Abstract
Background
Our current knowledge of modifiable risk factors to prevent myocardial infarction (MI) in young and middle-aged women is limited, and the impact of diet is largely unknown. Dietary flavonoids exert potential beneficial effects on endothelial function in short-term trials; however the relationship between habitual intake and risk of MI in women in unknown.
Methods and Results
We followed 93,600 women aged 25–42 years from the Nurses’ Health Study (NHS) II who were healthy at baseline (1989) to examine the relationship between anthocyanins and other flavonoids and risk of MI. Intake of flavonoid sub-classes were calculated from validated food-frequency questionnaires collected every 4 years using an updated and extended USDA database. During 18 years of follow-up, 405 cases of MI were reported. An inverse association between higher intake of anthocyanins and risk of MI was observed (Hazard Ratio [HR]: 0.68; 95% confidence interval [CI] 0.49–0.96; p=0.03 comparing highest versus lowest quintiles) after multivariate adjustment. The addition of intermediate conditions, including history of hypertension, did not significantly attenuate the relationship (HR 0.70; 0.50–0.97; p=0.03). Combined intake of two anthocyanin-rich foods, blueberries and strawberries, tended to be associated with a decreased risk of MI (HR 0.66; 0.40–1.08) comparing those consuming >3 servings/week to those with lower intake. Intakes of other flavonoid sub-classes were not significantly associated with MI risk.
Conclusions
A high intake of anthocyanins may reduce MI risk in predominately young women. Intervention trials are needed to further examine the health impact of increasing intakes of commonly consumed anthocyanin-rich foods.
Keywords: diet, anthocyanins, myocardial infarction, women, berries
Introduction
Coronary heart disease (CHD), a leading cause of death and disability worldwide, predominantly occurs in older age groups with a lower prevalence in women than men at middle-age 1. To date, most epidemiological studies on CHD have concentrated on older men and women, but risk factors may vary with age, particularly in women where menopause leads to several metabolic changes. For young and middle-aged women, previous studies have suggested use of oral contraceptives and smoking as factors that increase risk 2,3, but to our knowledge no prospective studies have examined the impact of dietary factors on MI in a large sample of well characterized middle-aged women with long-term follow-up and repeated measures of dietary intake. The mechanisms underlying CHD in young/middle-aged women may also differ from older women, as coronary vasospasm - a consequence of endothelial dysfunction – may play a particularly important role 4, 5.
From a dietary perspective, growing evidence supports beneficial effects of dietary flavonoids on endothelial function and blood pressure 6–8 suggesting that flavonoids might be more likely than other dietary factors to lower risk of CHD in predominately young women6–8. Specific flavonoids appear to improve endothelial function by exerting anti-inflammatory effects, inhibiting LDL oxidation and endothelial NADPH oxidase, modulating NOS activity/expression and augmenting NO status 6, 7. Flavonoids are widely distributed in many plant-based foods/beverage including fruits, vegetables, tea and wine and the sub-classes commonly consumed in the US diet include flavanones, anthocyanins, flavan-3-ols, flavonols, flavones and polymeric flavonoids.
Which classes of flavonoids might be associated with risk of CHD, if any, is uncertain. The limited available data from older adults suggest that the flavonoid sub-classes anthocyanins, flavonols and flavanones are associated with a reduction in CHD mortality 9, 10. In middle-aged and older women, the beneficial effects of anthocyanins on blood pressure were greatest in young/middle-aged women with a 12% reduction in incident hypertension comparing the extreme quintiles of intake, 11 supporting a role for flavonoids in lowering risk of CHD in this age group. A recent meta-analysis of intervention trials highlighted the cardioprotective effects of the flavan-3-ol sub-class on biomarkers of CHD risk including blood pressure, vascular function and insulin resistance 8, 12. Based on these data, we hypothesized that a higher intake of anthocyanins and flavan-3-ols would be associated with a reduced risk of MI in young and middle-aged women.
Methods
Study population
In 1989, 116,430 women aged 25 to 42 years were enrolled in the Nurses’ Health Study II (NHSII). By mail each participant returned a questionnaire on lifestyle and medical history and received follow-up questionnaires biennially to record newly diagnosed illnesses and to update lifestyle factors. Beginning in 1991, every four years they received semi-quantitative food frequency questionnaires (FFQ) 13, 14. Participants who reported a history of myocardial infarction, stroke, angina, other cardiovascular diseases or coronary bypass surgery or cancer (except non melanoma skin cancer) at baseline were excluded. Participants who were missing dietary data at baseline, or had implausible values for total caloric intake (<500 or >3500 kcal/d) were also excluded, resulting in the inclusion of 93,600 women in these analyses. The institutional review board at Brigham and Women’s Hospital reviewed and approved this study and participants provided implied consent by virtue of returning their questionnaires.
Outcome assessment
The outcome was incident MI which included non-fatal myocardial infarction (MI) and fatal CHD that occurred after the return of the 1991 questionnaire and before 2009. Non-fatal MI was confirmed if data in the medical records met World Health Organization criteria, based on symptoms plus either diagnostic electrocardiographic changes or elevated cardiac enzyme concentrations15. Fatal CHD was defined as a fatal MI if confirmed by hospital records, autopsy, or if CHD was listed on the death certificate as cause of death and evidence of previous CHD was available.
Dietary assessment
Dietary intake data were collected from NHSII participants in 1991 and subsequently every four years. A database for assessment of intake of the different flavonoid sub-classes was constructed as previously described 11 and was compiled prior to the release of the more recent flavonoid database, phenol-explorer database. Briefly, intakes of individual compounds were calculated as the sum of the consumption frequency of each food multiplied by the content of the specific flavonoid for the specified portion size. We derived intakes of the sub-classes commonly consumed in the US diet; specifically, flavanones (eriodictyol, hesperetin, naringenin), anthocyanins (cyanidin, delphinidin, malvidin, pelargonidin, petunidin, peonidin), flavan-3-ols (catechins, epicatachin), flavonols (quercetin, kaempferol, myricetin, isohamnetin), flavones (luteolin, apigenin) and polymers (including proanthocyanidins, theaflavins and thearubigins). Cumulative intakes (energy adjusted) were calculated for a given questionnaire cycle by averaging the intake for the current and preceding FFQs. The validity and reproducibility of the FFQs have been reported previously; and correlations between major dietary sources of flavonoids (fruits, vegetables, tea, wine) measured by diet-records and FFQ were 0.70, 0.50, 0.77 and 0.83 respectively 16, 17,18.
Statistical methods
Participants contributed person-time of follow-up from the date of return of the 1991 questionnaire to the date of MI diagnosis, death, or end of follow-up (June 2009). We used a left-truncated Cox proportional-hazard regression for time-varying covariates, with a counting process data structure and age in months as the time scale, stratifying additionally on calendar year19 to estimate the hazard ratio (HR) for flavonoid sub-class intake in relation to risk of MI using the lowest intake quintile as the referent group. Covariates were updated biennially20. We controlled for body mass index (BMI; <25, 25–29.9, or ≥30 kg/m2), physical activity (metabolic equivalents/week, in quintiles), alcohol consumption (0, 0.1–4.9, 5–14.9, 15–29.9, ≥30 g/day), energy intake (kcal/day, in quintiles), cereal fibre (g/day, in quintiles), saturated, trans, polyunsaturated and monounsaturated fat intake (g/day, in quintiles), caffeine (mg/day, in quintiles), use of aspirin (non-user, <6/week, 6+ per week), menopausal status (premenopausal, unknown menopause, postmenopausal), postmenopausal hormone use (never, past, or current hormone use), oral contraceptive use (never, past, or current hormone use), smoking (never, past and current (1–14 cigarettes per day, ≥ 15 cigarettes per day), and family history of MI. All analyses were conducted with the SAS software, version9 (SAS Institute, Inc., Cary, North Carolina). All p values were two sided. We examined the possible non-linear relationship between anthocyanin intake and risk of MI non-parametrically using stepwise restricted cubic splines21, 22. Tests for non-linearity used the likelihood ratio test, comparing the model with only the linear term to the model with the linear and cubic spline terms that were selected.
In secondary analyses, we additionally adjusted for potassium, folate, fruit and vegetable intake, both individually and in combination. We created several Cox proportional hazards regression models to measure the associations between flavonoid sub-classes intake and MI in the presence of intermediate outcome measures including history of hypertension, diabetes, angina, and hypercholesterolemia.
To identify risk factors that may modify the relationship between flavonoid sub-classes that were associated with MI risk we examined the associations among strata of smoking, physical activity, prevalent hypertension, alcohol and BMI. We also conducted food-based analyses on the main sources of anthocyanins, flavonols and flavonoid polymers – tea, onions, apples, strawberries and blueberries.
Results
During 18 years of follow-up among the 93,600 participants, we documented 405 cases of incident MI. Baseline characteristics of the participants according to quintiles of anthocyanin intake are shown in Table 1. The median age of cases at diagnosis was 48.9 years, with an age range of 33.8–60.8 years. Women with higher anthocyanin intake smoked less, exercised more, and had lower intakes of total fat, energy and higher wholegrain and fiber intakes. The flavonoid polymer sub-class contributed most to total flavonoid intake (58–643 mg/day), while anthocyanin intakes ranged from 2–35 mg/d.
Table 1.
Characteristics of the women from the Nurses’ Health Study II by quintile of anthocyanin intake at baseline
| Anthocyanin intake mg/d | ||||||
|---|---|---|---|---|---|---|
| Quintile 1 (n=18676) | Quintile 2 (n=18736) | Quintile 3 (n=18727) | Quintile 4 (n=18729) | Quintile 5 (n=18732) | P for trend | |
| Age (yrs) | 36.4 (4.7) | 36.4 (4.7) | 36.6 (4.7) | 36.7 (4.6) | 36.9 (4.6) | - |
| BMI (kg/m2) | 25.1 (5.8) | 24.8 (5.4) | 24.5 (5.1) | 24.4 (5.0) | 24.2 (4.8) | <0.0001 |
| Premenopausal % | 96.2 | 96.2 | 96.0 | 96.5 | 96.6 | <0.001 |
| Oral contraceptive use % | ||||||
| Never | 16 | 15 | 15 | 16 | 16 | 0.82 |
| Past | 74 | 74 | 73 | 74 | 73 | 0.12 |
| Current | 10 | 11 | 11 | 10 | 11 | 0.045 |
| Smoking % | ||||||
| Never | 61 | 66 | 67 | 68 | 66 | <0.0001 |
| Former | 20 | 21 | 22 | 23 | 24 | <0.0001 |
| Current,1–14 per day | 7 | 5 | 5 | 5 | 5 | <0.0001 |
| Current,15+ per day | 12 | 7 | 6 | 5 | 4 | <0.0001 |
| Physical activity (Mets/wk) | 16.4 (24.2) | 18.3 (24.2) | 20.7 (26.2) | 23.2 (29.3) | 25.8 (30.7) | <0.0001 |
| Family history of MI % | 23 | 21 | 22 | 21 | 21 | 0.0006 |
| History of hypertension % | 7.3 | 6.3 | 6.2 | 5.9 | 5.9 | <0.0001 |
| History of diabetes % | 0.9 | 0.9 | 1.1 | 0.9 | 1.0 | 0.64 |
| History of hypercholesterolemia % | 16.1 | 15.0 | 14.1 | 13.9 | 13.7 | <0.0001 |
| Energy intake (Kcal/d) | 1847 (594) | 1831 (486) | 1740 (606) | 1922 (523) | 1606 (461) | <0.0001 |
| Alcohol g/d | 3.0 (7.1) | 2.8 (5.5) | 3.0 (5.5) | 3.4 (6.0) | 3.3 (6.1) | <0.0001 |
| Total Fat g/d | 68.3 (25.2) | 65.7 (20.2) | 60.7 (23.7) | 66.0 (21.1) | 52.9 (17.3) | <0.0001 |
| Saturated Fat g/d | 24.7 (9.7) | 23.4 (7.7) | 21.5 (8.9) | 23.3 (8.0) | 18.5 (6.5) | <0.0001 |
| Monounsaturated Fat g/d | 26.1 (9.9) | 25.0 (8.1) | 23.0 (9.4) | 25.0 (8.4) | 19.9 (6.8) | <0.0001 |
| Polyunsaturated Fat g/d | 11.6 (4.8) | 11.6 (4.1) | 10.9 (4.6) | 12.0 (4.2) | 9.8 (3.6) | <0.0001 |
| Trans Fat g/d | 3.8 (1.8) | 3.5 (1.5) | 3.1 (1.6) | 3.3 (1.5) | 2.5 (1.1) | <0.0001 |
| Dietary Fiber g/day | 15.7 (6.6) | 17.6 (6.8) | 17.9 (7.7) | 20.6 (7.8) | 18.9 (8.0) | <0.0001 |
| Whole Grain g/d | 16.8 (15.4) | 19.6 (15.5) | 20.0 (16.3) | 23.1 (17.2) | 20.7 (16.3) | <0.0001 |
| Potassium mg/d | 2734 (924) | 2890 (860) | 2837 (995) | 3199 (961) | 2857 (929) | <0.0001 |
| Total flavonoids mg/d | 351 (541) | 366 (451) | 422 (502) | 402 (423) | 520 (519) | <0.0001 |
| Flavanones mg/d | 26.8 (34.3) | 30.4 (31.1) | 34.4 (35.4) | 34.4 (32.8) | 41.2 (38.5) | <0.0001 |
| Flavonols mg/d | 16.0 (13.7) | 17.2 (12.8) | 18.6 (13.2) | 19.3 (12.5) | 21.6 (13.9) | <0.0001 |
| Flavones mg/d | 1.2 (1.1) | 1.4 (1.0) | 1.6 (1.0) | 1.7 (1.0) | 1.9 (1.2) | <0.0001 |
| Flavan-3-ols mg/d | 58.1 (93.8) | 58.4 (83.7) | 62.6 (89.2) | 60.2 (77.6) | 71.1 (90.5) | <0.0001 |
| Polymers mg/d | 246.8 (439) | 254.0 (359) | 297.4 (403) | 273.3 (336) | 351.4 (415) | <0.0001 |
All values (except age) are age adjusted means ± SDs
After multivariate adjustment, we observed an inverse association between anthocyanin intake and MI risk (p for trend=0.047) and the greatest reduction in risk was 32% comparing participants in the highest versus lowest quintile of anthocyanin intake (Hazard Ratio [HR] 0.68; 95% confidence interval [CI] 0.49–0.96; p for trend=0.047) (Table 2). We tested for deviation from linearity and did not detect any significant deviations from linearity (p, test for deviation=0.41). The addition of intermediate conditions including history of hypertension, diabetes, angina or hypercholesterolemia to the model did not significantly attenuate the relationship (HR 0.70; 0.50–0.97). For every 15 mg increase in intake of anthocyanins, the RR of MI decreased by 17 % (HR 0.83; 0.68–1.00) in the multivariate model. We also examined the association between deciles of anthocyanin intake and risk of MI, and comparing the top versus bottom 10% of intake, (median intake 34.3 mg in the top decile) the RR was 0.53 (0.33–0.86) suggesting continual dose-response at higher levels of habitual intake.
Table 2.
The relationship between myocardial infarction and flavonoid intake (total and sub-classes in quintiles) in participants from the Nurses’ Health Study II (NHSII)
| Quintiles (Q) of flavonoid intake subclasses | ||||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | P for trend | |
| Flavonols (mg/d) | 7.8 | 11.6 | 15.3 | 20.5 | 33.2 | |
| No. of cases | 99 | 76 | 83 | 66 | 81 | |
| No. of person years | 329022 | 330993 | 331848 | 330730 | 328659 | |
| Age-adjusted model | 1.0 | 0.73 (0.54–0.99) | 0.78 (0.58–1.05) | 0.60 (0.44–0.81) | 0.71 (0.53–0.95) | 0.009 |
| Model 2* | 1.0 | 0.84 (0.62–1.13) | 0.93 (0.69–1.25) | 0.71 (0.52–0.98) | 0.81 (0.60–1.09) | 0.10 |
| Model 3† | 1.0 | 0.85 (0.63–1.15) | 0.95 (0.70–1.28) | 0.71 (0.51–0.99) | 0.79 (0.58–1.08) | 0.08 |
| Flavones (mg/d) | 0.6 | 1.0 | 1.4 | 1.9 | 2.9 | |
| No. of cases | 105 | 86 | 70 | 69 | 75 | |
| No. of person years | 327898 | 329943 | 330724 | 332407 | 330279 | |
| Age-adjusted model | 1.0 | 0.79 (0.60–1.05) | 0.62 (0.46–0.84) | 0.59 (0.43–0.79) | 0.60 (0.45–0.81) | 0.0001 |
| Model 2* | 1.0 | 0.95 (0.71–1.26) | 0.81 (0.59–1.10) | 0.82 (0.60–1.13) | 0.92 (0.68–1.26) | 0.36 |
| Model 3† | 1.0 | 0.98 (0.74–1.32) | 0.86 (0.62–1.17) | 0.89 (0.64–1.22) | 1.00 (0.72–1.40) | 0.75 |
| Flavanones (mg/d) | 6.6 | 15.5 | 25.5 | 40.1 | 71.1 | |
| No. of cases | 109 | 75 | 59 | 89 | 73 | |
| No. of person years | 328444 | 330702 | 331126 | 331375 | 329605 | |
| Age-adjusted model | 1.0 | 0.69 (0.51–0.93) | 0.52 (0.38–0.71) | 0.77 (0.58–1.01) | 0.60 (0.44–0.80) | 0.004 |
| Model 2* | 1.0 | 0.80 (0.59–1.07) | 0.64 (0.46–0.88) | 1.01 (0.76–1.34) | 0.85 (0.63–1.15) | 0.66 |
| Model 3† | 1.0 | 0.82 (0.61–1.11) | 0.67 (0.48–0.92) | 1.07 (0.80–1.44) | 0.91 (0.66–1.26) | 0.96 |
| Flavan-3-ols (mg/d) | 13.4 | 33.3 | 78.9 | 206.2 | 610.2 | |
| No. of cases | 99 | 64 | 81 | 77 | 84 | |
| No. of person years | 328919 | 330959 | 331080 | 330939 | 329355 | |
| Age-adjusted model | 1.0 | 0.66 (0.48–0.91) | 0.84 (0.63–1.13) | 0.79 (0.58–1.06) | 0.82 (0.61–1.09) | 0.40 |
| Model 2* | 1.0 | 0.76 (0.56–1.05) | 0.94 (0.70–1.26) | 0.88 (0.65–1.19) | 0.86 (0.64–1.15) | 0.53 |
| Model 3† | 1.0 | 0.77 (0.56–1.06) | 0.94 (0.70–1.27) | 0.87 (0.64–1.18) | 0.82 (0.61–1.11) | 0.37 |
| Anthocyanins (mg/d) | 2.5 | 5.0 | 8.4 | 13.5 | 25.1 | |
| No. of cases | 126 | 81 | 66 | 73 | 59 | |
| No. of person years | 324793 | 330336 | 331831 | 332148 | 332143 | |
| Age-adjusted model | 1.0 | 0.63 (0.47–0.83) | 0.50 (0.37–0.67) | 0.54 (0.40–0.72) | 0.42 (0.31–0.57) | <0.0001 |
| Model 2* | 1.0 | 0.76 (0.57–1.01) | 0.66 (0.49–0.90) | 0.77 (0.58–1.04) | 0.62 (0.45–0.86) | 0.006 |
| Model 3 † | 1.0 | 0.80 (0.60–1.07) | 0.71 (0.52–0.97) | 0.85 (0.63–1.15) | 0.68 (0.49–0.96) | 0.047 |
| Polymers (mg/d) | 65.4 | 110.1 | 160.9 | 256.7 | 578.6 | |
| No. of cases | 112 | 80 | 69 | 57 | 87 | |
| No. of person years | 327966 | 331209 | 331278 | 331145 | 329654 | |
| Age-adjusted model | 1.0 | 0.71 (0.53–0.95) | 0.61 (0.45–0.82) | 0.49 (0.36–0.68) | 0.72 (0.54–0.95) | 0.002 |
| Model 2* | 1.0 | 0.85 (0.64–1.14) | 0.77 (0.57–1.04) | 0.63 (0.45–0.86) | 0.84 (0.63–1.12) | 0.06 |
| Model 3† | 1.0 | 0.89 (0.66–1.19) | 0.80 (0.59–1.08) | 0.64 (0.46–0.89) | 0.83 (0.62–1.11) | 0.051 |
| Total Flavonoids (mg/d) | 117.4 | 187.1 | 260.7 | 389.9 | 804.7 | |
| No. of cases | 109 | 82 | 66 | 65 | 83 | |
| Person years | 328518 | 331254 | 330948 | 330890 | 329641 | |
| Age-adjusted model | 1.0 | 0.73 (0.55–0.98) | 0.58 (0.43–0.79) | 0.56 (0.41–0.76) | 0.69 (0.52–0.92) | 0.002 |
| Model 2* | 1.0 | 0.91 (0.68–1.22) | 0.76 (0.56–1.04) | 0.74 (0.54–1.01) | 0.84 (0.62–1.12) | 0.09 |
| Model 3† | 1.0 | 0.96 (0.72–1.28) | 0.80 (0.58–1.09) | 0.76 (0.55–1.05) | 0.83 (0.61–1.12) | 0.09 |
Model 2 – age, physical activity, smoking, BMI, alcohol, energy, menopausal status, PMH use, aspirin use, oral contraceptive use, family history of MI
Model 3 - model 2 plus cereal fiber, saturated fatty acids, trans fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, caffeine
Although intake of other sub-classes were not significantly associated with a reduction in MI, comparing the highest versus lowest quintiles, there was a trend towards a reduction in risk with a higher intake of flavonols and flavonoid polymers (Table 2). The results remained essentially unchanged after additional adjustment for potassium, folate, or total fruit and vegetable intake either individually or when added together to our final multivariate model (HR 0.71; 0.50–1.00).
In stratified analyses, the inverse association between anthocyanins and MI was stronger among women who never smoked compared to those who currently smoked, although this interaction was not significant (p-heterogeneity = 0.73) (Table 3). In other stratified analyses, we found the inverse association was similar in alcohol drinkers and non-drinkers, and in those without diabetes as well as those with a history of the disease although these interactions were also not significant (Table 3).
Table 3.
The associations between anthocyanin intake and risk of myocardial infarction across strata of risk factors for participants from the Nurses’ Health Study II
| Risk factor | Cases n | Person years n | Q5 v Q1 | P for trend | P for interaction |
|---|---|---|---|---|---|
| Age | |||||
| <55 years | 350 | 1540444 | 0.71 (0.50–1.03) | 0.16 | |
| ≥ 55 years | 55 | 110807 | 0.49 (0.22–1.12) | 0.07 | 0.42 |
| BMI mg/kg2 | |||||
| <25 | 123 | 823965 | 0.60 (0.33–1.09) | 0.10 | |
| ≥ 25 | 282 | 827287 | 0.73 (0.49–1.09) | 0.20 | 0.60 |
| Smoking | |||||
| never | 173 | 1010830 | 0.53 (0.31–0.92) | 0.04 | |
| past | 91 | 375119 | 0.99 (0.51–1.91) | 0.91 | |
| current | 141 | 265302 | 0.75 (0.42–1.34) | 0.47 | 0.73 |
| Physical activity | |||||
| < median | 259 | 825374 | 0.72 (0.47–1.11) | 0.19 | |
| > median | 146 | 825877 | 0.63 (0.36–1.08) | 0.12 | 0.68 |
| Alcohol | |||||
| Non-drinker | 204 | 668944 | 0.75 (0.46–1.25) | 0.61 | |
| Drinker | 201 | 982307 | 0.60 (0.38–0.94) | 0.03 | 0.26 |
| Prevalent hypertension | |||||
| No | 231 | 1398692 | 0.76 (0.49–1.18) | 0.33 | |
| Yes | 174 | 252560 | 0.59 (0.35–1.00) | 0.05 | 0.53 |
| History of diabetes | |||||
| No | 336 | 1609342 | 0.65 (0.45–0.95) | 0.04 | |
| Yes | 69 | 41910 | 0.80 (0.34–1.86) | 0.77 | 0.57 |
Multivariate model adjusted for age, physical activity, smoking, BMI, alcohol, energy, menopausal status, PMH use, aspirin use, oral contraceptive use, family history of myocardial infarction, Cereal fiber, saturated fatty acids, trans fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, caffeine
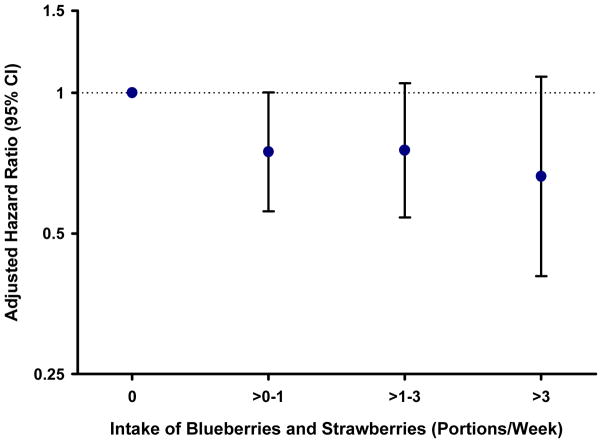
To confirm these findings and to relate the effects to public health and dietary guidelines, we conducted food-based analyses for the main dietary sources of anthocyanins. When we combined intakes of blueberries and strawberries, and compared those who consumed > 3 servings/week with those who rarely consumed these fruits, there was a trend towards a decrease in risk of MI (HR 0.66; 0.40–1.08; p=0.09) (Figure 1). For the other main foods that contributed to flavonoid intake, we did not observed a significant reduction in risk with increased intake, except for onions where intake ≥5 times per week was significantly associated with reduced risk (HR 0.27; CI 0.08–0.87; p=0.03).
Figure 1. Multivariate-adjusted relative risk of MI according to combined intake of strawberries and blueberries in the Nurses’ Health II Study.
Model adjusted for age, physical activity, smoking, BMI, alcohol, energy, menopausal status, PMH use, aspirin use, oral contraceptive use, family history of MI, cereal fibre, saturated fatty acids, trans fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, caffeine
Discussion
In this prospective cohort study of well characterized young and middle-aged women with 18 years of follow-up and repeated measures of dietary intake, we observed that a higher intake of anthocyanins was associated with a 32% reduction in risk of MI and this inverse association was independent of established dietary and non-dietary CVD risk factors. These compounds are present in red/blue colored fruits and vegetables, are readily incorporated into the habitual diet, and simple dietary change could have an impact on prevention efforts.
Our current knowledge of modifiable risk factors to prevent MI in young/middle-aged women is limited. Over the last few decades, several small case-control studies have reported on the increased risk of MI associated with use of oral contraceptives, heavy smoking, history of diabetes and hypertension in younger women 2, 3, 23–25. From a dietary perspective, the only available case-control data suggest a reduction in risk with moderate alcohol consumption and a modest increase in risk with very high coffee intake (≥ 10 cups per day) 26, 27. Our data suggest a potential role for flavonoids, specifically anthocyanins, in reducing risk.
To date, limited randomized controlled trials (RCTs) have examined the impact of anthocyanins on blood pressure and endothelial function relative to other sub-classes 8. However, a recent three month RCT in dyslipidemic patients showed that anthocyanin intake improved the lipoprotein profile resulting in an increase in HDL-cholesterol levels, a decrease in LDL-cholesterol levels, effects that were thought to be mediated via cholesteryl ester transfer protein (CETP) inhibition 28. A growing body of evidence from animal models and in vitro experiments support a cardioprotective role for anthocyanins and their degradation products or metabolites, however the concentrations used in many cell culture experiments are frequently higher than are physiologically achievable following dietary intake. In addition, many in vitro studies focus on the parent anthocyanin glycosides and their biological effects may not reflect that of the in vivo degradation and/or colonic metabolites which may be responsible for at least part of the cardiovascular bioactivity of anthocyanins7. In vitro, anthocyanins inhibit angiotensin converting enzyme activity 29, exert anti-inflammatory effects 30, 31, inhibit iNOS protein and mRNA expression, and the activation of NF-kB and NO production in a dose-dependent manner 32–34; some of these biological effects were also observed following exposure to their metabolites, including protocatechuic acid (PCA) 30, 31. In a rat heart model, anthocyanins increased cardiac glutathione concentrations, reduced infarct size following coronary occlusion and perfusion, which resulted in the myocardium being less susceptible to ischemia and reperfusion injury ex vivo 35. In an apo-E deficient mouse model, anthocyanins enhanced atherosclerotic plaque stabilization, suppressed the development of atherosclerotic lesions and the metabolite, PCA, directly inhibited atherosclerosis development 36–38. Anthocyanins have also been shown to act on a range of cells involved in atherosclerosis development including suppressed TNFα induced MCP-1 secretion in primary endothelial cells 39 and reduced expression of VEGF stimulated platelet-derived-growth-factor in vascular smooth muscle cells via de-activation of p38 MAP kinases and c-Jun N-terminal kinase 40.
These mechanistic insights for anthocyanins are important as the pathogenetic mechanisms underlying MI in young and middle-aged women may differ from older women and men. For example, risk in younger women may particularly reflect atherosclerosis with plaque disruption and ulceration leading to MI with nonobstructive coronary artery disease 4. Coronary artery spasm, a consequence of endothelial dysfunction, also may underlie CHD in younger women. Available data suggest it is a frequent cause of ACS, with coronary spasm identified in 50% of patients 5.
In an attempt to explore the biological pathways underlying the associations we observed, we added intermediate conditions including history of hypertension, hypercholesterolemia, diabetes and angina to the multivariate models, but the risk estimates were not substantially attenuated. This suggests that other mechanisms beyond these may be involved. There was a suggestion of a stronger association in those with prevalent hypertension although the interaction was not significant, (Table 3) potentially due to a lack of statistical power. The addition of other plant-based food constituents, including potassium or folate, to our model also did not substantially attenuate the relationship, nor did adjusting for total intake of fruits and vegetables. These latter models suggest that the benefits are specific to a food constituent in anthocyanin-rich foods (which includes blueberries, strawberries, eggplants, blackberries, blackcurrants) and not necessarily to non-specific benefits among participants who consume high intakes of fruits and vegetables.
We did not observe a substantial change in risk in the middle three quintiles of anthocyanin intake, but the difference in intake in these three quintiles was only 8 mg and with such small differences in intakes measurement error/misclassification is likely to be greatest. However when we examined the possible non-linear relationship across the entire distribution of anthocyanin intake we did not detect any significant deviations from linearity. To date, limited dose-response trials on anthocyanins and biomarkers of MI risk have been conducted, but given our knowledge of other sub-classes, including the isoflavones and flavan-3-ols, there is likely a threshold of intake for a biological effect and very low levels of intake are unlikely to be bioactive. When we compared extreme deciles of intake, those in the top decile had a 47% reduction in risk of MI suggesting a continual dose-response at higher levels of habitual intake.
In food-based analyses, we similarly observed a trend towards a reduction in risk of MI with increasing intake of the two main sources of anthocyanins, strawberries and blueberries, (these two foods equated to almost 60% of total anthocyanin intake) with a 34% decrease in risk for those who consumed > 3 portions per week compared to those who ate these fruits ≤ 1 per month. These data are important from a public health perspective as these fruits are readily incorporated into the habitual diet.
Previous prospective studies on flavonoids and CVD risk have been mixed, 41, 42 in part because until recently databases did not contain the comprehensive range of flavonoids present in the diet. Two recent studies suggest inverse associations between increased flavanone and anthocyanin intake and fatal CVD risk in older women 9, 10 although one study was based on the earlier 2003 USDA database. Our data included both fatal CHD and non-fatal MI, and we had insufficient power to examine the association between anthocyanin intake and fatal CHD risk (n=36 cases). In relation to age, in these middle-aged women, (median age 48.9 years at diagnosis and aged 25–42 years at baseline) we observed a 32% reduction in risk comparing extreme intake quintiles, findings which support our previous study where we observed the greatest magnitude of effect on blood pressure in younger/middle-aged women 11. This compares to the two previous studies of older women (mean age 69 years and age range 55–69 years respectively at baseline) where the magnitude of the association was lower; 18% and 9% reduction in risk respectively 9, 10.
We hypothesized that increased flavan-3-ol intake would also be associated with a reduction in risk, given the wealth of mechanistic support for this sub-class. 6, 8, 12 However, to the extent that we could assess intake, no relationship was apparent for habitual intake in this population. Our recent meta-analysis of flavan-3-ol RCTs and CVD risk biomarkers suggested that > 50mg/d of epicatechin, one of the main flavan-3-ol compounds, is required for beneficial effects on systolic and diastolic blood pressure 12. By their very nature, FFQ’s cannot capture all sources of flavonoids and some sources of flavan-3-ols may not have been accurately captured. Specifically, dark chocolate is one of the main sources of flavan-3-ols, but its overall consumption in the 1990s was quite low and most FFQs of that era did not assess different chocolate types.
There was a trend towards a reduction in risk of MI with increasing intake of flavonoid polymers and flavonols although these did not reach statistical significance. The association with flavonoid polymer intake is intriguing as to date, many of these compounds remain poorly defined and we currently know little about their biological activity and bioavailability. This sub-class includes proanthocyanidins, theaflavins and thearubigins, found predominantly in the habitual diet in tea and apples and the relative impact of these different constituents on biomarkers of CVD risk merits further investigation in future RCTs.
The strengths of this study include the prospective design, the focus on middle-aged and not older women, large sample size with long-term follow-up, repeat measures of dietary intake, detailed data on important risk factors and confounders for CVD risk and comprehensive assessment of the range of flavonoid sub-classes present in the habitual diet. The limitations of our study also warrant discussion. We adjudicated cases of acute MI using standard criteria but we did not collect catherization results, therefore although we speculated that coronary spasm may underlie CHD in younger women, we were unable to delineate which women had evidence of coronary spasm during their infarction. Although we adjusted for possible confounders that are strongly associated with MI risk (including BMI, smoking, family history), there is still the possibility of residual or unmeasured confounding from additional unmeasured factors and this may be greatest when comparing those consuming the highest v lowest anthocyanin intakes. However given our detailed and updated adjustment for potential confounders it is unlikely that these would account fully for the observed results, and in our stratified analyses we showed that even in non-smokers, and in normal weight/physically active participants the point estimates remained similar (Table 3). Of all the flavonoid sub-classes assessed, only anthocyanin intake was associated with a reduction in MI risk, suggesting something specific about this sub-class. Our FFQ assesses intake of each food up to six times per day, therefore even amongst the few participants who consume that many servings of blueberries/strawberries we would be able to accurately assess their intakes. Our data may underestimate the true benefits of lowering blood pressure as we only have a dichotomous variable. It is likely that anthocyanin intake exerts benefits across the entire range of blood pressure, and not just at an artificial threshold set for hypertension diagnosis. We used repeated measurements of diet to obtain a more accurate assessment of long-term flavonoid intake and to reduce measurement error. Mean cumulative dietary flavonoid intakes were calculated from a database developed using the most recent USDA databases 11, with additional input from other sources. These datasets allowed us to quantify a broad range of flavonoid sub-class intakes more robustly than previous analyses. The flavonoid content of foods varies depending on growing conditions and manufacturing processes, but despite this variation, these data allow us to rank order intakes and compare high and low intakes in large population groups. Although correlations between the major dietary sources of flavonoids (fruits, vegetables, tea, wine) have been determined for our FFQ 16, 17 our FFQ has not been specifically validated for the intake of flavonoid sub-classes. However in a recent study, the sum of seven flavonoid biomarkers measured in 24hr urine samples were correlated with intakes of fruits and vegetables (0.43–0.66)43, correlations similar to our validation studies. There are currently no specific biomarkers for anthocyanins as there is currently a limited understanding of their degradation and metabolism following ingestion. It is possible that our findings for anthocyanins might be due to other constituents found in the foods that contribute most to this subclass however the addition of other potentially beneficial constituents of fruits, including potassium, folate and total fruit and vegetable intake to our multivariate model did not substantially attenuate the relationship between anthocyanins and MI risk, suggesting that anthocyanins may be another important cardioprotective constituent. However in a population based study like ours it is impossible to disentangle the relative influence of all constituent of fruits and vegetables.
Our findings suggest that bioactive compounds present in red/blue colored fruits and vegetables commonly consumed in the habitual diet may be associated with a reduced risk of MI in young and middle-aged women. Further prospective studies are needed to confirm these associations including studies with biomarkers of CHD risk to elucidate mechanisms. Randomized trials focussing on commonly consumed anthocyanin-rich foods are also needed, to examine dose-response effects and be of long enough duration to assess clinically relevant endpoints.
Acknowledgments
Funding sources: This study was supported by Public Health Service grant NCI CA050385, HL091874 from the National Institutes of Health, Department of Health and Human Services, USA and the Biotechnology and Biological Sciences Research Council (BBSRC REF: BB/J004545/1), UK.
Footnotes
Reprints will not be available from the author
Contributions: LL, AC, KM, EBR conducted the statistical analysis, interpreted the data and drafted the paper. KM also adjudicated cases of myocardial infarction. MF, AC and EBR developed the flavonoid database. All authors critically reviewed the manuscript and agreed the final version.
Disclosures: none
References
- 1.Roger VLGA, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Executive summary: Heart disease and stroke statistics--2012 update: A report from the american heart association. Circulation. 2012;125:188–197. doi: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- 2.WHO. Acute myocardial infarction and combined oral contraceptives: Results of an international multicentre case-control study. Lancet. 1997;349:1202–1209. [PubMed] [Google Scholar]
- 3.Rosenberg LKD, Helmrich SP, Miller DR, Stolley PD, Shapiro S. Myocardial infarction and cigarette smoking in women younger than 50 years of age. J Am Med Assoc. 1985;253:2965–2969. [PubMed] [Google Scholar]
- 4.Reynolds HRSM, Iqbal SN, Slater JN, Mancini GB, Feit F, Pena-Sing I, Axel L, Attubato MJ, Yatskar L, Kalhorn RT, Wood DA, Lobach IV, Hochman JS. Mechanisms of myocardial infarction in women without angiographically obstructive coronary artery disease. Circulation. 2011;124:1414–1425. doi: 10.1161/CIRCULATIONAHA.111.026542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ong PAA, Hill S, Vogelsberg H, Voehringer M, Sechtem U. Coronary artery spasm as a frequent cause of acute coronary syndrome: The caspar (coronary artery spasm in patients with acute coronary syndrome) study. J Am Coll Cardiol. 2008;52:523–527. doi: 10.1016/j.jacc.2008.04.050. [DOI] [PubMed] [Google Scholar]
- 6.Schewe TSY, Sies H. How do dietary flavanols improve vascular function? A position paper. Arch Biochem Biophys. 2008;476:102–106. doi: 10.1016/j.abb.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 7.de Pascual-Teresa SMD, García-Viguera C. Flavanols and anthocyanins in cardiovascular health: A review of current evidence. Int J Mol Sci. 2010;11:1679–1703. doi: 10.3390/ijms11041679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hooper L, Rimm EB, Cohn JS, Harvey I, Le Cornu KA, Ryder JJ, Hall WL, Cassidy A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: A meta-analysis of randomized controlled trials. Am J Clin Nutr. 2008;88:38–50. doi: 10.1093/ajcn/88.1.38. [DOI] [PubMed] [Google Scholar]
- 9.Mink PJ, Scrafford CG, Barraj LM, Harnack L, Hong CP, Nettleton JA, Jacobs DR., Jr Flavonoid intake and cardiovascular disease mortality: A prospective study in postmenopausal women. Am J Clin Nutr. 2007;85:895–909. doi: 10.1093/ajcn/85.3.895. [DOI] [PubMed] [Google Scholar]
- 10.McCullough ML, Peterson JJ, Patel R, Jacques PF, Shah R, Dwyer JT. Flavonoid intake and cardiovascular disease mortality in a prospective cohort of us adults. Am J Clin Nutr. 2012;95:454–464. doi: 10.3945/ajcn.111.016634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cassidy A, O’Reilly ÉJ, Kay C, Sampson L, Franz M, Forman JP, Curhan G, eb R. Habitual intake of flavonoid subclasses and incident hypertension in adults. Am J Clin Nutr. 2011;93:338–347. doi: 10.3945/ajcn.110.006783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hooper L, Kay C, Abdelhamid A, Kroon PA, Cohn JS, Rimm EB, Cassidy A. Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: A systematic review and meta-analysis of randomized trials. Am J Clin Nutr. 2012;95:740–751. doi: 10.3945/ajcn.111.023457. [DOI] [PubMed] [Google Scholar]
- 13.Colditz GA, Manson JE, Hankinson SE. The nurses’ health study: 20-year contribution to the understanding of health among women. J Womens Health. 1997;6:49–62. doi: 10.1089/jwh.1997.6.49. [DOI] [PubMed] [Google Scholar]
- 14.Willett WC. Nutritional epidemiology. New York (NY): Oxford University Press; 1998. [Google Scholar]
- 15.Rose GABH. Cardiovascular survey methods. World Health Organization monograph series. 1982:56. [PubMed] [Google Scholar]
- 16.Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, Willett WC. Food-based validation of a dietary questionnaire: The effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;18:858–867. doi: 10.1093/ije/18.4.858. [DOI] [PubMed] [Google Scholar]
- 17.Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, Willett WC. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93:790–796. doi: 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- 18.Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, Willett WC. Dietary fat and coronary heart disease: A comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiology. 1999;149:531–540. doi: 10.1093/oxfordjournals.aje.a009849. [DOI] [PubMed] [Google Scholar]
- 19.Therneau TM, Grambach PM. Modeling survival data. New York, New York: Springer; 2000. The counting process form of a cox model; pp. 68–77. [Google Scholar]
- 20.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Amer Statistical Assoc. 1999;94:496–509. [Google Scholar]
- 21.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 22.Govindarajulu U, Spiegelman D, Thurston S, Eisen E. Comparing smoothing techniques for modeling exposure-response curves in cox models. Stat Med. 2007;26:3735–3752. doi: 10.1002/sim.2848. [DOI] [PubMed] [Google Scholar]
- 23.Mann JI, Doll R, Thorogood M, Vessey MP, Waters WE. Risk factors for myocardial infarction in young women. Br J Prev Soc Med. 1976;30:94–100. doi: 10.1136/jech.30.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.La Vecchia C, Franceschi S, Decarli A, Pampallona S, Tognoni G. Risk factors for myocardial infarction in young women. Am J Epidemiol. 1987;125:832–843. doi: 10.1093/oxfordjournals.aje.a114599. [DOI] [PubMed] [Google Scholar]
- 25.Rosenberg L, Palmer JR, Rao RS, Shapiro S. Low-dose oral contraceptive use and the risk of myocardial infarction. Arch Intern Med. 2001;161:1065–1070. doi: 10.1001/archinte.161.8.1065. [DOI] [PubMed] [Google Scholar]
- 26.Rosenberg L, Slone D, Shapiro S, Kaufman DW, Miettinen OS, Stolley PD. Alcoholic beverages and myocardial infarction in young women. Am J Public Health. 1981;71:82–85. doi: 10.2105/ajph.71.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palmer JR, Rosenberg L, Rao RS, Shapiro S. Coffee consumption and myocardial infarction in women. Am J Epidemiol. 1995;141:724–731. doi: 10.1093/oxfordjournals.aje.a117494. [DOI] [PubMed] [Google Scholar]
- 28.Qin Y, Xia M, Ma J, Hao Y, Liu J, Mou H, Cao L, Ling W. Anthocyanin supplementation improves serum ldl- and hdl-cholesterol concentrations associated with the inhibition of cholesteryl ester transfer protein in dyslipidemic subjects. Am J Clin Nutr. 2009;90:485–492. doi: 10.3945/ajcn.2009.27814. [DOI] [PubMed] [Google Scholar]
- 29.Ojeda D, Jimenez-Ferrer E, Zamilpa A, Herrera-Arellano A, Tortoriello J, Alvarez L. Inhibition of angiotensin convertin enzyme (ace) activity by the anthocyanins delphinidin- and cyanidin-3-o-sambubiosides from hibiscus sabdariffa. J Ethnopharmacol. 2010;127:7–10. doi: 10.1016/j.jep.2009.09.059. [DOI] [PubMed] [Google Scholar]
- 30.Min SW, Ryu SN, Kim DH. Anti-inflammatory effects of black rice, cyanidin-3-o-beta-d-glycoside, and its metabolites, cyanidin and protocatechuic acid. Int Immunopharmacol. 2010;10:959–966. doi: 10.1016/j.intimp.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 31.Hidalgo M, Martin-Santamaria S, Recio I, Sanchez-Moreno C, de Pascual-Teresa B, Rimbach G, de Pascual-Teresa S. Potential anti-inflammatory, anti-adhesive, anti/estrogenic, and angiotensin-converting enzyme inhibitory activities of anthocyanins and their gut metabolites. Genes Nutr. 2012;77:295–306. doi: 10.1007/s12263-011-0263-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pergola C, Rossi A, Dugo P, Cuzzocrea S, Sautebin L. Inhibition of nitric oxide biosynthesis by anthocyanin fraction of blackberry extract. Nitric Oxide. 2006;15:30–39. doi: 10.1016/j.niox.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Hamalainen M, Nieminen R, Vuorela P, Heinonen M, Moilanen E. Anti-inflammatory effects of flavonoids: Genistein, kaempferol, quercetin, and daidzein inhibit stat-1 and nf-kappab activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only nf-kappab activation along with their inhibitory effect on inos expression and no production in activated macrophages. Mediators Inflamm. 2007;2007:45673. doi: 10.1155/2007/45673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang D, Zou T, Yang Y, Yan X, Ling W. Cyanidin-3-o-beta-glucoside with the aid of its metabolite protocatechuic acid, reduces monocyte infiltration in apolipoprotein e-deficient mice. Biochem Pharmacol. 2011;82:713–719. doi: 10.1016/j.bcp.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 35.Toufektsian MC, de Lorgeril M, Nagy N, Salen P, Donati MB, Giordano L, Mock HP, Peterek S, Matros A, Petroni K, Pilu R, Rotilio D, Tonelli C, de Leiris J, Boucher F, Martin C. Chronic dietary intake of plant-derived anthocyanins protects the rat heart against ischemia-reperfusion injury. J Nutr. 2008;138:747–752. doi: 10.1093/jn/138.4.747. [DOI] [PubMed] [Google Scholar]
- 36.Xia X, Ling W, Ma J, Xia M, Hou M, Wang Q, Zhu H, Tang Z. An anthocyanin-rich extract from black rice enhances atherosclerotic plaque stabilization in apolipoprotein e-deficient mice. J Nutr. 2006;136:2220–2225. doi: 10.1093/jn/136.8.2220. [DOI] [PubMed] [Google Scholar]
- 37.Miyazaki K, Makino K, Iwadate E, Deguchi Y, Ishikawa F. Anthocyanins from purple sweet potato ipomoea batatas cultivar ayamurasaki suppress the development of atherosclerotic lesions and both enhancements of oxidative stress and soluble vascular cell adhesion molecule-1 in apolipoprotein e-deficient mice. J Agric Food Chem. 2008;56:11485–11492. doi: 10.1021/jf801876n. [DOI] [PubMed] [Google Scholar]
- 38.Wang D, Wei X, Yan X, Jin T, Ling W. Protocatechuic acid, a metabolite of anthocyanins, inhibits monocyte adhesion and reduces atherosclerosis in apolipoprotein e-deficient mice. J Agric Food Chem. 2010;58:12722–12728. doi: 10.1021/jf103427j. [DOI] [PubMed] [Google Scholar]
- 39.Garcia-Alonso M, Minihane AM, Rimbach G, Rivas-Gonzalo JC, de Pascual-Teresa S. Red wine anthocyanins are rapidly absorbed in humans and affect monocyte chemoattractant protein 1 levels and antioxidant capacity of plasma. J Nutr Biochem. 2009;20:521–529. doi: 10.1016/j.jnutbio.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 40.Oak MH, Bedoui JE, Madeira SV, Chalupsky K, Schini-Kerth VB. Delphinidin and cyanidin inhibit pdgf(ab)-induced vegf release in vascular smooth muscle cells by preventing activation of p38 mapk and jnk. Br J Pharmacol. 2006;149:283–290. doi: 10.1038/sj.bjp.0706843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arts IC, Hollman PC. Polyphenols and disease risk in epidemiologic studies. Am J Clin Nutr. 2005;81:317S–325S. doi: 10.1093/ajcn/81.1.317S. [DOI] [PubMed] [Google Scholar]
- 42.Lin J, Rexrode KM, Hu F, Albert CM, Chae CU, Rimm EB, Stampfer MJ, Manson JE. Dietary intakes of flavonols and flavones and coronary heart disease in us women. Am J Epidemiol. 2007;165:1305–1313. doi: 10.1093/aje/kwm016. [DOI] [PubMed] [Google Scholar]
- 43.Krogholm KS, Bysted A, Brantsaeter AL, et al. Evaluation of flavonoids and enterolactone in overnight urine as intake biomarkers of fruits, vegetables and beverages in the Inter99 cohort study using the method of triads. Br J Nutr. 2012:1–9. doi: 10.1017/S0007114512000104. [DOI] [PubMed] [Google Scholar]