Abstract
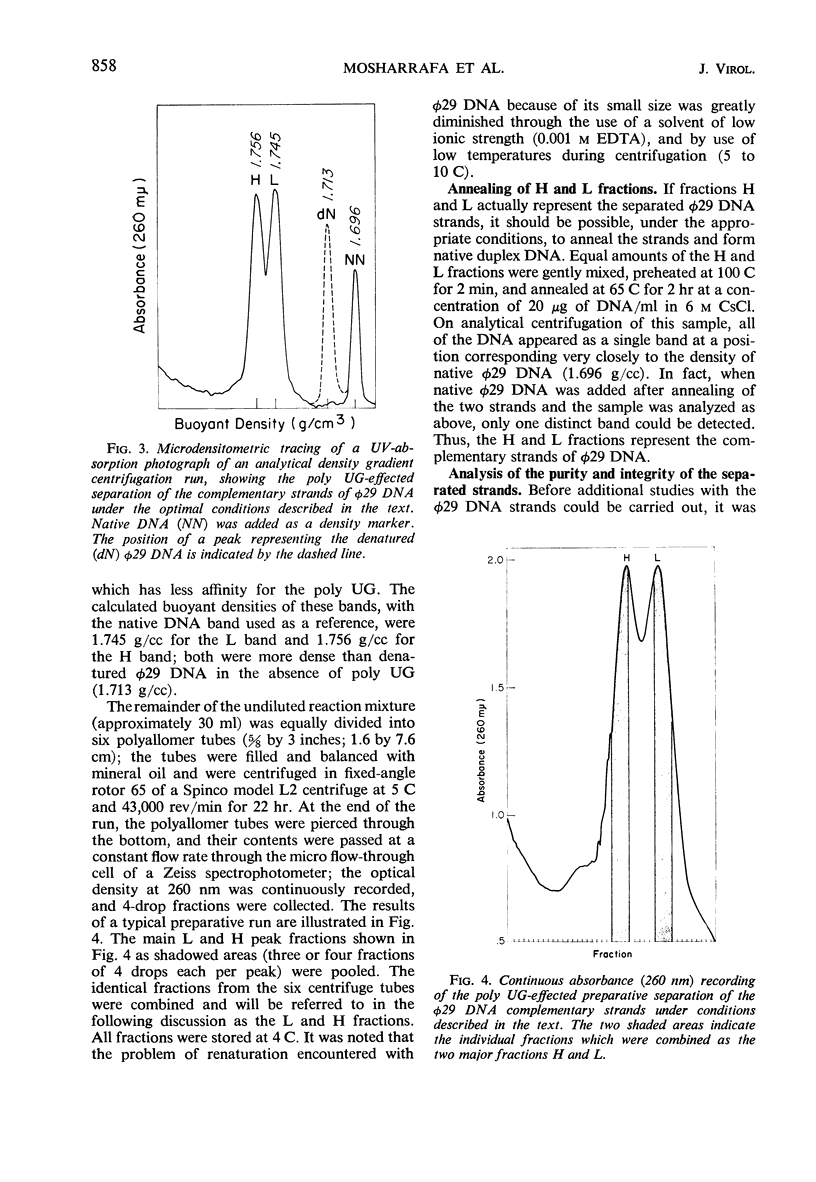
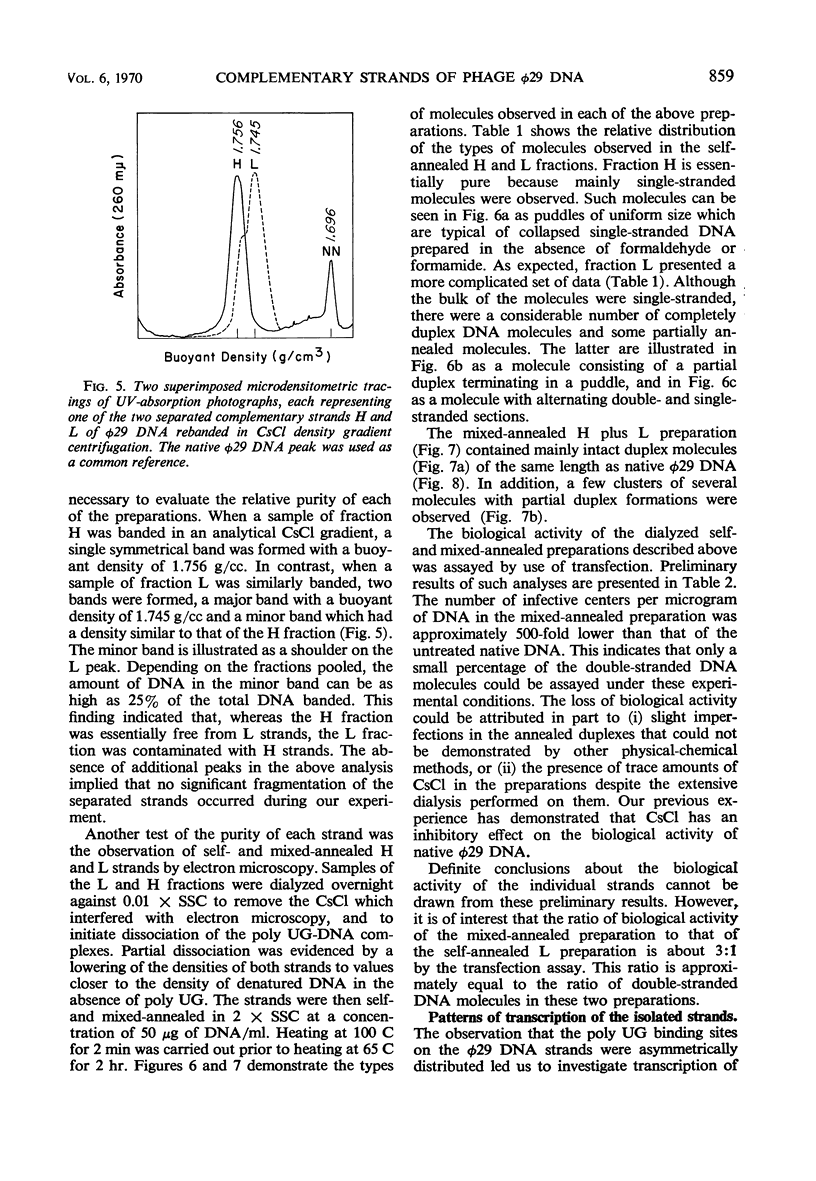
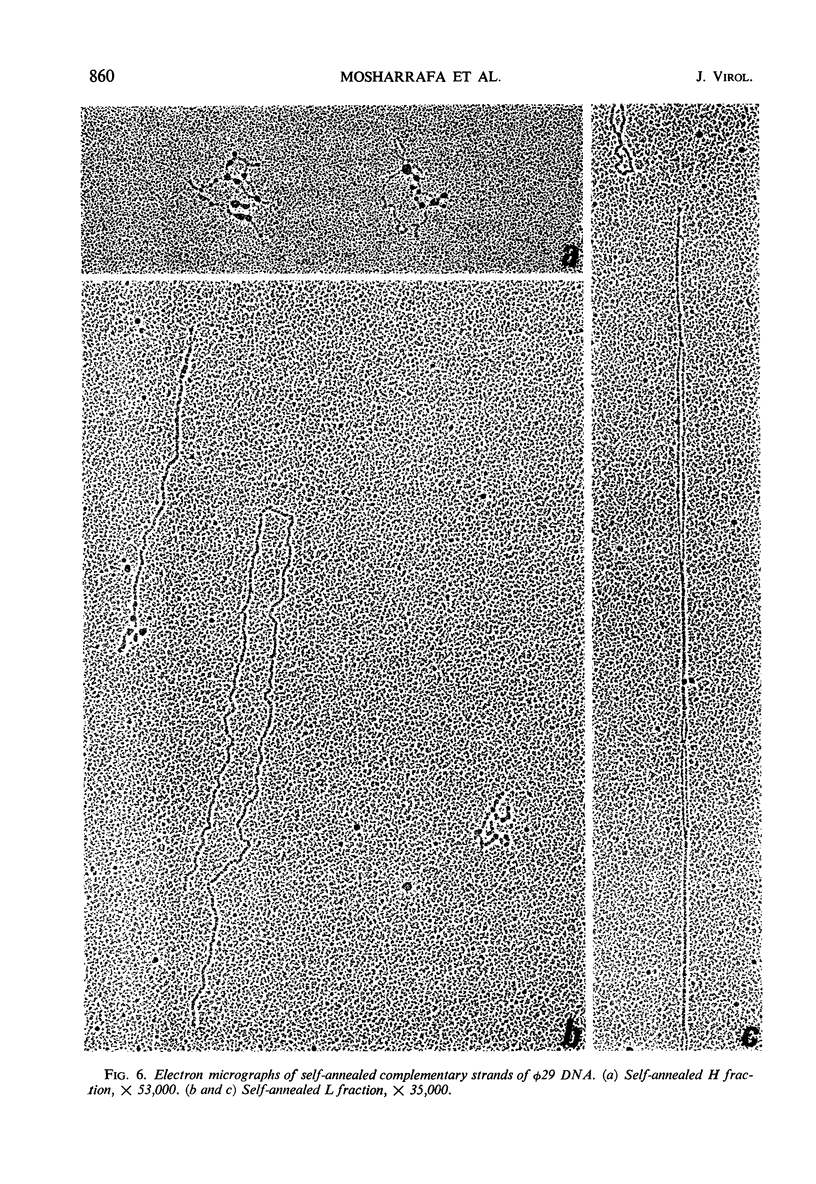
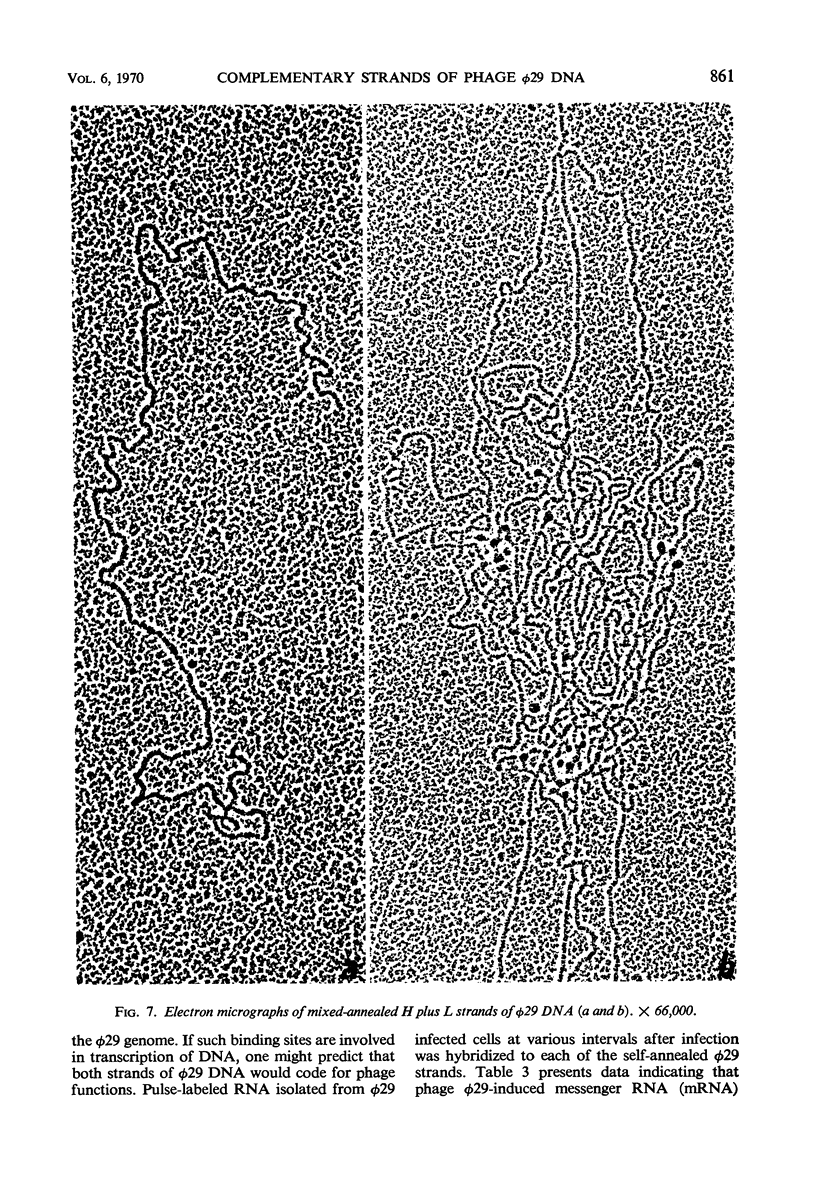
Bacillus subtilis phage φ29 has a nonpermuted, duplex deoxyribonucleic acid (DNA) with cohesive ends and a molecular weight of 11 × 106. Denaturation of this DNA yielded two intact polynucleotide chains. Preferential binding of the polyribonucleotide polyuridylic-guanylic acid (poly UG) to the complementary strands of denatured φ29 DNA permitted separation of the strands in neutral CsCl gradients. In analytical CsCl density gradient centrifugation, the separated strands with poly UG appeared as two symmetrical bands, both heavier than the normal denatured DNA band. The strands differed in density by 11 mg/cc. Preparative separation of the φ29 DNA strands resulted in two fractions, heavy (H) and light (L). The H fraction was essentially free from L contamination, whereas L contained up to 25% of H, as determined both by rebanding the separated fractions in CsCl and by electron microscopic examination of self- and mixed-annealed fractions. Pulse-labeled ribonucleic acid (RNA) prepared at intervals after infection was hybridized with the self-annealed DNA strands. Preliminary experiments indicated that both strands of φ29 DNA are transcribed during the development of the virus. Early transcribed φ29-specific RNA hybridizes only with the L strand; at later times, transcription occurs from both the L and H strands.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. L., Hickman D. D., Reilly B. E. Structure of Bacillus subtilis bacteriophage phi 29 and the length of phi 29 deoxyribonucleic acid. J Bacteriol. 1966 May;91(5):2081–2089. doi: 10.1128/jb.91.5.2081-2089.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. L., Mosharrafa E. T. Physical and biological properties of phage phi 29 deoxyribonucleic acid. J Virol. 1968 Oct;2(10):1185–1190. doi: 10.1128/jvi.2.10.1185-1190.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal N., Kleinschmidt A. K., Spatz H. C., Trautner T. A. Physical properties of the DNA of bacteriophage SP50. Mol Gen Genet. 1967;100(1):39–55. doi: 10.1007/BF00425774. [DOI] [PubMed] [Google Scholar]
- CORDES S., EPSTEIN H. T., MARMUR J. Some properties of the deoxyribonucleic acid of phage alpha. Nature. 1961 Sep 9;191:1097–1098. doi: 10.1038/1911097b0. [DOI] [PubMed] [Google Scholar]
- GREEN D. M. INFECTIVITY OF DNA ISOLATED FROM BACILLUS SUBTILIS BACTERIOPHAGE, SP82. J Mol Biol. 1964 Dec;10:438–451. doi: 10.1016/s0022-2836(64)80065-6. [DOI] [PubMed] [Google Scholar]
- Guha A., Szybalski W. Fractionation of the complementary strands of coliphage T4 DNA based on the asymmetric distribution of the poly U and poly U,G binding sites. Virology. 1968 Apr;34(4):608–616. doi: 10.1016/0042-6822(68)90082-2. [DOI] [PubMed] [Google Scholar]
- HAYASHI M., SPIEGELMAN S. The selective synthesis of informational RNA in bacteria. Proc Natl Acad Sci U S A. 1961 Oct 15;47:1564–1580. doi: 10.1073/pnas.47.10.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogness D. S., Doerfler W., Egan J. B., Black L. W. The position and orientation of genes in lambda and lambda dg DNA. Cold Spring Harb Symp Quant Biol. 1966;31:129–138. doi: 10.1101/sqb.1966.031.01.020. [DOI] [PubMed] [Google Scholar]
- KENNELL D. PERSISTENCE OF MESSENGER RNA ACTIVITY IN BACILLUS MEGATERIUM TREATED WITH ACTINOMYCIN. J Mol Biol. 1964 Sep;9:789–800. doi: 10.1016/s0022-2836(64)80185-6. [DOI] [PubMed] [Google Scholar]
- KLEINSCHMIDT A. K., LANG D., JACHERTS D., ZAHN R. K. [Preparation and length measurements of the total desoxyribonucleic acid content of T2 bacteriophages]. Biochim Biophys Acta. 1962 Dec 31;61:857–864. [PubMed] [Google Scholar]
- Lozeron H. A., Szybalski W. Congruent transcriptional controls and heterology of base sequences in coliphages lambda and phi-80. Virology. 1969 Nov;39(3):373–388. doi: 10.1016/0042-6822(69)90085-3. [DOI] [PubMed] [Google Scholar]
- MARMUR J., GREENSPAN C. M. TRANSCRIPTION IN VIVO OF DNA FROM BACTERIOPHAGE SP8. Science. 1963 Oct 18;142(3590):387–389. doi: 10.1126/science.142.3590.387. [DOI] [PubMed] [Google Scholar]
- NYGAARD A. P., HALL B. D. FORMATION AND PROPERTIES OF RNA-DNA COMPLEXES. J Mol Biol. 1964 Jul;9:125–142. doi: 10.1016/s0022-2836(64)80095-4. [DOI] [PubMed] [Google Scholar]
- REILLY B. E., SPIZIZEN J. BACTERIOPHAGE DEOXYRIBONUCLEATE INFECTION OF COMPETENT BACILLUS SUBTILIS. J Bacteriol. 1965 Mar;89:782–790. doi: 10.1128/jb.89.3.782-790.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva S. C. Asymmetric transcription of B. subtilis phage SPP1 DNA in vitro. Biochem Biophys Res Commun. 1969 Mar 31;34(6):824–830. doi: 10.1016/0006-291x(69)90254-x. [DOI] [PubMed] [Google Scholar]
- Riva S., Polsinelli M., Falaschi A. A new phage of Bacillus subtilis with infectious DNA having separable strands. J Mol Biol. 1968 Jul 28;35(2):347–356. doi: 10.1016/s0022-2836(68)80029-4. [DOI] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Schachtele C. F., Oman R. W., Anderson D. L. Effect of elevated temperature on deoxyribonucleic acid synthesis in bacteriophage phi-29-infected Bacillus amyloliquefaciens. J Virol. 1970 Oct;6(4):430–437. doi: 10.1128/jvi.6.4.430-437.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtele C. F., Rogers P. Canavanine death in Escherichia coli. J Mol Biol. 1965 Dec;14(2):474–489. doi: 10.1016/s0022-2836(65)80197-8. [DOI] [PubMed] [Google Scholar]
- Summers W. C. Effect of size and aggregation of guanine-rich polyribonucleotides on the practical aspects of DNA strand separation in CsCl density gradients. Biochim Biophys Acta. 1969 May 20;182(1):269–272. doi: 10.1016/0005-2787(69)90548-6. [DOI] [PubMed] [Google Scholar]
- Szybalski W., Kubinski H., Sheldrick P. Pyrimidine clusters on the transcribing strand of DNA and their possible role in the initiation of RNA synthesis. Cold Spring Harb Symp Quant Biol. 1966;31:123–127. doi: 10.1101/sqb.1966.031.01.019. [DOI] [PubMed] [Google Scholar]
- Taylor K., Hradecna Z., Szybalski W. Asymmetric distribution of the transcribing regions on the complementary strands of coliphage lambda DNA. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1618–1625. doi: 10.1073/pnas.57.6.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westmoreland B. C., Szybalski W., Ris H. Mapping of deletions and substitutions in heteroduplex DNA molecules of bacteriophage lambda by electron microscopy. Science. 1969 Mar 21;163(3873):1343–1348. doi: 10.1126/science.163.3873.1343. [DOI] [PubMed] [Google Scholar]





