Abstract
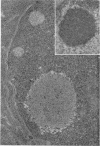
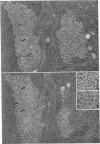
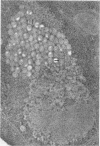
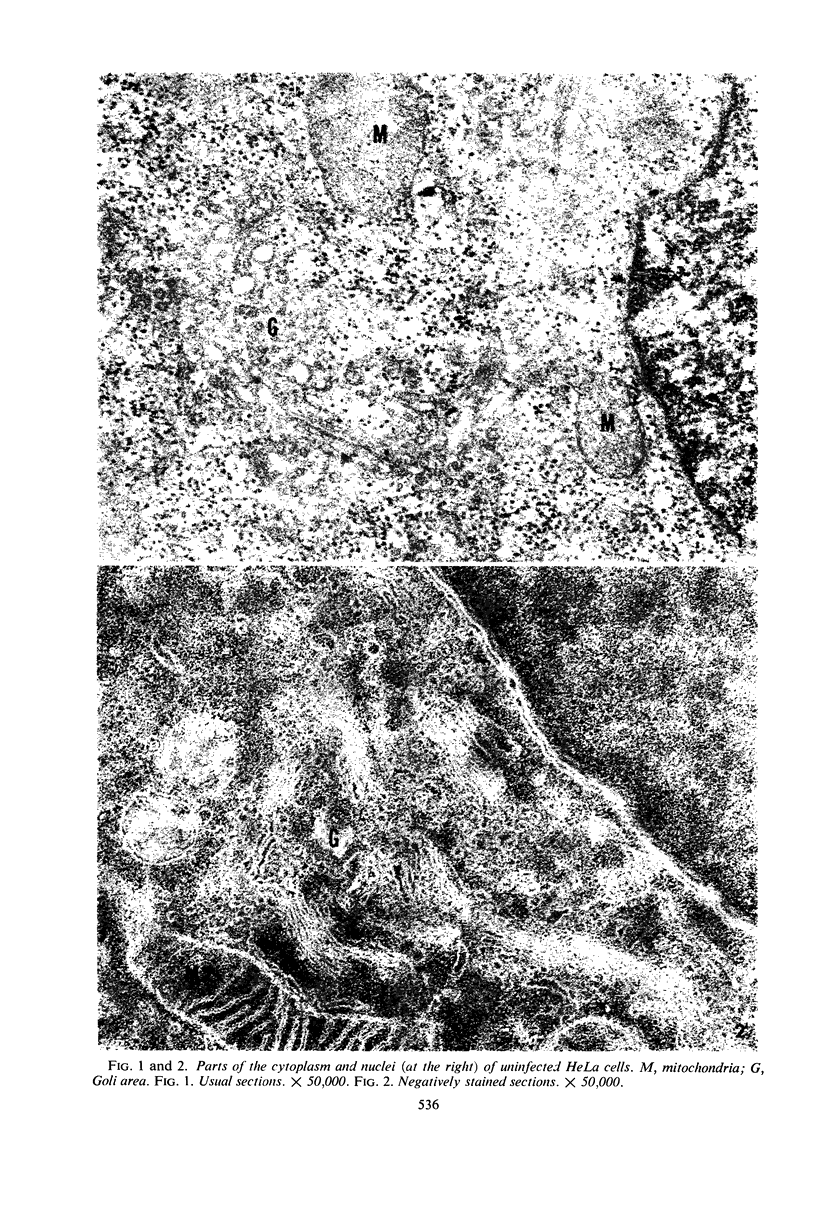
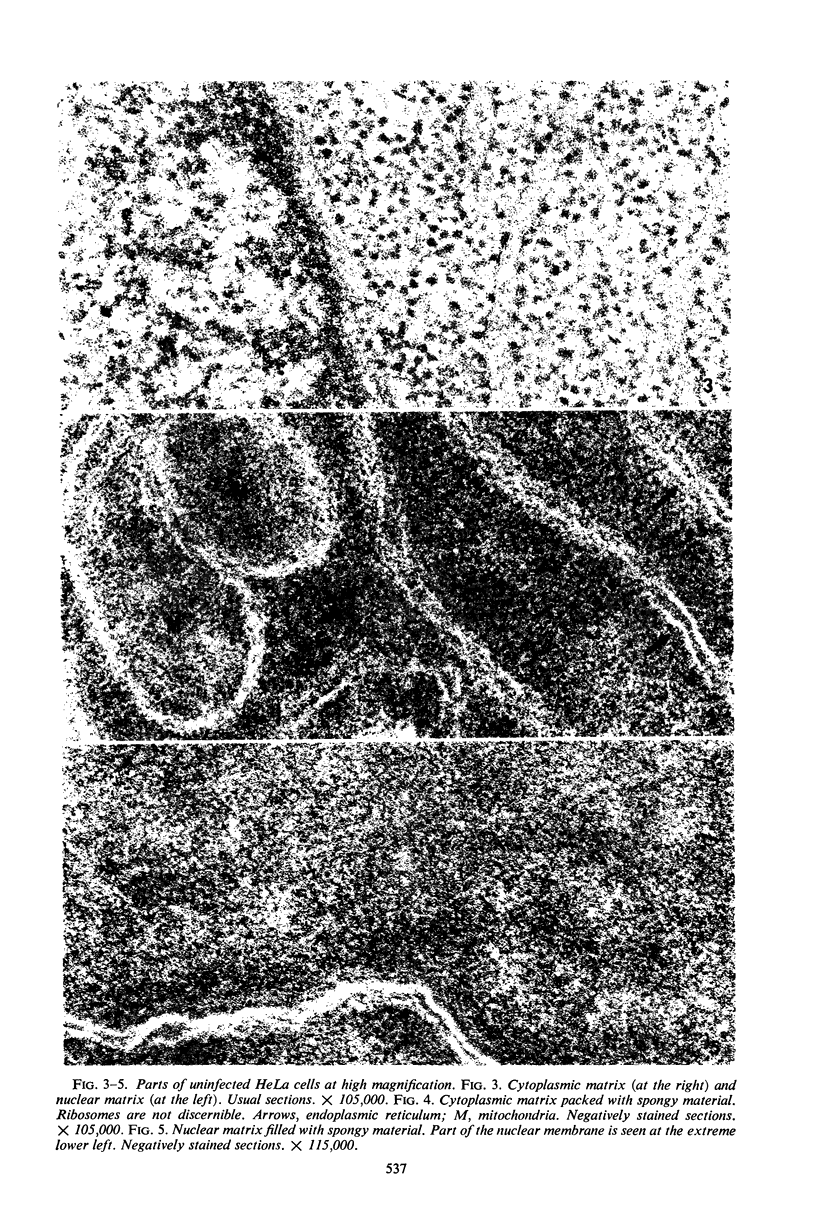
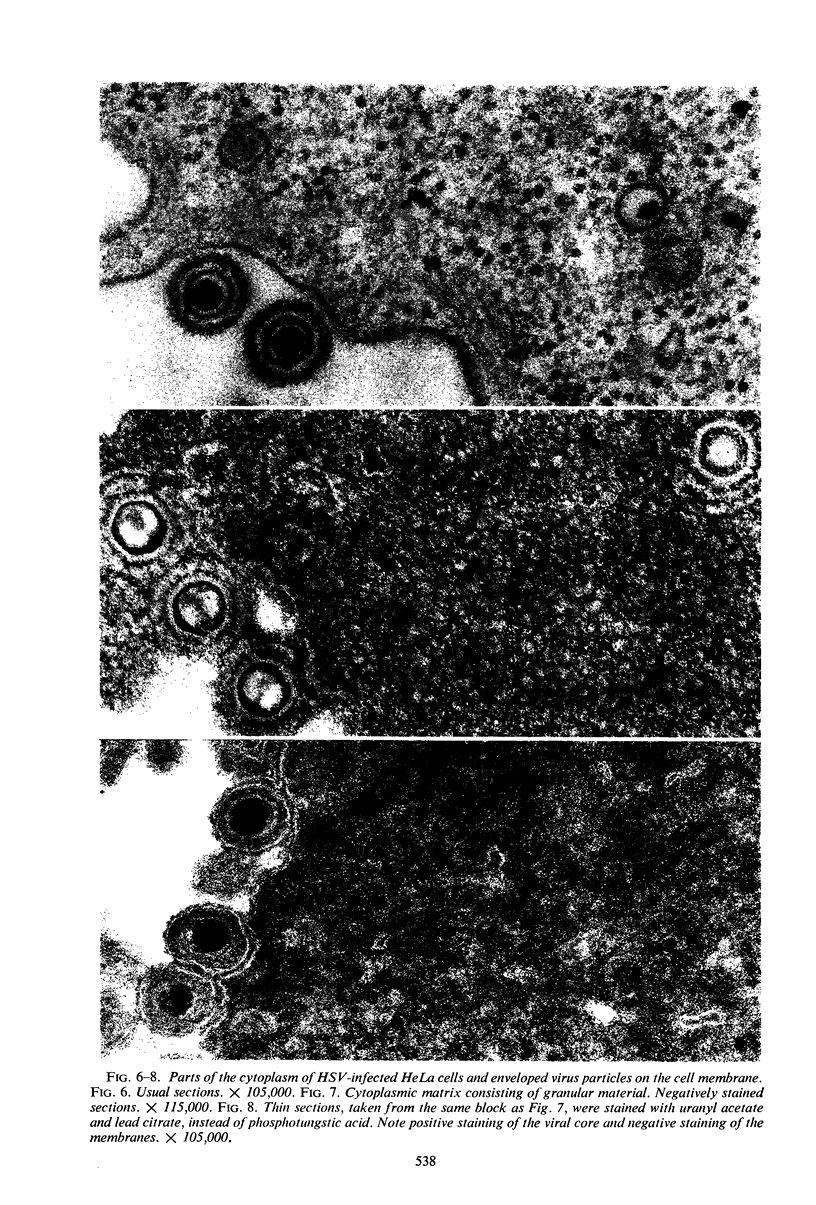
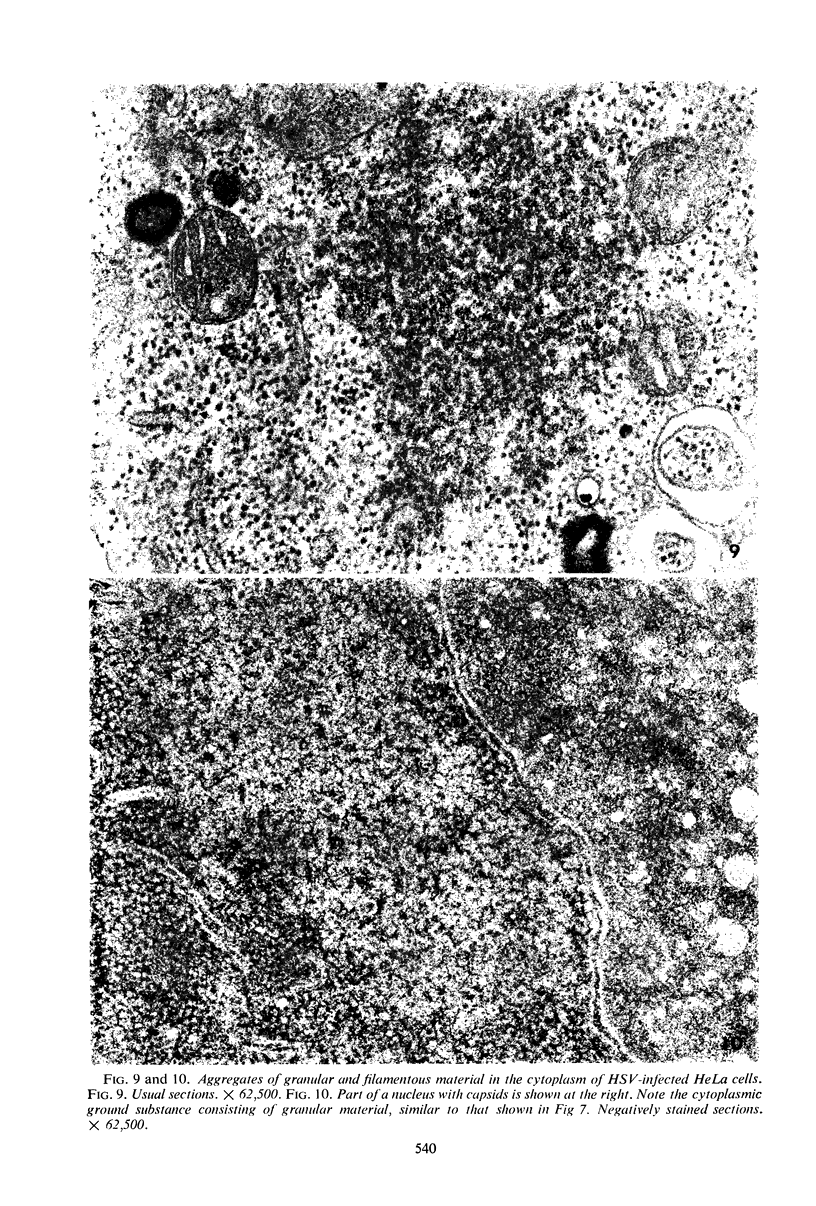
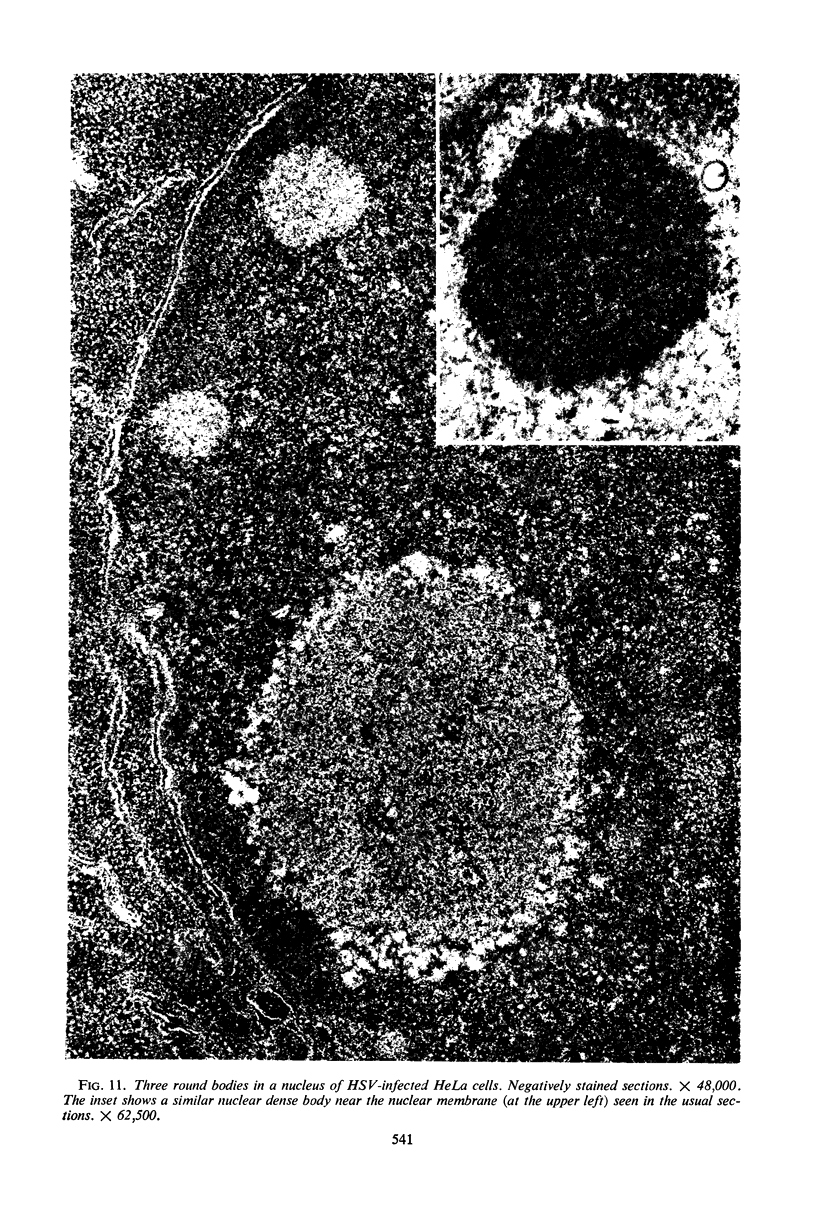
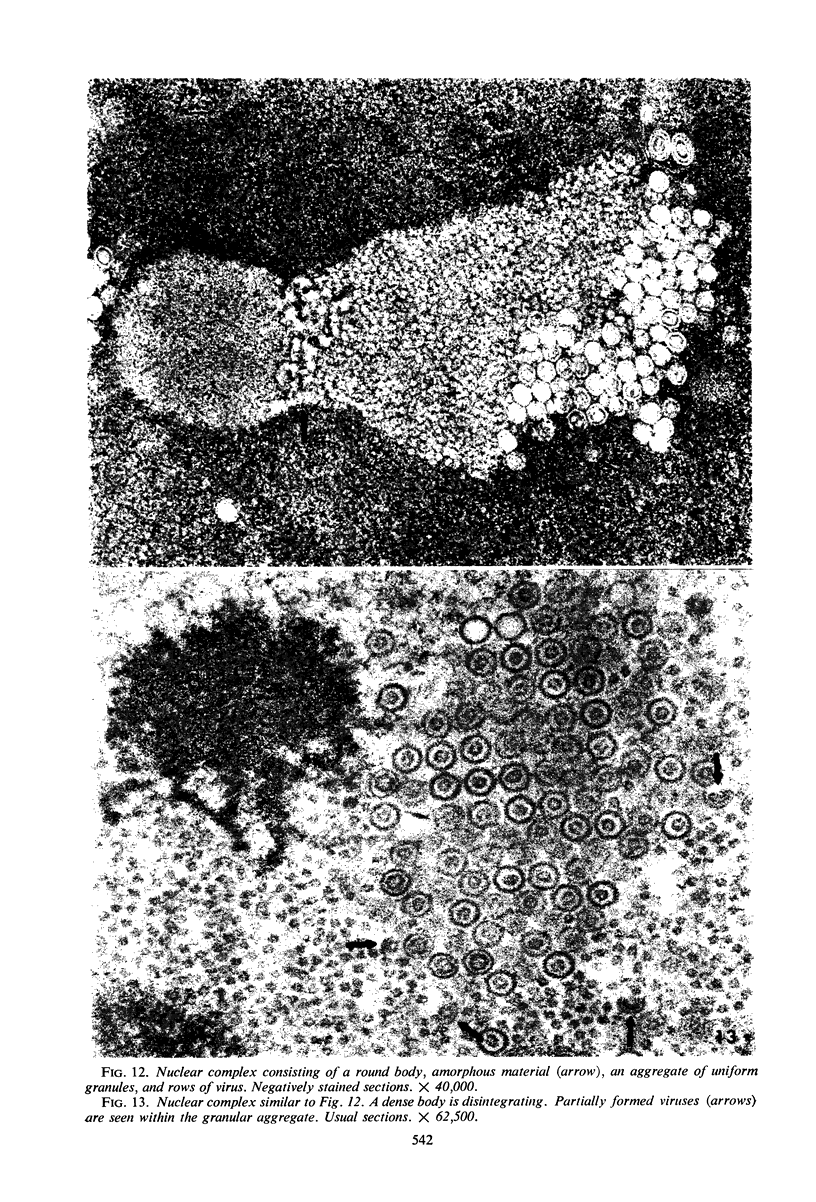
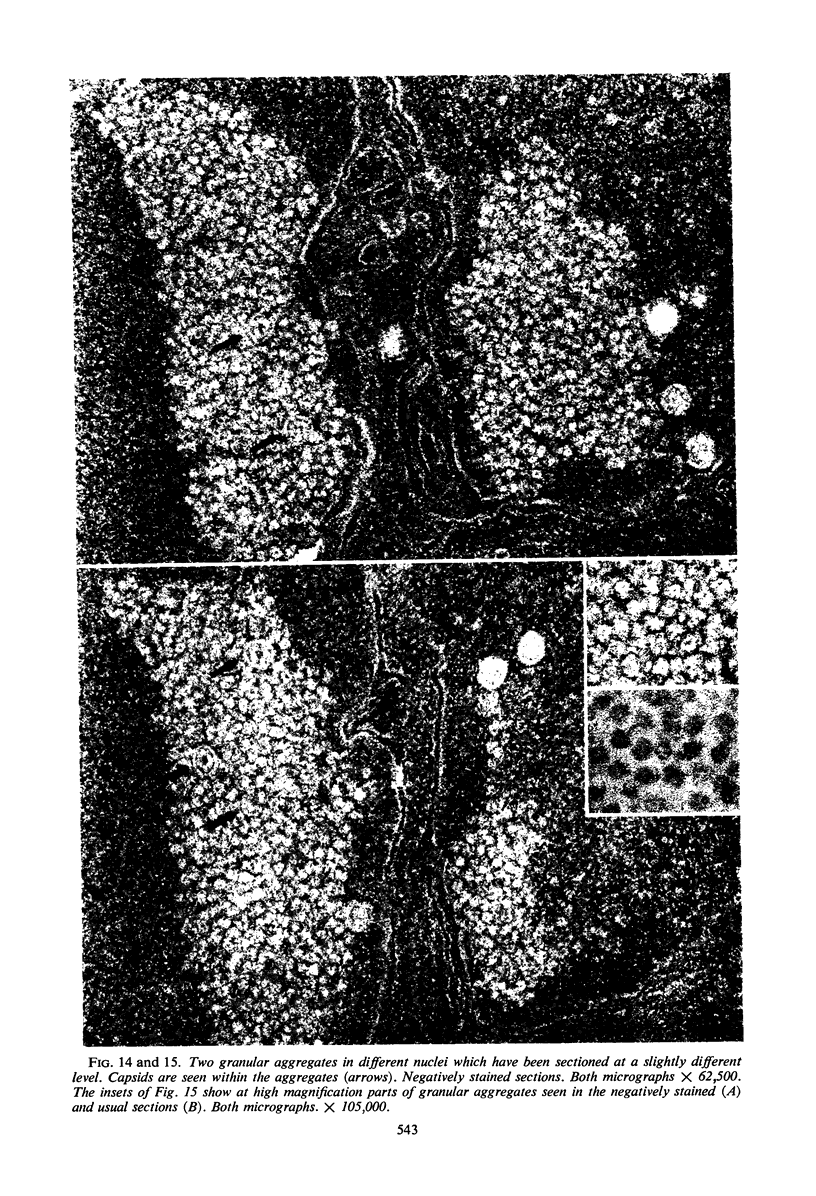
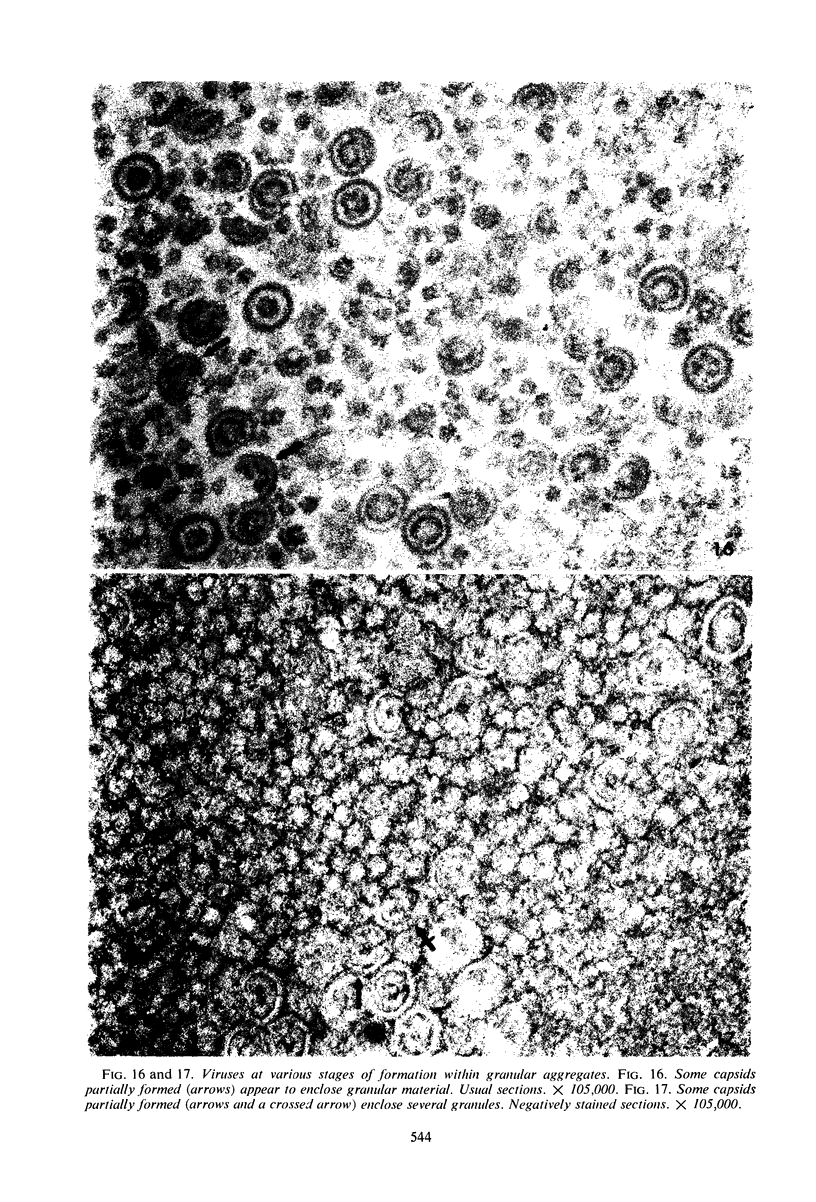
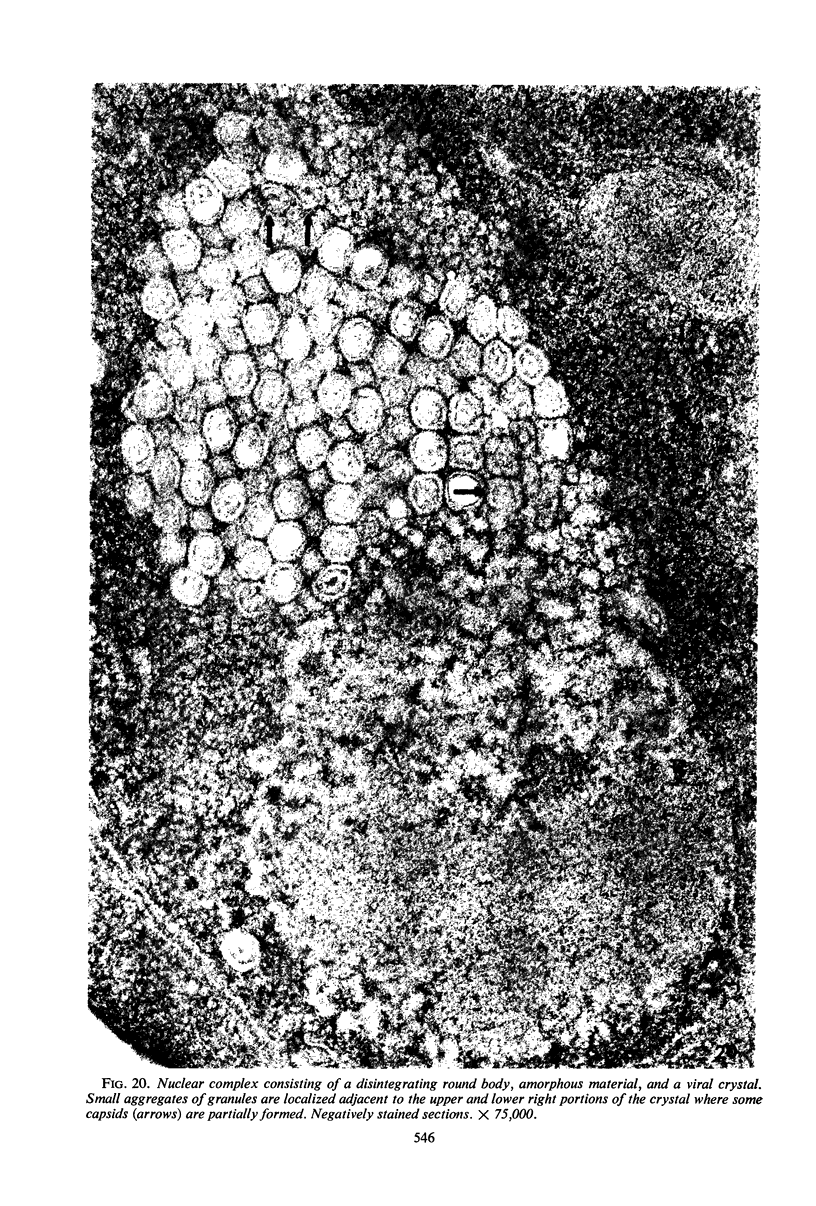
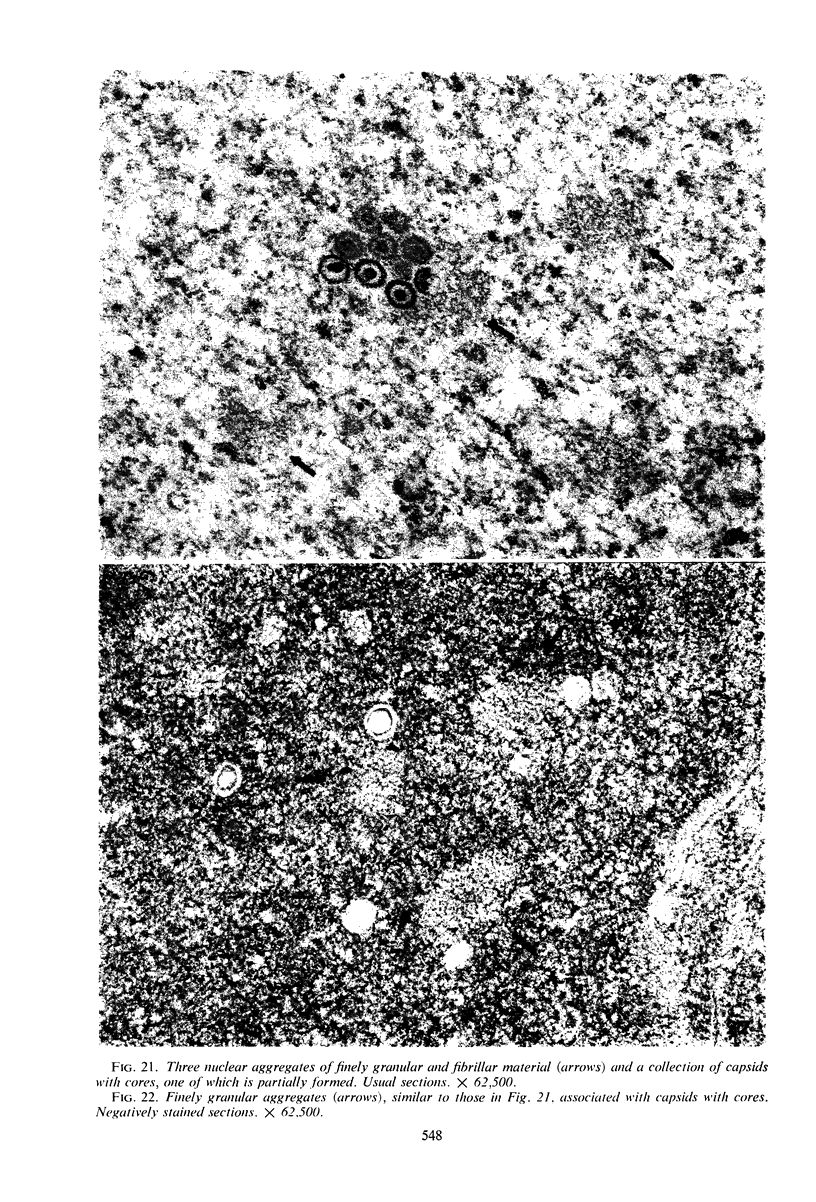
Structural alterations induced in HeLa cells by herpes simplex virus and the mechanism whereby the virus is formed in the nucleus in crystal arrays were studied by electron microscopy with both the usual and negatively stained sections. Aggregates of granular and filamentous material were observed in the cytoplasm of infected cells with both sections. On the other hand, no remarkable alterations in appearance of the cytoplasmic ground substance were observed with the usual sections of infected cells. However, the cytoplasmic ground substance of infected cells when negatively stained consisted of granular material which was different in appearance from the spongy material constituting the cytoplasmic matrix of uninfected cells. In the nucleus of infected cells, complexes consisting of round bodies, amorphous material, aggregates of uniform granules in rows, and viral crystals were often observed near the nuclear membrane in both types of sections. Examinations of the granular aggregates with negatively stained sections suggested that each granule represents a subunit and that the several adjoining subunits (approximately eight) constitute the requirement for formation of a single viral capsid with a core. Thus, rapid and simultaneous formation of the core and capsid within the aggregate would replace the rows of the granules with the viral crystal. The advantages of negative staining of thin sections for visualization of fine structural alterations are discussed.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham A., Tegtmeyer P. Morphological changes in productive and abortive infection by feline herpesvirus. J Virol. 1970 May;5(5):617–623. doi: 10.1128/jvi.5.5.617-623.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedoya V., Rabson A. S., Grimley P. M. Growth in vitro of herpes simplex virus in human lymphoma cell lines. J Natl Cancer Inst. 1968 Sep;41(3):635–652. [PubMed] [Google Scholar]
- Epstein M. A., Achong B. G., Churchill A. E., Biggs P. M. Structure and development of the herpes-types virus of Marek's disease. J Natl Cancer Inst. 1968 Sep;41(3):805–820. [PubMed] [Google Scholar]
- Hummeler K., Tomassini N., Zajac B. Early events in herpes simplex virus infection: a radioautographic study. J Virol. 1969 Jul;4(1):67–74. doi: 10.1128/jvi.4.1.67-74.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORGAN C., JONES E. P., HOLDEN M., ROSE H. M. Intranuclear crystals of herpes simplex virus observed with the electron microscope. Virology. 1958 Jun;5(3):568–571. doi: 10.1016/0042-6822(58)90047-3. [DOI] [PubMed] [Google Scholar]
- MORGAN C., ROSE H. M., HOLDEN M., JONES E. P. Electron microscopic observations on the development of herpes simplex virus. J Exp Med. 1959 Oct 1;110:643–656. doi: 10.1084/jem.110.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto K., Morgan C., Hsu K. C., Hampar B. Differentiation by immunoferritin of herpes simplex antigens with the use of rabbit 7S and 19S antibodies from early (7-day) and late (7-week) immune sera. J Natl Cancer Inst. 1971 Mar;46(3):629–646. [PubMed] [Google Scholar]
- Morris J. E. Dehydrated cysts of Artemia salina prepared for electron microscopy by totally anhydrous techniques. J Ultrastruct Res. 1968 Oct;25(1):64–72. doi: 10.1016/s0022-5320(68)80060-7. [DOI] [PubMed] [Google Scholar]
- Nazerian K., Lee L. F., Witter R. L., Burmester B. R. Ultrastructural studies of a herpesvirus of turkeys antigenically related to Marek's disease virus. Virology. 1971 Feb;43(2):442–452. doi: 10.1016/0042-6822(71)90316-3. [DOI] [PubMed] [Google Scholar]
- Nii S., Morgan C., Rose H. M., Hsu K. C. Electron microscopy of herpes simplex virus. IV. Studies with ferritin-conjugated antibodies. J Virol. 1968 Oct;2(10):1172–1184. doi: 10.1128/jvi.2.10.1172-1184.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nii S., Rosenkranz H. S., Morgan C., Rose H. M. Electron microscopy of herpes simplex virus. 3. Effect of hydroxyurea. J Virol. 1968 Oct;2(10):1163–1171. doi: 10.1128/jvi.2.10.1163-1171.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olshevsky U., Becker Y. Herpes simplex virus structural proteins. Virology. 1970 Apr;40(4):948–960. doi: 10.1016/0042-6822(70)90141-8. [DOI] [PubMed] [Google Scholar]
- Pease D. C. The preservation of unfixed cytological detail by dehydration with "inert" agents. J Ultrastruct Res. 1966 Feb;14(3):356–378. doi: 10.1016/s0022-5320(66)80054-0. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J., Roizman B. Similarities and Differences in the Development of Laboratory Strains and Freshly Isolated Strains of Herpes Simplex Virus in HEp-2 Cells: Electron Microscopy. J Virol. 1969 Dec;4(6):879–889. doi: 10.1128/jvi.4.6.879-889.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegert R., Falke D. Elektronenmikroskopische Untersuchungen über die Entwicklung des Herpesvirus hominis in Kulturzellen. Arch Gesamte Virusforsch. 1966;19(2):230–249. [PubMed] [Google Scholar]
- Sirtori C., Bosisio-Bestetti M. Nucleolar changes in KB tumor cells infected with herpes simplex virus. Cancer Res. 1967 Feb;27(2):367–376. [PubMed] [Google Scholar]
- Stackpole C. W. Herpes-type virus of the frog renal adenocarcinoma. I. Virus development in tumor transplants maintained at low temperature. J Virol. 1969 Jul;4(1):75–93. doi: 10.1128/jvi.4.1.75-93.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. L., Craighead J. E., Reynolds E. S. Electron microscopic observations on Herpesvirus hominis (herpes simplex virus) encephalitis in man. Lab Invest. 1966 Dec;15(12):1966–1981. [PubMed] [Google Scholar]