation by [6]-gingerol. Cancer Res. 2001;61:850–853. [PubMed] [Google Scholar]
- 18.Huang C, Ma WY, Dong Z. Requirement for phosphatidylinositol 3-kinase in epidermal growth factor-induced AP-l transactivation and transformation in JB6 P + cells. Mol Cell Biol. 1996;16:6427–6435. doi: 10.1128/mcb.16.11.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Surh YJ. Anti-tumor promoting potential of selected spice ingredients with antioxidative and anti-inflammatory activities: a short review. Food Chem Toxicol. 2002;40:1091–1097. doi: 10.1016/s0278-6915(02)00037-6. [DOI] [PubMed] [Google Scholar]
- 20.Yoshimi N, Wang A, Morishita Y, Tanaka T, Sugie S, Kawai K, Yamahara J, Mori H. Modifying effects of fungal and herb metabolites on azoxymethane-induced intestinal carcinogenesis in rats. Jpn J Cancer Res. 1992;83:1273–1278. doi: 10.1111/j.1349-7006.1992.tb02758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park EJ, Pezzuto JM. Botanicals in cancer chemoprevention. Cancer Metastasis Rev. 2002;21:231–255. doi: 10.1023/a:1021254725842. [DOI] [PubMed] [Google Scholar]