Abstract
Prebiotic oligosaccharides, with a degree of polymerization (DP) of mostly less than 10, exhibit diverse biological activities that contribute to human health. Currently available prebiotics are mostly derived from disaccharides and simple polysaccharides found in plants. Subtle differences in the structures of oligosaccharides can cause significant differences in their prebiotic proper-ties. Therefore, alternative substances supplying polysaccharides that have more diverse and complex structures are necessary for the development of novel oligosaccharides that have actions not present in existing prebiotics. In this review, we show that structural polysaccharides found in plant cell walls, such as xylans and pectins, are particularly potential resources supplying broadly diverse polysaccharides to produce new prebiotics.
Keywords: Prebiotics, Oligosaccharides, Plant cell wall, Complex polysaccharides, Xylans, Pectins
INTRODUCTION
Since Japanese researchers had demonstrated that specif-ic non-digestible oligosaccharides were selectively fermented by bifidobacteria and had the capacity, upon feeding, in stimu-lating their growth in human faeces in the 1980’s, prebiotic oligosaccharides have attracted the interest of industrial as well as academic scientists (Mitsuoka et al., 1987; Roberfroid et al., 2010). Prebiotics is defined as ‘a selectively fermented ingredient that results in specific changes, in the composition and/or activity of the gastrointestinal mibrobials, thus confer-ring benefit(s) upon host health’ by International Scientific As-sociation for Probioitics and Prebiotics (ISAPP) (6th meeting in Ontario, Canada, November 2008). Disaccharides, such as lactose and sucrose, and storage polysaccharides in plants, such as starch and fructans, are important resources for pro-ducing prebiotic oligosaccharides that are applied to the food and drink industry or towards investigating biological activities related to human health (Crittenden and Playne, 1996; Nauta and Schoterman, 2009). Oligosaccharides are categorized as prebiotics when they fulfill the following 3 criteria (Gibson and Roberfroid, 1995; Lomax and Calder, 2009): (1) resistance to hydrolysis or absorption in the upper gastrointestinal tract, (2) fermentation by intestinal microbials, or (3) selective stimula-tion of the growth and/or activity of beneficial intestinal microbials classified into probiotics defined as ‘living non-pathogen-ic micro-organisms, which as food ingredients (supplements) also beneficially affect host’s health’. These microbials may be lactic acid bacteria, such as lactobacilli, lactococci and bifido-bacteria, or yeasts such as Saccharomyces cerevisiae sub-spp. boulardii (Bongaerts et al., 2005, Roberfroid et al., 2010).
According to the definition of prebiotics, prebiotics appears to be merely food additives or nutraceuticals. However, pre-biotics causes intestinal fermentation and the promotion of growth of beneficial gut microbials, these compound could improve host health through diverse direct or indirect biologi-cal activities, including the stimulation of host immunity and protection against pathogens, as well as facilitating mineral absorption by hosts (Gibson and Roberfroid, 1995; Lenoir-Wijinkoop et al., 2007). In addition to these general activities of prebiotics, some prebiotic oligosaccharides have other bio-logical activities, such as anti-inflammatory and anticarcino-genic properties (Nurmi et al., 2005). Therefore, prebiotics has become a popular research topic in the biomedical fields re-cently (Roberfroid et al., 2010) and the potentiality of prebiot-ics as therapeutics is increased. For a simple example, for the treatement of hepatic encephalopathy, prebiotics associated with probitcs might be an alternative therapeutics to reduce ammonia production by urease-positive bacteria Sloga in-stead of antibiotics resulting in survival of ammonia-producing antibiotic-resistant bacteria (Bongaerts et al., 2005).
Subtle differences in structure, such as changes in size and the presence of branch(s) and differences in the complexity of the substituents, can cause significant changes in the prebi-otic properties of oligosaccharides (Kabel et al., 2002a; Kabel et al., 2002b; Holck et al., 2011). Most currently available pre-biotics are derived from disaccharides and simple polysaccha-rides. Therefore, to develop novel prebiotics with other biologi-cal activities, which are not present in existing prebiotics, an alternative resource of polysaccharides is required that sup-plies oligosaccharides with more diverse and complex struc-tures. In this context, heteropolysaccharides, which form plant cell walls, are ideal substances to supply polysaccharides with more diverse and complex structures. The exact nature and relative abundance of each component of the plant cell wall, including xylans and pectins, varies according to plant spe-cies, as well as the types of tissues and organs used (Ebring-erova and Heinze, 2000; Bauer et al., 2006; Popper, 2008). Furthermore, within a plant, the composition of oligosaccha-rides varies dynamically according to developmental stages (Ebringerova and Heinze, 2000), as well as environmental factors, such as temperature and pathogens (Zablackis et al.,1995).
In this paper, we discuss the potential application of 2 class-es of heteropolysaccharides of plant cell walls, xylans and pectins, as substances to supply diverse polysaccharides. In addition, we review the general process used to produce oli-gosaccharides from polysaccharides, including the fragmen-tation of polysaccharides, as well as the separation and iden-tification of resultant oligosaccharides. The bioassays used to select prebiotics among available oligosaccharides are not considered within the scope of this review.
BIOLOGICAL ACTIVITIES OF PREBIOTICS
Selective stimulation of beneficial bacteria in the gut
In the gut, the selective nature of prebiotics stimulates in-digenous beneficial bacteria (Saulnier et al., 2009). For exam-ple, β-(2-1) fructans, one of prebiotics stimulates the growth of Lactobacillus and Bifidobacterium species (Kruse et al., 1999; Kleessen et al., 2001). In addition, β-(2-1) fructans is selec-tively fermented by Lactobacillus species (Kaplan and Hut-kins, 2000) and Bifidobacterium species (Gibson et al., 1995). These bacteria produce enzymes, such as intracellular fructo-sylfructofuranosidase, which hydrolyzes the β-(2-1) glycosidic bond in β-(2-1) fructans. Such fermentation by beneficial bac-terial leads to the production of short chain fatty acids (SCFA), such as butyrate. Recent studies have shown that other gut species, such as Faecalibacterium prausnitzii, also have the ability to degrade prebiotic oligosaccharides (Ramirez-Farias et al., 2009). Regarding selectivity of prebiotics, it is possible probiotics have enzymes for hydrolysis and fermentation, as well as a mechanism of oligosaccharide up-take, such as transporters of specific oligosaccharides. By increasing the number of beneficial probiotics, prebiotics help outcompete pathogenic bacteria for nutrients and binding sites on the in-testinal epithelium, thus suppressing the survival of the patho-genic strains (Lomax and Calder, 2009). Furthermore, probiot-ics are able to produce antibacterial substances that inhibit the growth and survival of pathogens (Gibson and Wang, 1994). Through altering bacterial flora in the intestines, pathogenic bacteria become less abundant (Kruse et al., 1999; Kleessen et al., 2001).
The fermentation products of prebiotics, such as SCFAs, cause the acidification of the colonic environment, which is detrimental to some pathogenic bacteria (Blaut, 2002). Fur-thermore, this acidification process improves mucosal mor-phology by increasing mucin production, as well as decreasing pathogenic bacteria colonization and translocation (Barcelo et al., 2000).
Ultimately, because of the stimulation of probiotic growth and fermentation, prebiotics might contribute to host health, particularly with respect to defense against pathogenic bac-teria.
Stimulation of host immunity
An increase in the number of beneficial microbials by prebi-otics stimulates host immune cells. It has been suggested that bacteria might cross the host intestinal barrier onto the Peyer’s patch, and activate immune cells in that region. In contrast, it has been suggested that microbial substances, such as bac-terial cell wall components, rather than micro-organisms, act as the stimulating factor (Berg, 1985; De Simone et al., 1987). Besides, prebiotics may influence the immune system directly. Although there is currently little evidence, the potential direct ligation of pattern recognition receptors on immune cells by prebiotic oligosaccharide may result in immunomodulation (Brown and Gordon, 2001; Herre et al., 2004; Roberfroid et al., 2010). Fermentation product of prebiotics, SCFAs, such as butylate, might participate in the stimulation of host immu-nity. Butylate bound to SCFA receptors, including GPR41 and GPR43 on leukocytes, particularly neutrophils, within the gut-lymphoid tissues (GALT) may regulate leukocyte function in the immune system (Brown et al., 2003; Nilsson et al., 2003). Alternatively, SCFA might alter the signaling of epithelial cells to the mucosal immune system (Sanderson, 2007). Finally, butylate alters epithelial cell gene expression, such as IL-8 and monocyte chemo-attractant protein 1. Regarding to the gene expression participating in immunemodulating, some genes such as major histocompatibility complex class I and II, interferon and phosphatidulinositol metabolites participat-ing in the intestinal immunemodulating were identifi ed by DNA microarray analysis in mice fed with prebiotics manufactured from sucrose (Fukasawa et al., 2007).
Prevention of pathogen adherence to the epithelial mem-brane of the gut
Some oligosaccharides bind to receptors on pathogenic bacteria and prevent them from attaching to the same sugar on the epithelial membrane of the gut (Ouwehand et al., 2005; Shoaf-Sweeney and Hutkins, 2009). For example, galacto-oligosaccharide reduces the adherence of enteropathogenic E. coli to cultured cells, such as HeP cells (Shoaf et al., 2006).
Promotion of mineral absorption in the gut
Prebiotics promote host absorption of minerals, such as Ca2+ and Mg2+ ion. For example, β-(2-1) fructans promotes the absorption of Ca2+ by the colonic mucosa in human (Abrams et al., 2007). For Ca2+ absorption, SCFA contributed to lower luminal pH in the large intestine which, in turn, elicits a modi-fication of Ca2+ speciation and hence solubility in the luminal phase so that its passive diffusion is improved (Lopez et al., 2000). Besides, prebiotics may directly modulate transcellular active Ca2+ transport by increasing expression of the carrier protein, calbindin D9K in the cecum and colorectum (Taka-saki et al., 2000). This promoting effect of prebiotics might be useful in the prevention of osteoporosis (Scholz-Ahrens et al., 2001).
Furthermore, other indirect biological activities of prebi-otics have been reported. For instance, the fermentation products of prebiotics appear to have anti-inflammatory and anticarcinogenic properties on colonic cancer cells and the enterocyte-like cell line Caco-2 (Nurmi et al., 2005; Ewaschuk et al., 2006). Using laboratory animal models, the effects of prebiotics on inflammation were studied. Prebiotic treatment decreased proinflammatory cytokine, IL-1β, but increased im-munomodularoty molecules, TGF-β in cecal tissues of HLA-B27 transgenic rats, which develop spontaneous colitis un-der specific pathogen-free conditions (Hoentjen et al., 2005.) Prebiotics in food reduced the both numbers of cells stained immunehistochemically with monoclonal antibodies to CCR4 and mast cells, and the edema formation rate in the duode-num of ovaalbumin-induced food allergy model Nc/jic mice (Fujitani et al., 2007).
EXISTING PREBIOTIC OLIGOSACCHARIDES
Prebiotic oligosaccharides derived from disaccharides
Many prebiotics that are currently applied to the food in-dustry are mostly derived from disaccharides. These disac-charides are converted to prebiotic oligosaccharides that have a small DP (degree of polymerization) and a simple structure following treatment with enzymes.
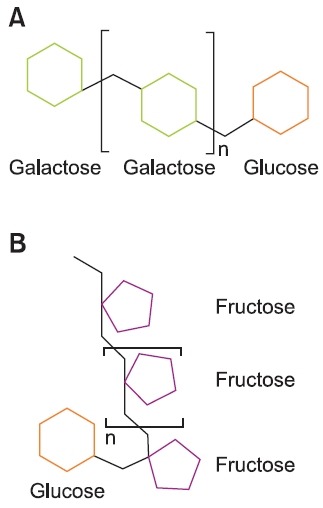
Galacto-oligosaccharides (GOS) are produced from lactose by β-galactosidase mediated hydrolysis and polymerization of β-linked sugars (Nauta and Schoterman, 2009). GOS is one of the most commonly produced prebiotic oligosaccharides. β-Galactosidase reacts with lactose to form an oligosaccha-ride, and liberates a glucose molecule. The successive trans-galactosylations with lactose, or previously formed oligosac-charides, as a donor results in the production of mixtures of GOS with DP up to 8 (Nauta and Schoterman, 2009) (Fig.1A). Interestingly, GOS are similar to oligosaccharides found in human milk, which also appear to have prebiotic activities (Sharon and Ofek, 2000).
Fig. 1. (A) Schematic illustration of galacto-oligosaccharide. (B) Schematic illustration of inulin. n: Degree of polymerization.
Galacto-fructose is a disaccharide made through the isom-erization of lactose (Montgomery and Hudson, 1929). Dur-ing the isomerization process, the glucose unit of lactose is converted into fructose. Since the β-(1-4) glycosidic linkage connecting to galacto-fructose disaccharide is not cleaved by human intestinal enzymes, galacto-fructose disaccharide reaches the colon intact (Tamura et al., 1993).
Lactosucrose is a non-reducing trisaccharide (Gal β-(1-4) Glc α-(1-2) Fru) produced from sucrose and lactose. The fructopuranosyl residue in sucrose is transferred to a glucose moiety of lactose by β-fructofuranosidase (Fujita et al., 1992).
Glycosyl sucrose is a trisaccharide made from sucrose and maltose by cyclomaltodextrin glucanotransferase (Crittenden and Playne, 1996).
Prebiotic oligosaccharides derived from plant storage polysaccharides
Both starch, which is insoluble polyglucan formed in the plastid, and fructans, which is a soluble polyfructan formed in the vacuole as a storage compound (Heldt, 2005), comprise other reliable sources of prebiotics that are currently available
to the food and health industries. The glucose molecules in starch are connected to each other by α-(1-4) and α-(1-6)linkages. In fructans, especially 1-kestose type, the fructose residue of sucrose is connected with other fructoses by β-(2-1) linkages (Heldt, 2005). These simple structured storage poly-saccharides are fragmented and modified into the following prebiotic oligosaccharides by enzymatic processes.
Isomalto-oligosaccharides (IMO) are produced from starch through a two-stage enzymatic process (Crittenden and Playne, 1996). Starch is converted to malto-oligosaccharides by the sequential treatment of α-amylase and β-amylase. Then, α-glucosidase catalyzes the transglycosylation to con-vert the α-(1-4) linkage into a α-(1-6) linkage. While most IMO contain only α-(1-6) linkages with a DP range of 2 to 6, trisac-charide, panose (Glu α-(1-6) Glu α-(1-4) Glu) contains both α-(1-4) and α-(1-6) linkages.
Gentio-oligosaccharides contain α-(1-6) linked glucoses with DP of 2 to 6. They are produced from glucose syrup, which is made from the hydrolysis of starch by enzymatic transglucosylation (Playne and Crittenden, 1996; Wichienchot et al., 2009).
β(2-1) Fructans such as inulin (Fig. 1B) and oligofructose, which are also known as short-chain fructo-oligosaccharides, are fructose polymers that originate from sucrose. β-(2-1) Fructans play several important roles towards enhancing the tolerance of plants against cold and draught (Ritsema and Smeekens, 2003). Inulin is a linear molecule, which contains β-(2-1) fructosyl-fructose linkages with a terminal glucose (De LeenHeer and Hoebregs, 1994). In particular, inulins found in chicory contain β-(2-1) linked fructoses with DP ranges from 2 to 60. In contrast, oligofructoses derived from inulin by en-zymatic hydrolysis contain fructoses with DP from 2 to 7 (De LeenHeer and Hoebregs, 1994).
Plant cell wall polysaccharides
The minor discrepancy in the structures of oligosaccharides results in significant changes in the properties of prebiotic oli-gosaccharides (Kabel et al., 2002a; Kabel et al., 2002b; Holck et al., 2011). Therefore, to develop novel prebiotics, alterna-tive sources of polysaccharides are necessary, from which
oligosaccharides with diverse structures are derived. In this context, hetero-polysaccharide, which forms the plant cell wall, might be of interest.
The plant cell wall contains cells that are unique in the plant. They perform many essential biological roles, such as the regulation of cell expansion, control of tissue attachment, ion exchange, and defense against pathogenic microbes (Popper, 2008). The plant cell wall is composed of various types of poly-saccharides and proteins, as well as lignin, a polymer of phen-ylpropane derivatives such as cumaryl alcohol, coniferyl alco-hol, and sinapyl alcohol. However, polysaccharides constitute the greater part of the plant cell wall. These polysaccharides are often classified into (1) cellulose, which is composed of β-(1-4) linked glucan chains organized in more or less crystal-line microfibrils, (2) hemicelluloses, and (3) pectin (Vázquez et al., 2000; Scheller and Ulvskov, 2010). Both hemicellulose and pectin together constitute a matrix in which cellulose microfi-brils are embedded (Harholt et al., 2010). However, the nature of each cell wall component, including these polysaccharides, varies dynamically according to plant species and the types of organs, as well as the stage of plant development and various environmental factors (Zablackis et al., 1995; Ebringerova and Heinze, 2000; Bauer et al., 2006; Popper, 2008).
Xylans
Hemicelluloses comprise several different polymers, in-cluding xylans, xyloglucans, and (gluco)mannans, which are characterized by a backbone of β-(1-4) linked sugars with an equatorial linkage configuration (Bauer et al., 2006; Scheller and Ulvskov, 2010). Among these compounds, xylans are ma-jor hemicellulosic polysaccharides in the secondary cell walls of dicots and in all types of cell walls of grasses (Faik, 2010). The content of xylans is 25-35% of the dry mass of woody tis-sue of dicots and lignified tissue of monocots. In cereal grains, the content of xylans is up to 50% (Moure et al., 2006). In higher plants, xylans are partially acetylated in the native state (Wende and Fry, 1997). Even if diverse types of xylans (such as those listed below) are present in plants, certain types are common to certain plant families (Ebringerova and Heinze, 2000).
4-O-methyl glucuronoxylan (GX) contains a single side chain of 2-linked-4-O-methyl-α-D-glucopyranosyl uronic acid units (MeGlcA) (Fig. 2). GX is common in the wood of dicots (Ebringerova and Heinze, 2000). The ratio of Xylp to MeGlcA in GX isolated from different woods is 4-16:1 (Timell, 1964).
Fig. 2. Schematic illustration of three types of xylans found in plant cell walls. GX: Glucuronoxylan AGX: Arabino (glucurono) xylan AX: Neutral arabinoxylan Fer: Ferulic acid OAc: Acetylation OMe: Methylation.
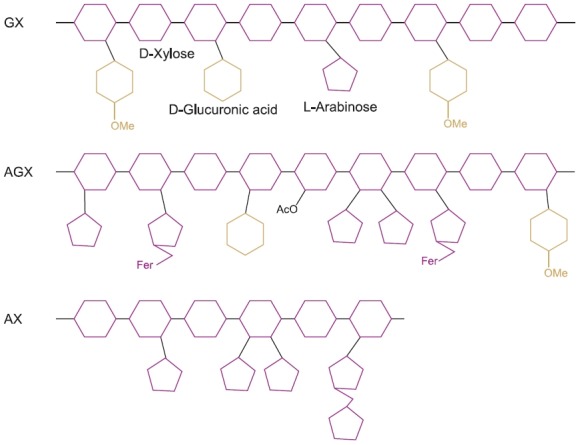
Arabino(glucurono)xylan (AGX) contains a single side chain of 2-O-linked-α-D-glucopyranosyl uronic acid (GlcA) and/or its 4-O-methyl derivative (MeGlcA), in addition to 3-O-linked-α-L-arabinofuranosyl units. AGX is common in gymnosperms and in the supporting tissues of monocots (Ebringerova and Heinze, 2000). Water-soluble AGX contains terminating β-D-xylopyranosyl residue and disaccharide side chains com-posed of 2-O-β-D-xylopyranosyl-α-L-arabinofuranose next to the single Araf and MeGlcA side chain. This disaccharide is usually esterified by ferulic acid at position O-5 of the Araf unit.
Neutral arabinoxylan (AX) has xylofyranosyl residues that are substituted at position 3 and/or at both positions 2 and 3 of Xylp by α-L-arabinofuranosyl units (Fig. 2). In contrast, water insoluble AX contains a relatively low amount of Araf units, which are mainly positioned on mono-substituted Xylp residues. AX is one of the major types of xylans in grasses, and is mainly found in the starch endosperm of cereal grains (Ebringerova et al., 2005; Faik, 2010).
Especially, in sorghum, glucurono(arabino)xylan (GAX) comprises the structural features of AX, and contains disac-charide moieties composed of 2-O-α-L-arabinofuranosy-Larabinofuranose linked at position O-3 to the xylan backbone (Fig. 2) (Ebringerova and Heinze, 2000). In particular, grasses have type II walls that are rich in GAXs (Ebringerova et al., 2005).
Heteroxylan (HX) is the most structurally diverse xylan. The HX backbone is highly branched with Xylp-, Araf-, and Galp-containing monosaccharide, disaccharide, and trisaccharide side chains, as well as phenolic acid, which is mainly ferulic acid esterified to Araf units. HX is commonly found in cereals and seeds (Wikie, 1979).
Pectins
Pectin is a hetero-polysaccharide that con-tains a high content of GlcA units. Various types of pectins, such as homoga-lacturonan (HG), xylogalacturonan (XGA), rhamnogalacturonan I (RG I), and rhamnogalacturo-nan II (RG II), are present in the plant cell wall (Buchholt et al., 2004; Harholt et al., 2010). These types of pectins are not separate polymers, but are covalently linked together. While the ratio in the amounts of these pectins is variable, HG is usually the most abundant type. The content of HG type is up to 65% of total pectin, while GR I type constitute 20-35% (Mohnen, 2008). XGA and RG II are minor components, with each type constituting less than 10% (Zandleven et al., 2007; Mohnen, 2008).
Homogalacturonan (HG) is a linear homologous chain ofα-(1-4) linked GlcAs (Fig. 3). GlcA residues in the galacturo-nan backbone are substituted at various positions with sugar moieties, such as Xylp and apiofuranose (Harholt et al., 2010). In the pure galacturonan backbone, GlcA is further modified by methyl esterifi cation at the C-6 carboxyl position and by O-acetylation at the O-2 or O-3 position.
Fig 3. Schematic illustration of four types of pectins found in plant cell walls. HG: Homogalacturonan RG I: Rhamnogalacturonan I XGA: Xylogalacturonan RG II: Rhamnogalacturonan II Kdo: 3-Deoxy-D-manno-2-octulosonic acid DHA: 3-Deoxy-D-lyxo-2-heptulosaric acid OAc: Acetylation OMe: Methylation.
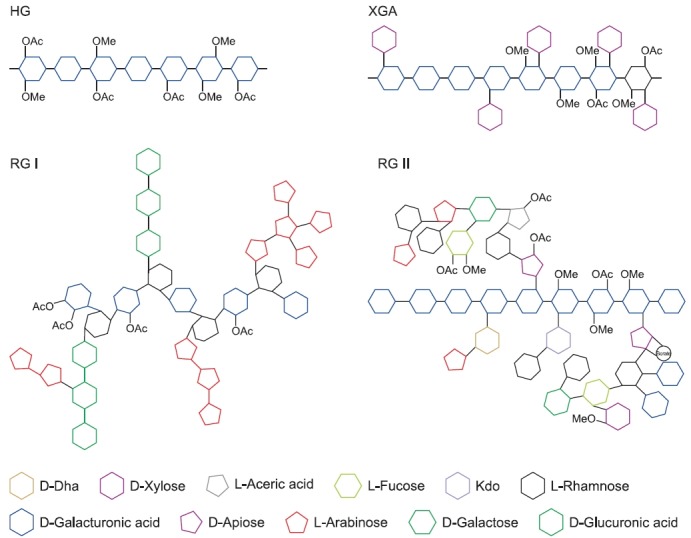
Rhamnogalacturonan I (RG I) is a branched oligosaccha-ride with a backbone composed of disaccharide (α-(1-4)-D-GlcA-α-(1-2)-L-Rha) repeats (Fig. 3). Therefore, RG I is the only type of pectin that is not built on a pure galacturonan backbone. About 20-80% of the rhamnose residues in the RG I backbone are substituted with side chains, such as β-(1-4) galactan, branched arabinan, and/or arabinogalactan. In con-trast to RG II, the structure of the side chains of RG I vary depending on plant species (Harholt et al., 2010). In particular, in the side chains of RG I of sugar beet, arabinose residues substituted with ferulic acid are rich. While GlcA residues in the pure galacturonan backbone are modified by both meth-ylation and acetylation, GlcA residues in the RG I backbone are only acetylated at the O-3 position (Komalavilas and Mort, 1989; Ishii, 1997).
In xylogalacturonan (XGA), a single Xylp is attached to the O-3 position of some GlcA residues in the HG backbone, and additional Xylp residues are attached to the first Xylp with β-(1-4) linkage (Zandleven et al., 2006) (Fig. 3). XGA is abundantly present in reproductive tissues, and is also found in other tis-sues, such as leaves (Zandleven et al., 2007). In aquatic an-giosperms, including Lemna and Spirodela, D-apiofuranose is attached to the O-2 or O-3 position of GlcA resides in the HG backbone (Hart and Kindel, 1970; Longland et al., 1989).
In rhamnogalacturonan II (RG II), a cluster of complex side chains is attached to the O-2 or O-3 position of GlcA residues in the HG backbone (Fig. 3). The side chains are composed of 12 different glycosyl residues that are linked together by at least 22 different glycosidic bonds (Harholt et al., 2010). These glycosyl residues, such as 2-O-methyl-L-fucose and L-aceric acid, and glycosidic linkages, such as α-(1-3)-xylofura-nose, are rare and unique in plant polysaccharides. Despite its complexity, the structure of RG II is highly conserved among vascular plants (Matsunaga et al., 2004).
OLIGOSACCHARIDES DERIVED FROM XYLANS AND PECTINS
Reports about the oligosaccharides derived from complex heteropolysaccharides, xylans, and pectins remain limited. The properties of XOS (oligosaccharides derived from xylans), particularly with respect to fermentation by probiotic bacteria, have been found to significantly vary according to structure. The velocity of XOS fermentation derived from Eucalyptus wood and the spent grain of breweries, was faster for those with a linear structure than those with branched structures. The velocity of fermentation of acetylated XOS and XOS with substituents in its structure, such as 4-O-MeGlcA, was slower than non-substituted linear and branched XOS (Kabel et al., 2002a). The fermentation product also varied depend-ing on the structure of XOS. While acetate and lactate were major fermentation products of both non-substituted linear and branched XOS, substituted XOS produced more pro-pionate and butyrate and less lactate (Kabel et al., 2002b). Other properties of XOS such as the activities according to the structure remain to inquire further. Acidic XOS consists of a linear polymer of β-(1-4) linked xylose branched with gluc-uronic acid showed suppressive effect on contact hypersensi-tivity in mice (Yoshino et al., 2006). On the other hands, XOS including glycosylated daidzein, which produced by cultured cells of Catharanthus roseus and Aspergillus sp. β-xylosidase showed superoxide-radical scavenging antioxidant activity (Shimoda et al., 2011).
With respect to biological activities, prebiotic oligosaccha-rides derived from xylans and pectins appear to be involved in a range of activities that are different from those of classical prebiotics (Moure et al., 2006). For example, XOS exhibited activities, such as healing effect against atopic dermatitis (Izu-mi et al., 2004), enhancement of collagen production (Azumi and Ikemizu, 2004), and the prevention of type II diabetes (Monsan et al., 2004). Other novel effects of pectic oligosac-charides (POS) include reports of the stimulation of urinary excretion of toxic metals (Zhao et al., 2008) and the suppres-sion of liver lipid accumulation rates (Yamaguchi et al., 1994).
FRAGMENTATION, SEPARATION, AND IDENTIFICA-TION OF NOVEL OLIGOSACCHARIDES FROM PLANT CELL WALL POLYSACCHARIDES
In the final section of this paper, we briefly review the gen-eral processes to obtain new oligosaccharides from complex plant cell wall heteropolysaccharides.
Fragmentation
The fragmentation of polysaccharides that are present in the plant cell wall into oligosaccharides is a prerequisite for the separation and identification of novel oligosaccharides de-rived from xylans and pectins. There are several methods to fragment polysaccharides, including: 1) chemical treatments, 2) enzymatic hydrolysis, and 3) a combination of chemical and enzymatic methods (Moure et al., 2006). Chemical treatments typically involve autohydrolysis with water or steam, diluted solutions of mineral acids, or alkaline solutions (Aachary and Prapulla, 2011). For example, the autohydrolysis of xylan-containing lignocellulosic materials (LCM) can cause cleav-age of the hemicellulosic chain via the hydrolytic reaction of hydronium ions, which mainly produce oligosaccharides as a soluble material, and both cellulose and lignin as solid materi-als (Gullón et al., 2008). Most oligosaccharides produced by the autohydrolysis reaction tend to have DP ranging from 3 to 10 (Moure et al., 2006). The reaction conditions may also af-fect the molecular distribution of oligosaccharides (Nabarlatz et al., 2005). Harsh reaction conditions decrease DP and in-crease the decomposition of oligosaccharides. Therefore, the production of XOS from LCMs may be carried out by combined chemical-enzymatic treatments (Yuan et al., 2004). Treatment to LCMs with some alkalis, such as NaOH, KOH, and am-monium, may produce xylan or soluble xylan fragments. Once soluble forms of xylan are isolated, enzymatic reduction ef-ficiently yields XOS (Hiroyuki et al., 1995).
Polysaccharide degrading enzymes are also useful tools for the fragmentation of xylan and pectin. The application of enzymes is much more specific, and under less drastic con-ditions than the chemical fragmentation of polysaccharides. However, degrading enzymes must be completely pure, or at least free from undesirable activity (Bauer et al., 2006). Many degrading enzymes with properties to digest specific polysac-charides and/or sites in polysaccharides have been purified from various microbial sources, such as plant pathogens and parasites (Shin et al., 2009). Alternatively, genes encoding such enzymes isolated from Aspergillus nidulans, Neurospora crassa, and Bacillus licheniformis are expressed in suitable hosts, such as Pichia pastoris (Bauer et al., 2006; Holck et al., 2011). In nature, xylanolytic enzyme systems consist of endo-1,4-β-D-xylanase, which is an enzyme that degrades the xylan backbone into XOS. In turn, exo-β-D-xylosidase is a debranching enzyme that degrades XOS into xyloses, and es-terase (Shin et al., 2009). To prepare XOS, the enzyme com-plex should have low exo-xylanase activity. High exo-xylanase activity results in the suppression of XOS production, through the accumulation of high amount of xyloses (Vázquez et al., 2002). For the fragmentation of pectins, single enzymatic treatments are carried out. For example, for the preparation of POS derived from citrus pectin, endo-polygalacturonase iso-lated from Aspergillus pulverulentus is treated solely in the en-zyme membrane reactor (Olano-Martin et al., 2001). Recently, the sequential enzymatic fragmentation method was devel-oped, which uses various enzymes to produce sugar beet POS with a defined DP (Holck et al., 2011). Through treat-ment with Pectin lyase, the first POS is released from both HG and RG I type pectins. Through treatment with side chain degrading enzymes, such as β-galactosidase, β-galactanase, α-L-arabinofuranosidase, and α-arabinanase, the second POS originates from the side chain of RG I, with polysaccha-rides of the RG I backbone without side chains also being pro-duced concurrently. Finally, through treatment with RG I lyase, the final POS derived from the RG I backbone is produced. However, enzymatic fragmentation of the backbone of highly branched RG I has only limited success, as the presence of side chains typically prevents enzymes from digesting adja-cent regions of the backbone (Mutter et al., 1998). In this case, an appropriate chemical method to generate RG I fragments, such as the selective depolymerization of the methyl-esterified RG I backbone by β-elimination, must be combined with enzy-matic fragmentation (Deng et al., 2006).
Separation
A variety of compounds, such as monosaccharides, oligo-saccharides, and diverse forms of polysaccharides, as well as inorganic materials and proteins, might be produced after fragmentation processes (Vegas et al., 2005). Thus, the puri-fication of oligosaccharides might prove challenging, requiring multiple stages of processing to obtain high quality prebiotic oligosaccharides. Several approaches have been used to separate oligosaccharides, including: 1) solvent extraction, 2) chromatographic separation, 3) absorption, and 4) mem-brane technology. Solvent extraction, which is also termed as liquid-liquid extraction, is a technique used to separate mixed compounds based on their different solubility in different im-miscible phases (Swennen et al., 2005). Typical solvents for this method are water and organic solvents. Solvent extraction can be used to separate desired oligosaccharides from un-wanted non-saccharide components or volatile fractions. The separation of oligosaccharides has also been conducted using chromatographic methods, such as a variety of column, size-exclusion, and ion-exchange chromatography methods which provide a reasonably good separation of oligosaccharides with advantages of scales from micrograms to kilograms and relatively the low coast (Kabel et al., 2002b). Often, combined multiple separation steps are required to acquire desirable level of oligosaccharides (Katapodis et al., 2003). Size-exclu-sion chromatography (SEC) is a method in which mixed com-pounds, including oligosaccharides in solution, are separated based on size or molecular weight (Mehrlaender et al., 2002). Ion-exchange chromatography is based on charge-charge interactions between oligosaccharides and the charges im-mobilized on resins (Kuhn and Maugeri, 2010). Alternatively, absorption has been utilized to separate oligosaccharides that contain solvents (Vázquez et al., 2000). Typical adsorbents in this method include activated charcoal, acid clay, benton-ite, and porous synthetic materials. Membrane technology showed the most interesting method to separate oligosaccha-rides. Nano-filtration through a ceramic membrane along with a mass cutoff of 1 kDa, facilitates the simultaneous concentra-tion and purification of oligosaccharides (Vegas et al., 2006).
Identification
Both NMR and mass spectrometric studies now have a central role in the elucidation of oligosaccharides, particularly XOS structures (Jones et al., 1994). NMR together with con-ventional MS and MS/MS data provide sufficient structural information about oligosaccharides (Hounsell et al., 1997). For example, one- and two-dimensional NMR techniques can provide information for the identification of individual sugar residues, anomeric configuration, interglycosidic linkages, se-quencing,and the site of appended moiety. NMR experiments have also revealed the complete structures of oligosaccha-rides and polysaccharides isolated from milled aspen wood (Teleman et al., 2000). The first 2 polysaccharides were identi-fied as O-acetyl-(4-O-methylglucurono) xylans, and the third fraction was found to be acetylated XOS. Tandem mass spec-trometry with ESI has been used to elucidate the structure of underivatized neutral and acidic oligosaccharides. Preliminary structures of about 30 neutral oligosaccharides have been characterized using matrix-assisted laser desorption/ioniza-tion (MALDI) and infrared multi-photon dissociation tandem mass spectrometry (MS) (Li et al., 2011).
CONCLUSION
Without doubt, diverse types of xylans and pectins that are found in plant cell walls are potential resources for the devel-opment of novel prebiotic oligosaccharides. The potential of xylans and pectins as resources could be developed by the appropriate modification of heteropolysaccharide structures through region-selective chemical and enzymatic modification (Ebringerova and Heinze, 2000). Recent progress towards understanding the biosynthesis of plant cell wall components at the gene and genomics levels (Faik, 2010; Harholt et al., 2010; Sheller and Ulvskov, 2010) might further expand on the potential applications of these polysaccharides via transgen-ic techniques. However, it is necessary to advance existing methods of fragmentation, separation, and isolation of oligo-saccharides from the plant cell wall component to realize the optimal potential of these polysaccharides.
Acknowledgments
This paper was supported by Konkuk University in 2009.
References
- 1.Aachary A. A. Prapulla S. G. Xylooligosaccharides (XOS) as an emerging prebiotic: Microbial synthesis utilization structural charcterization bioactive properties and applications. Compr. Rev. Food Sci. F. (2011);10:2–16. doi: 10.1111/j.1541-4337.2010.00135.x. [DOI] [Google Scholar]
- 2.Abrams S. A. Griffin I. J. Hawth K. M. Ellis K. J. Effect of prebiotic supplementation and calcium intake on body mass index. J. Pediatr. (2007);151:293–298. doi: 10.1016/j.jpeds.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 3.Azumi N. Ikemizu S. Collagen production promoters con-taining acidic xylooligosaccharides. Japan Patent JP 2004210664 (2004)
- 4.Barcelo A. Claustre J. Moro F. Chayvialle J. A. Cuber J. C. Plaisancie P. Mucin secretion is modulated by luminal fac-tors in the isolated vascularly perfused rat colon. Gut. (2000);46:218–224. doi: 10.1136/gut.46.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bauer S. Vasu P. Persson S. Mort A. J. Somerville C. R. Development and application of a suite of polysaccharide-degrading enzymes for analyzing cell walls. Proc. Natl. Acad. Sci. USA. (2006);103:11417–11422. doi: 10.1073/pnas.0604632103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berg R. D. Indigenous intestinal microflora and the host im-mune response. EOS J. Immunol. Immunopharmacol. (1985);4:161–168. [Google Scholar]
- 7.Blaut M. Relationship of prebiotics and food to intestinal micro-flora. Eur. J. Nutr. (2002);41(Suppl 1):I11–I16. doi: 10.1007/s00394-002-1102-7. [DOI] [PubMed] [Google Scholar]
- 8.Bongaertsa G. Severijnenb R. Timmermanc H. Effect of antibiotics prebiotics and probiotics in treatment for hepatic en-cephalopathy. Med. Hypothesis. (2005);64:64–68. doi: 10.1016/j.mehy.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 9.Brown G. D. Gordon S. Immune recognition. A new recep-tor for beta-glucans. Nature. (2001);413:36–37. doi: 10.1038/35092620. [DOI] [PubMed] [Google Scholar]
- 10.Brownt A. J. Goldsworthy S. M. Barnes A. A. Eilert M. M. Tcheang L. Daniels D. Muir A. I. Wigglesworth M. J. Kinghorn I. Fraser N. J. Pike N. B. Strumi J. C. Steplewski K. M. Murdock P. R. Holder J. C. Marshall F. H. Szekeres P. G. Wilson S. Ignar D. M. Foord S. M. Wise A. Dowell S. J. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. (2003);278:11312–11319. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- 11.Buchholt H. C. Christensen T. M. I. E. Fallesen B. Ralet M. C. Thibault J. F. Preparation and properities of enzymatically and chemically modified sugar beet pectin. Carbohyd. Polym. (2004);58:149–161. doi: 10.1016/j.carbpol.2004.06.043. [DOI] [Google Scholar]
- 12.Crittenden R. G. Playne M. J. Prodution proterties and application of food-grade oligosaccharides. Trends Food Sci. Tech. (1996);7:353–361. doi: 10.1016/S0924-2244(96)10038-8. [DOI] [Google Scholar]
- 13.De Leenheer L. Hoebregs H. Progress in the elucidation of the composition of chicory inulin. Starch. (1994);46:193–196. [Google Scholar]
- 14.De Simone C. Vesely R. Negri R. Bianchi Salvadori B. Zanzoglu S. Cilli A. Lucci L. Enhancement of immune response of mu-rine Peyer’s patches by a diet supplemented with yogurt. Immuno-pharm. Immunot. 9:87–100. doi: 10.3109/08923978709035203. [DOI] [PubMed] [Google Scholar]
- 15.Deng C. Neill M. A. O. York W. S. Selective chemical depolymerization of rhamnogalacturonans. Carbohyd. Res. (2006);341:474–484. doi: 10.1016/j.carres.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Ebringerova A. Heinze T. Xylan and xylan derivatives -biopolymers with valuable properties 1. Naturally occurring xylans structures isolation procedures and properties. Macromol. Rapid Commun. (2000);21:542–556. doi: 10.1002/1521-3927(20000601)21:9˂542::AID-MARC542˃3.0.CO;2-7. [DOI] [Google Scholar]
- 17.Ebringerova A. Hromadkova Z. Heinze T. Hemicellulose. Adv. Polym. Sci. (2005);186:1–67. doi: 10.1007/b136816. [DOI] [Google Scholar]
- 18.Ewaschuk J. B. Walker J. W. Diaz H. Madsen K. L. Bio-production of conjugated linoleic acid by probiotic bacteria occurs in vitro and in vivo in mice. J. Nutr. (2006);136:1483–1487. doi: 10.1093/jn/136.6.1483. [DOI] [PubMed] [Google Scholar]
- 19.Faik A. Xylan Biosynthesis: News from the Grass. Plant Physi-ol. (2010);153:396–402. doi: 10.1104/pp.110.154237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujita K. Kitahata S. Kozo H. Hotoshi H. Production of lactosucrose and its properties. In Carbohydrates in industrial synthesis; (Proceeding of the symposium of the division of carbohy-drate chemistry of the American chemical society) (Ckarke, M.A.,ed.)..Berlin Germany.: Bartens; (1992). pp. 68–76. [Google Scholar]
- 21.Fujitani S. Ueno K. Kamiya T. Tsukahara T. Ishihara K. Ki-tabayashi T. Itabashi K. Increased number of CCR4-positive cells in the duodenum of ovalbumin-induced food allergy model NC/jic mice and antiallergic activity of fructooligosaccha-rides. Allergol. Int. (2007);2:131–138. doi: 10.2332/allergolint.O-06-450. [DOI] [PubMed] [Google Scholar]
- 22.Fukasawa T. Murashima K. Matsumoto I. Hosono A. Ohara H. Nojiri C. Koga J. Kubota H. Kanegae M. Kaminogawa S. Abe K. Kono T. Identification of marker genes for in-testinal immunomodulating effect of a fructooligosaccharide by dna microarray analysis. J. Agric. Food Chem. (2007);55:3174–3179. doi: 10.1021/jf062814q. [DOI] [PubMed] [Google Scholar]
- 23.Gibson G. R. Roberfroid M. B. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutr. (1995);125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 24.Gibson R. G. Wang X. Enrichment of bifidobacteria from human gut contents by oligofructose using continuous culture. FEMS Microbiol. Lett. (1994);118:121–127. doi: 10.1111/j.1574-6968.1994.tb06813.x. [DOI] [PubMed] [Google Scholar]
- 25.Gullon P. Moura P. Esteves M. P. Girio F. M. Dominguez H. Parajo J. C. Assessment on the fermentability of xylooligo-saccharides from rice husks by probiotic bacteria. J. Agric. Food Chem. (2008);56:7482–7487. doi: 10.1021/jf800715b. [DOI] [PubMed] [Google Scholar]
- 26.Harholt J. Suttangkakul A. Scheller H. V. Biosynthesis of Pectin. Plant Physiol. (2010);153:384–395. doi: 10.1104/pp.110.156588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hart D. A. Kindel P. K. Isolation and partial characteriza-tion of apiogalacturonans from cell wall of Lemna minor. Biochem. J. (1970);116:569–579. doi: 10.1042/bj1160569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heldt H. W. Plant biochemistry. 3rd Ed Elsevieracademic press.; London UK.: (2005). pp. 265–269. [Google Scholar]
- 29.Herre J. Gordon S. Brown G. D. Dectin-1 and its role in the recognition of β-glucans by macrophages. Mol. Immunol. (2004);40:869–876. doi: 10.1016/j.molimm.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 30.Hiroyuki H. Masayasu T. Toshiro S. Agent for Improving Glucose Tolerance Disorder. Japanese Patent JP 19957324036 (1995)
- 31.Hoentjen F. Welling G. W. Harmsen H. J. Zhang X. Snartn J. Tannock G. W. Lien K. Churchill T. A. Lupicki M. Dieleman L.A. Reduction of colitis by prebiotics in HLA-B27 transgenic rats is associated with microfl ora changes and immunomodulation. Inflamm. Bowel. Dis. (2005);11:977–985. doi: 10.1097/01.mib.0000183421.02316.d5. [DOI] [PubMed] [Google Scholar]
- 32.Holck J. Hjernø K. Lorentzen A. Vigsnæs L. K. Hemmingsen L. Licht T. R. Mikkelsen J. D. Meyer A. Tailored enzymatic production of oligosaccharides from sugar beet pectin and evidence of differential effects of a single DP chain length differ-ence on human faecal microbiota composition after in vitro fermen-tation. Process Biochem. (2011);46:1039–1049. doi: 10.1016/j.procbio.2011.01.013. [DOI] [Google Scholar]
- 33.Hounsell E. F. Young M. Davies M. J. Glycoprotein changes in tumours: a renaissance in clinical applications. Clin. Sci. (1997);93:287–293. doi: 10.1042/cs0930287. [DOI] [PubMed] [Google Scholar]
- 34.Ishii T. O-Acetylated oligosaccharides from pectins of potato tuber cell wall. Plant Physiol. (1997);113:1265–1272. doi: 10.1104/pp.113.4.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Izumi Y. Azumi N. Kido Y. Nakabo Y. Oral preparations for atopic dermatitis containing acidic xylooligosaccharides. Japan Patent JP 2004210666 (2004)
- 36.Jones C. Previato J. O. Mendonça-Previato L. Wait R. The use of NMR spectroscopy in the structure determination of a Leptomonas samueli glycosylphosphosphingolipid-derived oligo-saccharide. Braz. J. Med. Biol. Res. (1994);27:219–226. [PubMed] [Google Scholar]
- 37.Kabel M. A. Carvalheiro F. Garrote G. Avgerinos E. Koukios E. Parajó J. C. Girio F. M. Schols H. A. Voragen A. G. J. Hydrothermally treated xylan rich by-products yield differ-ent classes of xylo-oligosaccharides. Carbohyd. Polym. (2002a);50:47–56. doi: 10.1016/S0144-8617(02)00045-0. [DOI] [Google Scholar]
- 38.Kabel M. A. Kortenoeven L. Schols H. A. Voragen A. G. J. In vitro fermentation of differently substituted xylo-oligos-accharides. J. Agric. Food Chem. (2002b);50:6205–6210. doi: 10.1021/jf020220r. [DOI] [PubMed] [Google Scholar]
- 39.Kaplan H. Hutkins R. W. Fermentation of fructooligosac-charides by lactic acid and bifidobacteria. Appl. Environ. Microbiol. (2000);66:2682–2684. doi: 10.1128/AEM.66.6.2682-2684.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katapodis P. Vardakou M. Kalogeris E. Kekos D. Macris B. J. Christakopoulos P. Enzymic production of a feruloylated oli-gosaccharide with antioxidant activity from wheat flour arabinox-ylan. Eur. J. Nutr. (2003);42:55–60. doi: 10.1007/s00394-003-0400-z. [DOI] [PubMed] [Google Scholar]
- 41.Kleesseen B. Hartmann L. Blaut M. Oligofructose and long-chain inulin: influence on the gut microbial ecology of rats as-sociated with a human faecal flora. Br. J. Nutr. (2001);86:291–300. doi: 10.1079/BJN2001403. [DOI] [PubMed] [Google Scholar]
- 42.Komalavilas P. Mort A. J. The acetylation at O-3 of ga-lacturonic acid in the raamnose-rich portion of pectins. Carbohyd. Res. (1989);189:261–272. doi: 10.1016/0008-6215(89)84102-3. [DOI] [Google Scholar]
- 43.Kruse H. P. Kleessen B. Blaut M. Oligofructose and long-chain inulin: influence on the gut microbial ecology of rats associ-ated with a human faecal flora. Br. J. Nutr. (2001);86:291–300. doi: 10.1079/BJN2001403. [DOI] [PubMed] [Google Scholar]
- 44.Kuhn R. C. Maugeri F. F. Separation of fructooligosac-charides using zeolite fixed bed columns. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. (2010);878:2023–2028. doi: 10.1016/j.jchromb.2010.05.039. [DOI] [PubMed] [Google Scholar]
- 45.Lenoir-Wijnkoop I. Sanders M. E. Cabana M. D. Caglar E. Corthier G. Rayes N. Sherman P. M. Timmerman H. M. Vaneechoutte M. Van Loo J. Wolvers D. A. W. Probiotic and prebiotic influence beyond the intestinal tract. Nutr. Rev. (2007);65:469–489. doi: 10.1111/j.1753-4887.2007.tb00272.x. [DOI] [PubMed] [Google Scholar]
- 46.Li B. Russell S. C. Zhang J. Hedrick J. L. Lebrilla C. B. Structure determination by MALDI-IRMPD mass spectrometry and exoglycosidase digestions of O-linked oligosaccharides from Xen-opus borealis egg jelly. Glycobiology. (2011);21:877–894. doi: 10.1093/glycob/cwr003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lomax A. R. Calder P. C. Probiotics immune function infection and inflammation: a review of the evidence from studies conducted in humans. Curr. Pharm. Des. (2009);15:1428–518. doi: 10.2174/138161209788168155. [DOI] [PubMed] [Google Scholar]
- 48.Longland J. M. Fry S. C. Trewavas A. J. Developmental control of apiogalacturonan biosynthesis and UDP-apiose produc-tion in a duck-weed. Plant Physiol. (1989);90:972–976. doi: 10.1104/pp.90.3.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lopez H. W. Coudray C. Bellaanger J. Levrat-verny M. A. Demi-gne C. Rayssiguier Y. Remesy C. Resistant starch improves mineral assimilation in rats adapted to a wheat bran diet. Nutr. Res. (2000);20:141–155. doi: 10.1016/S0271-5317(99)00146-3. [DOI] [Google Scholar]
- 50.Matsunaga T. Ishii T. Matsumoto S. Higuchi M. Darvill A. Alber-sheim P. O’Neill M. A. Occurrence of the primary cell wall polysaccharide rhamnogalacturonan II in pteridophytes lyco-phytes and bryophytes: implication for the evolution of vascular plants. Plant Physiol. (2004);134:339–351. doi: 10.1104/pp.103.030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mehrlaender K. Dietrich H. Sembries S. Dongowski G. Will F. Structural characterization of oligosaccharides and polysac-charides from apple juices produced by enzymatic pomace lique-faction. J. Agric. Food Chem. (2002);50:1230–1236. doi: 10.1021/jf011007i. [DOI] [PubMed] [Google Scholar]
- 52.Mitsuoka T. Hidaka H. Eida T. Effect of fructo-oligosac-charides on intestinal microflora. Mol. Nutr. Food Res. (1987);31:427–436. doi: 10.1002/food.19870310528. [DOI] [PubMed] [Google Scholar]
- 53.Mohnen D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. (2008);11:266–277. doi: 10.1016/j.pbi.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 54.Monsan P. Valet P. Remaud S. M. Saulnier B. J. S. Use of prebiotics for the prevention of onset of type II diabetes. Fr. Patent FR 20042844453 (2004)
- 55.Montgomery E. Hudson C. S. Transformation of lactose to a new disaccharide lactoketose. Science. (1929);69:556–557. [Google Scholar]
- 56.Moure A. Gullón P. Domínguez H. Parajó J. C. Ad-vandes in the manufacture purification and applications of xylo-oligosaccharides as food additives and nutraceuticals. Process Biochem. (2006);41:1913–1923. doi: 10.1016/j.procbio.2006.05.011. [DOI] [Google Scholar]
- 57.Mutter M. Colquhoun I. J. Beldman G. Schols H. A. Bakx E. J. Voragen A. G. Characterization of Recombinant Rhamnoga lacturonan α-l-Rhamnopyranosyl-(14)-α-d-Galactopy-rano syluro nide Lyase from Aspergillus aculeatus: An Enzyme That Frag ments Rhamnogalacturonan I Regions of Pectin. Plant Physi-ol. (1998);117:141–152. doi: 10.1104/pp.117.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nabarlatz D. Farriol X. Montane D. Autohydrolysis of almond shells for the production of xylo-oligosaccharides: Prod-uct characteristics and reaction kinetics. Ind. Eng. Chem. Res. (2005);44:7746–7755. doi: 10.1021/ie050664n. [DOI] [Google Scholar]
- 59.Nauta A. Schoterman M. H. C. Galacto-oligosaccharides. In Handbook of prebiotics and probiotics Ingredients (Ed. Jardine,S.) 2nd Ed. CRC Press; Boca Raton London New York.: (2009). pp. 75–88. [Google Scholar]
- 60.Nilsson N. E. Kotarsky K. Owman C. Olde B. Identifica-tion of a free fatty acid receptor FFA2R expressed on leukocytes and activated by short-chain fatty acids. Biochem. Biophys. Res. Commun. (2003);303:1047–1052. doi: 10.1016/S0006-291X(03)00488-1. [DOI] [PubMed] [Google Scholar]
- 61.Nurmi J. T. Puolakkainene P. A. Rautonen N. E. Bifido-bacterium lactis sp. 420 up-regulates cyclooxygenase (COX)1 and down-regulates COX-2 gene expression in a Caco-2 cell culture model. Nutr. Cancer. (2005);51:83–92. doi: 10.1207/s15327914nc5101_12. [DOI] [PubMed] [Google Scholar]
- 62.Olano-Martin E. Mountzouris G. R. Gibson G. R. Rastall R.A. Continuous production of pectic oligosaccharides in an enzyme membrane reactor. J. Food Sci. (2001);46:1035–1042. [Google Scholar]
- 63.Ouwehand A. C. Derrien M. de Vos W. Tiionen K. Rautonene N. Prebiotics and other microbial subsrates for gut function-ality. Curr. Opin. Biotechol. (2005);16:212–217. doi: 10.1016/j.copbio.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 64.Playne M. J. Crittenden R. Commercially available oligos-accharides. Bull. Int. Dairy Fed. (1996);313:10–22. [Google Scholar]
- 65.Popper Z. A. Evolution and diversity of green plant cell walls. Curr. Opin. Plant Biol. (2008);11:286–292. doi: 10.1016/j.pbi.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 66.Ramirez-Farias C. Slezak K. Fuller Z. Duncan A. Holtrop G. Louis P. Effect of inulin on the human gut microbiota: stimu-lation of Bifidobacterium adolescentis and Faecalibacterium praus-nitzii. Br. J. Nutr. (2008);101:541–550. doi: 10.1017/S0007114508019880. [DOI] [PubMed] [Google Scholar]
- 67.Roberfroid M. Gibson G. R. Hoyles L. McCartney A. L. Rastall R. Rowland I. Wolvers D. Watzl B. Szajewska H. Stahl B. Guarner F. Respondek F. Whelan K. Coxam V. Davicco M. J. Léotoing L. Wittrant Y. Delzenne N. M. Cani P. D. Neyrinck A. M. Meheust A. Prebiotic effects: metabolic and health benefits. Br. J. Nutr. (2010);104:s1–s63. doi: 10.1017/S0007114510003363. [DOI] [PubMed] [Google Scholar]
- 68.Sanderson I. R. Dietary modulation of GALT. J. Nutr. (2007);137:2557S–2562S. doi: 10.1093/jn/137.11.2557S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saulnier D. M. Kolida S. Gibson G. R. Microbiology of the human intestinal tract and approaches for its dietary modula-tion. Curr. Pharm. Des. (2009);15:1403–1414. doi: 10.2174/138161209788168128. [DOI] [PubMed] [Google Scholar]
- 70.Scheller H. V. Ulvskov P. Hemicellulose. Annu. Rev. Plant Biol. (2010);61:263–289. doi: 10.1146/annurev-arplant-042809-112315. [DOI] [PubMed] [Google Scholar]
- 71.Scholz-Ahrens K. E. Schaafsma G. van den Heuvel E. G. Schrezenmeir J. Effects of prebiotics on mineral metabo-lism. Am J. Clin. Nutr. (2001);73:459–464. doi: 10.1093/ajcn/73.2.459s. [DOI] [PubMed] [Google Scholar]
- 72.Sharon N. Ofek I. Safe as mother's milk: Carbohydrates as future anti-adhesion drugs for bacterial diseases. Glycoconju-gate J. (2000);17:659–664. doi: 10.1023/A:1011091029973. [DOI] [PubMed] [Google Scholar]
- 73.Shimoda K. Hamada H. Hamada H. Synthesis of xyloo-ligosaccharides of daidzein and their anti-oxidant and anti-allergic activities. Int. J. Mol. Sci. (2011);12:5616–5625. doi: 10.3390/ijms12095616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shin J. H. Choi J. H. Lee O. S. Kim Y. M. Lee D. S. Kwak Y. Y. Kim W. C. Rhee I. K. Thermostable xylanase from Streptomyces thermocyaneoviolaceus for optimal production of xy-looligosaccharides. Biotechnol. Bioprocess Eng. (2009);14:391–399. doi: 10.1007/s12257-008-0220-3. [DOI] [Google Scholar]
- 75.Shoaf K. Mulvey G. L. Armstrong G. D. Hutkins R. W. Prebiotic galactooligosaccharides reduce adherence of entero-pathogenic Escherichia coli to tissue culture cells. Infect Immun. (2006);74:6920–6928. doi: 10.1128/IAI.01030-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shoaf-Sweeney K. D. Hutkins R. W. Adherence anti-ad-herence and oligosaccharides preventing pathogens from sticking to the host. Adv. Food Nutr. Res. (2009);55:101–161. doi: 10.1016/S1043-4526(08)00402-6. [DOI] [PubMed] [Google Scholar]
- 77.Swennen K. Courtin C. M. Bruggen B. V. Vandecasteele C. Delcour J. A. Ultrafiltration and ethanol precipitation for isolation of arabinoxylooligosaccharides with different structures. Carbohyd. Polym. (2005);62:283–292. doi: 10.1016/j.carbpol.2005.08.001. [DOI] [Google Scholar]
- 78.Takasaki M. Inaba H. Ohta A. Motohashi Y. Sakai K. Morris H. Sakuma K. Dietary short-chain fructooligosaccharides increase calbindin-D9k levels only in the large intestine in rats in-dependent of dietary calcium deficiency or serum 1,25 dihydroxy vitamin D levels. Int. Vitam. Nutr. Res. (2000);70:206–213. doi: 10.1024/0300-9831.70.5.206. [DOI] [PubMed] [Google Scholar]
- 79.Teleman A. Lundqvist J. Tjerneld F. Stålbrand H. Dahlman O. Characterization of acetylated 4-O-methylglucuronoxylan isolated from aspen employing 1H and 13C NMR spectroscopy. Car-bohyd. Res. (2000);329:807–815. doi: 10.1016/S0008-6215(00)00249-4. [DOI] [PubMed] [Google Scholar]
- 80.Timell T. E. Wood Hemicelluloses: Part I. Adv. Carbohyd. Chem. (1964);19:247–302. doi: 10.1016/S0096-5332(08)60284-2. [DOI] [PubMed] [Google Scholar]
- 81.Vázquez M. J. Alonso J. L. Domínguez H. Parajó J. C. Xylooligosacchrides: manufacture and application. Trends Food Sci. Technol. (2000);11:387–393. doi: 10.1016/S0924-2244(01)00031-0. [DOI] [Google Scholar]
- 82.Vázquez M. J. Alonso J. L. Domínguez H. Parajo J. G. Enzymatic processing of crude xylooligomer solutions obtained by autohydrolysis of eucalyptus wood. Food Biotechnol. (2002);16:91–105. doi: 10.1081/FBT-120014321. [DOI] [Google Scholar]
- 83.Vegas R. Alonso J. L. Dominguez H. Parajo J. C. Manufacture and refining of oligosaccharides from industrial solid wastes. Ind. Eng. Chem. Res. (2005);44:614–620. [Google Scholar]
- 84.Vegas R. Luque S. Alvarez J. R. Alonso J. L. Domínguez H. Parajó J. C. Membrane-assisted processing of xyloo-ligosaccharide-containing liquors. J. Agric. Food Chem. (2006);54:5430–5436. doi: 10.1021/jf060525w. [DOI] [PubMed] [Google Scholar]
- 85.Wende G. Fry S. C. 2-O-beta-D-xylopyranosyl-(5-O-feruloyl)-L-arabinose a widespread component of grass cell walls. Phytochem. (1997);44:1019–1030. doi: 10.1016/S0031-9422(96)00649-8. [DOI] [Google Scholar]
- 86.Wichienchot S. Prasertsan P. Hongpattarakere T. Rastall R. A. Manufacture of gluco-oligosaccharide prebiotic by Glu-conobacter oxydans NCIMB 4943. Songklanakarin J. Sci. Technol. (2009);31:597–603. [Google Scholar]
- 87.Wilkie K. C. B. The hemicelluloses of grasses and cereals. Adv. Carbohyd. Chem. Biochem. (1979);36:215–264. doi: 10.1016/S0065-2318(08)60237-1. [DOI] [Google Scholar]
- 88.Yamaguchi F. Shimizu N. Hatanaka C. Preparation and physiological effect of low-molecular-weight pectin. Biosci. Biotech. Biochem. (1994);58:679–682. doi: 10.1271/bbb.58.679. [DOI] [Google Scholar]
- 89.Yoshino K. Higashi N. Koga K. Preventive effects of acidic xylooligosaccharide on contact hypersensitivity in mice. J. Health Sci. (2006);5:628–632. [Google Scholar]
- 90.Yuan Q. P. Zhang H. Qian Z. M. Yang X. J. Pilot-plant production of xylo-oligosaccharides from corncob by steaming en-zymatic hydrolysis and nanofiltration. J. Chem. Technol. Biot. (2004);79:1073–1079. doi: 10.1002/jctb.1071. [DOI] [Google Scholar]
- 91.Zablackis E. Huang J. Muller B. Darvill A. G. Albersheim P. Characterization of the cell-wall polysaccharides of Arabi-dopsis thaliana leaves. Plant Physiol. (1995);107:1129–1138. doi: 10.1104/pp.107.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zandleven J. Beldman G. Bosveld M. Schols H. A. Voragen A. G. J. Enzymatic degradation studies of xylogalacturo-nans from apple and potato using xylogalacturonan hydrolase. Carbohyd. Polym. (2006);65:495–503. doi: 10.1016/j.carbpol.2006.02.015. [DOI] [Google Scholar]
- 93.Zandleven J. Sorensen S. O. Harholt J. Beldman G. Schols H. A. Scheller H. V. Voragen A. J. Xylogalacturonan ex-ists in cell walls from various tissues of Arabidopsis thaliana. Phy-tochem. (2007);68:1219–1226. doi: 10.1016/j.phytochem.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 94.Zhao Z. Y. Liang L. Fan X. Yu Z. Hotchkiss A. T. Wilk B. J. Eliaz I. The role of modified citrus pectin as an effective chelator of lead in children hospitalized with toxic lead levels. Al-tern. Ther. Health M. (2008);14:34–38. [PubMed] [Google Scholar]


