Abstract
Extracellular signals play essential roles in controlling the proliferation and differentiation of oligodendrocyte progenitor cells in the developing central nervous system. However, the intracellular pathways that transduce these extrinsic signals remain to be elucidated. In this study, we showed that conditional ablation of the nonreceptor tyrosine phosphatase Shp2 in Olig1-expressing oligodendrocyte lineage resulted in dramatic reduction in the generation and proliferation of oligodendrocyte progenitor cells in the spinal cord. Maturation and myelination of oligodendrocytes were also compromised in the Shp2 mutants. The deficits in oligodendrocyte development in Shp2 mutants nearly phenocopied those observed in PDGF-A mutants, suggesting that Shp2 is a crucial component in transducing PDGFRα signals in the control of oligodendrocyte proliferation and maturation.
Keywords: Shp2, oligodendrocyte, spinal cord, proliferation, differentiation
Introduction
In the mouse spinal cord, early oligodendrocyte progenitor cells (OPCs) originate from the ventral pMN progenitor domain of the ventricular zone at around E12.5 after motor neuron genesis is completed (Miller 2002; Richardson et al. 2000; Rowitch 2004). About two days later, a second wave of OPCs arises from the dorsal progenitor domains (Cai et al. 2005; Vallstedt et al. 2005). As OPCs migrate away from the ventricular zone, they continue to divide until they populate the entire spinal cord (Levison et al. 1993; Noll and Miller 1993). At perinatal stages, a significant percentage of OPC cells exit cell cycle and differentiate into mature myelin-forming cells.
The progression of oligodrocyte lineage is controlled by both intrinsic mechanism and environmental factors. A variety of enviromental growth factors, including CNTF (Barres et al. 1993; Mayer et al. 1994; Vos et al. 1996), fibroblast growth factors (FGFs) (Bogler et al. 1990; McKinnon et al. 1990; Noble et al. 1990) and platelet derived growth factor alpha (PDGF-A) (Noble et al. 1988; Raff et al. 1988; Richardson et al. 1988), have been shown to regulate oligodendrocyte development. For instance, PDGF-A growth factor promotes OPC proliferation and survival (Calver et al., 1998; Fruttiger et al., 1999), whereas FGF signaling inhibits OPC differentiation (Bogler et al. 1990; Noble et al. 1990). One of the key questions that remain to be addressed is how the signals from extracellular growth factors are transduced and integrated in oligodendrocyte cells to control their delicate development.
Shp2 (also named Ptpn11, SAP-2, SH-PTP2, SH-PTP3, Syp) is a nonreceptor protein tyrosine phosphatase (PTP) which contains two SH2 domains at its amimo-terminal and a phosphatase domain at the C-terminal (Feng and Pawson 1994; Neel et al. 2003). The Drosophila homologue of Shp2, Corkscrew (Csw), is a required positive component acting downstream of the Torso receptor tyrosine kinase signaling pathway that is essential for the development of embryonic tissues (Lu et al. 1993; Perkins et al. 1996; Perkins et al. 1992). Like Csw, Shp2 is ubiquitously expressed in the developing mouse embryos, and is important for the development of various embryonic tissues (Allard et al. 1996; Feng et al. 1993). Multiple studies demonstrated that Shp2 can bind to a variety of receptor tyrosine kinases (RTKs) in response to the stimulation by growth factors and cytokines (Feng 1999). For example, it can be implicated in positive signaling from platelet-derived growth factor receptor beta (PDGFRβ) by directly binding to PDGFR and becoming tyrosine-phosphorylated (Bennett et al. 1994; Lechleider et al. 1993a; Lechleider et al. 1993b). Recently, it is reported that the conditional gene deletion of Shp2 in neural progenitor cells or the knockdown of Shp2 expression in cortical precursors resulted in defective development of neurons and astroglial cells (Gauthier et al. 2007; Ke et al. 2007). However, the specific role of Shp2 in oligodendrocyte development has not been systematically characterized.
In this study, we investigated the in vivo function of Shp2 in oligodendrocyte lineage development in the mouse model in which the Shp2 is selectively deleted in Olig1-expressing cells in the ventral spinal cord. The conditional mutation of Shp2 lead to a dramatic reduction in the number of both OPCs and mature oligodendrocytes, indicating that Shp2 is a key positive regulator of oligodendrocyte proliferation and differentiation. In addition, our results suggested that Shp2 is probably involved in regulating the migration of a subset of early differentiated oligodendrocytes in the ventral spinal cord.
Materials and Methods
Animals and tissue collection
All of the mice used in this study were handled according to protocols approved by University of Louisville. The Olig1Cre mice were generously provided by Dr. Charles Stiles (Lu et al., 2002). Genomic DNA extracted from tails was used for genotyping by Southern analysis or by PCR. Genotyping of Shp2loxP/loxP mice was described earlier (Zhang et al. 2004). Briefly, A forward primer (5′-ACG TCA TGA TCC GCT GTC AG-3′) and a reverse primer (5′-ATG GGA GGG ACA GTG CAG TG-3′) were used to detect the Shp2flox allele and a forward primer (5′-CAG TTG CAA CTT TCT TAC CTC-3′) and a reverse primer (5′-GCA GGA GAC TGC AGC TCA GTG ATG-3′) were used to detect the wild-type allele.
The Shp2loxP/+:Olig1Cre/+ double heterozygous animals were used to produce the control and Shp2 mutant mice. In this study, the Shp2flox/flox:Olig1+/- mice were referred to as the Shp2 mutant mice, and the control littermates were either Shp2+/+:Olig1Cre/+ or Shp2+/flox:Olig1Cre/+ animals. Embryos were fixed in 4% paraformaldehyde (PFA) at 4 °C for 2–6 hours, cryoprotected in 20% sucrose/PBS at 4 °C over night, and embedded in Tissue-Tek Optimal Cuttig Temperature (O.C.T.) media (SAKURA in Torrance, CA). To collect postnatal tissues, the anesthetized mice were perfused with 4% PFA/PBS, postfixed in 4% PFA/PBS at 4 °C over night, cryoprotected in 20% sucrose/PBS at 4 °C overnight, and embedded in O.C.T. All the tissue blocks were stored at −80 °C and sectioned on a cryostat.
In situ hybridization
RNA in situ hybridization (ISH) was performed as described by Schaeren-Wiemers and Gerfin-Moser (Nicole and Andrea 1993) with minor modifications. The spinal cord sections from the thoracic level were subjected to ISH with riboprobes. The plasmid templates were linearized and transcribed in vitro with RNA polymerase to obtain the riboprobe, which was labeled by digoxigenin-labeled nucleotide and purified with RNA purification column (Roche).
Immunofluorescence staining
The spinal cord sections from the thoracic level were rinsed in PBS, blocked in PBS with 3%BSA and 0.1% Triton X-100 at room temperature (RT) for 30 minuets, and incubated with primary antibody diluted in PBS with 3% BSA at 4 °C over night. On the next day, sections were washed three times in PBS for 10 minutes each, incubated with the secondary antibody (1:2000 diluted in PBS with 5% goat serum) at RT for one hour, washed again in PBS three times (10 minutes each), and mounted in Mowiol mounting medium.
In vivo cell proliferation assay
The short-term 5-Bromo-2′-deoxy-uridine (BrdU) labeling was used to compare cell proliferation rate between control and mutant tissues. Briefly, pregnant mice were intraperitoneally (IP) injected with BrdU (Sigma, 75μg/g body weight) two hours before the embryos were dissected. For double labeling experiments, spinal cord sections were sequentially incubated with anti-Olig2 and Alexa-594 conjugated secondary antibody as described above, and fixed in absolute methanol for 10 min at RT. Air-dried slides were treated with 2N HCl for 20 min at RT, washed twice in PBS for 5 min each, and neutralized in 0.1M sodium borate buffer (pH 8.0) for 10 min at RT. Following a brief wash in PBS, sections were immunolabeled with anti-BrdU supernatant and Alexa-488 conjugated secondary antibody.
Western blotting
Spinal cord tissues were removed from the anesthetized P15 mice and lysed in tissue lysis buffer (Sigma) with protease inhibitor cocktail (Sigma). 30 μg protein from control and mutant tissues were loaded for SDS-PAGE electrophoresis and subsequently detected with Rabbit anti-MBP (Millipore) and mouse anti-β-actin (Sigma) antibodies according to the standard protocol.
TEM Microscopy
Control and mutant mice littermates at P15 were anesthetized and perfused with PB buffer followed by 3% gluteraldehyde in 0.1M sodium cacodylate buffer. The spinal cord was dissected out, postfixed overnight and washed in sodium cacodylate buffer. After being postfixed in 1% osmium tetroxide/0.1M cacodylate buffer for 1 hr and dehydrated through a series of graded alcohol, the samples were embedded in epony plastic. The ultra-thin sections stained with uranium acetated and lead citrate were examined under a Philips CM12 electron microscpe. Calculation of the percentage of myelinated axons was performed on the randomly selected images from the ventral white matter of the spinal cord. At least 200 axons of 0.5 μm or greater diameter were counted from 3 individual images per animal.
RT-PCR
The OPCs were prepared as previously described (Cao et al. 2005). Total RNA was prepared from OPCs and wild type mouse brain of P15 with the RNeasy kit (Roche) and the reverse-transcription was performed with SuperScript III First Strand Synthesis System Kit (sigma) according to the manual. The PCR primers for Shp2 are Shp2RTUP 5′ CTGGGGACTACTATGACCTCTATG 3′ and Shp2RTDP 5′ GACTTGCCGTCGTTGCTC 3′, and the PCR conditions are 95°C for 5 min; 30 cycles of 94°C for 30 s, 50°C for 30 sec, 72°C for 1 min, followed by incubation at 72°C for 10 min. GAPDH was the control.
Antibodies and chemicals
The rabbit polyclonal anti-Olig2 (1:20,000) and the guinea-pig polyclonal anti-Sox10 (1:6000) were generously provided by Dr. Charles Stile (Harvard Medical School, Boston, MA) and by Dr. Wegner Michael (Universitat Erlangen-Nurnberg, Erlangen, Germany), respectively. The anti-BrdU supernatant was prepared in our lab. The mouse anti-APC (1:600) was obtained from Calbiochem. The rabbit anti-activated Caspase-3 (1:200) and mouse anti-Pdgfra (1:300) were purchased from BD. The Alexa-488 and Alexa594 conjugated secondary antibodies were obtained from Molecular Probes (Eugene, OR). Nucleic acid dye 4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI) was from Rocha (Mannheim, Germany).
Results
Developmental abnormalities of Olig1Cre/+ Shp2flox/flox conditional mutants
It has been reported that Shp2 is expressed in embryonic neural tube as early as E10.5 (Feng et al. 1993), suggesting its possible involvement in the early development of the central nervous system. RT-PCR results also confirmed that Shp2 is expressed in OPC cells (Supp. Fig. 1). To characterize the role of Shp2 in oligodendroglia development, we generated the Olig1Cre/+: Shp2flox/flox conditional mutant mice by sequential interbreeding. In Olig1Cre/+ knock-in mouse line, the Cre-mediated recombination occurs in Olig1-expressing ventral neuroepithelial cells at as early as embryonic day 10.5 (Lu et al. 2002). Therefore, in the Olig1Cre/+: Shp2flox/flox mutants, the Shp2 expression is inactivated in the pMN domain of neural progenitor cells, from which motor neurons and oligodendrocytes are sequentially produced. The Shp2 mutant mice were viable but significantly smaller than their littermates starting from P7, and the mutants weighed about 40% of their control littermates at P21 (Supp. Fig. 2). In addition to the smaller body sizes, the mutants showed minor shaking and unbalanced movements at early postnatal stages. Most of the mutants died at around P21, but few survived into adulthood.
Impaired oligodendrocyte development in Shp2 conditional mutants
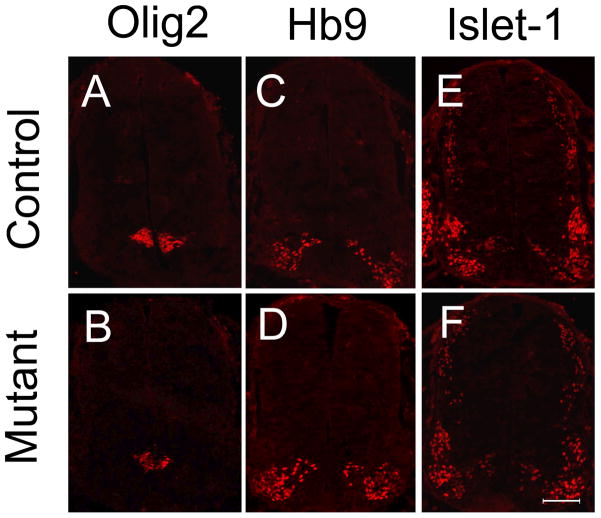
To examine the possible effects of the Shp2 mutation on the regional patterning and cell fate specification in the ventral neural tube, we first studied the expression of Olig2 in the spinal cord of Shp2 conditional mutant mice. At E11.5, Olig2 is predominantly expressed in the pMN domain of neuroepithelial cells (Lu et al. 2000), and there was no obvious difference in Olig2 expression between the control and mutant embryos (figure 1A, B). Consistently, a similar number of HB9+ and Islet1+ motor neurons were produced from the pMN domain in the control and Shp2 conditional mutant spinal cord at E11.5 (Fig 1C, D, E, F) and E12.5 (Sup Fig 3B–F). These results indicated that Shp2 activity is not required for the initial patterning and subsequent neurogenesis of the pMN domain.
Figure 1.
Normal development of neural progenitor cells in the pMN domain and motor neurons in Shp2 mutant spinal cord. The spinal cord sections from control (A, C, E) and the mutant mice (B, D, F) at E11.5 were immunostained with anti-Olig2 (A, B), anti-Hb9 (C, D) and anti-Islet-1 (E, F). Scale bar, 100 μm.
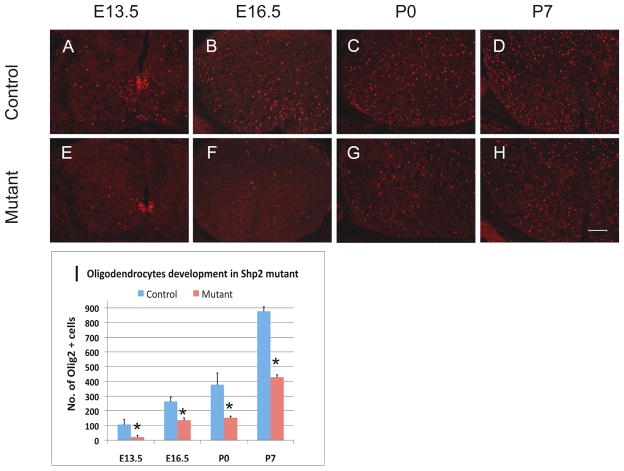
Since the pMN domain also gives rise to oligodendrocyte cells at later gliogenesis stages, we next examined the oligodendroglial development in the Shp2 conditional mutants. Olig2 was used as a specific marker for cells of oligodendrocyte lineage, including both oligodendrocyte progenitor cells (OPCs) and mature oligodendrocytes (Lu et al. 2000; Takebayashi et al. 2000; Zhou et al. 2000). At E12.5, a few Olig2+ OPCs started to migrate out of the pMN domain in the control spinal cord, whereas none could be detected in the mutant tissues (Supp. Fig. 3A, B). At E13.5, many Olig2+ OPCs were observed in the surrounding region of the control spinal cord. By contrast, few were detected outside the pMN domain in the mutants (Fig. 2-A, E), suggesting that fewer OPC cells were produced from the pMN domain in the absence of Shp2 expression. From E15.5 to postnatal day 7 (P7), the number of Olig2+ cells was consistently and significantly decreased in the Shp2 mutant spinal cords (Fig. 2).
Figure 2.
Reduced Olig2+ cells in Shp2-Olig1Cre mutant spinal cords. (A–H) Olig2 immunofluorescent staining in the control (A–D) and mutant (E–H) spinal cords from E13.5 to P7; Scale bar, 100 μm; (I) Quantitative analysis of the number of Olig2+ cells migrating out of the ventricular zone. Olig2+ cells were counted under 10X magnification. At least 3 sections were counted for each animal at each stage (*P<0.08).
To further confirm the defective generation of OPCs in the Shp2 mutants, we examined the expression of PDGFRα (a specific marker for OPCs) in spinal cord tissues prepared from E13.5 to P7. At all embryonic and postnatal stages examined, the number of PDGFRα+ cells was dramatically decreased in the mutant spinal cords as compared to their littermate controls (Fig. 3). Thus, Shp2 activity is required for the normal generation of OPC population in the spinal cord.
Figure 3.
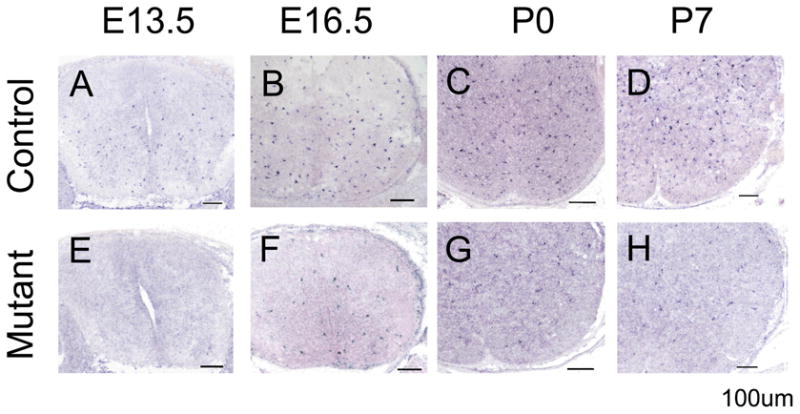
Decreased expression of PDGFR in the Shp2-Olig1Cre mutants. Spinal cord sections from E13.5 to P7 control (A–D) and mutant (E–H) animals were subjected to RNA in situ hybridization. The number of PDGFRα+ OPC cells was drastically reduced in the mutants at all stages examined. Scale bar, 100 μm.
Reduced OPC proliferation in the Shp2 mutants
The reduced number of OPC cells in Shp2 conditional mutants is reminiscent of the defective oligodendrocyte development observed in the PDGF-A mutant spinal cord tissues ( Fruttiger et al. 1999; Fu et al. 2002), raising the possibility that Shp2 may function downstream of PDGF-A signaling in support of OPC proliferation and survival. To test whether Shp2 signaling is involved in regulating OPC proliferation, we carried out a 2-hour short-term BrdU labeling in E16.5 mouse embryos, followed by double-immunostaining with anti-BrdU and anti-Olig2 antibodies (Fig 4A, B). Our results revealed that the percentage of dividing OPCs (Olig2+ cells that were positive for BrdU) in the Shp2 mutants (10.44%±1.38%) was about 26% less than that in the controls (14.02%±1.17%) (Fig 4E), indicating a slower cycling rate of OPC cells in the mutants.
Figure 4.
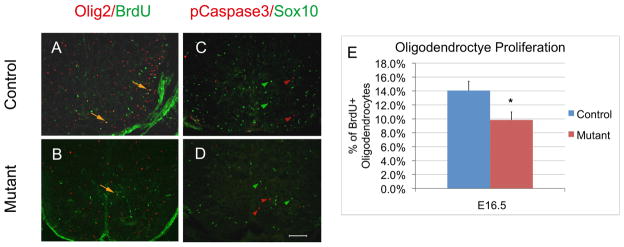
Assays of oligodendrocyte proliferation and apoptosis in E16.5 spinal cords. A–B. Double immunofluorescent staining with anti-BrdU and anti-Olig2. Double-stained cells are represented by arrows. C–D Double immunostaining with anti-pCaspase 3 and anti-Sox10. Green and red arrowheads represent oligodendrocytes and apoptotic cells, respectively. Scale bar, 100 μm. (E) The percentage of BrdU labeled Olig2+ cell at E16.5. More than 400 Olig2-positive and BrdU/Olig2 double positive cells from 4 different sections were counted under 10X magnification (*P<0.01).
It is conceivable that increased cell death could also contribute to the smaller population size of OPC cells in the Shp2 mutant spinal cords at late embryonic stages. This possibility was examined by anti-Sox10 and anti-phosphate-Caspase-3 double immunofluorescent staining on E16.5 and P0 spinal cord sections. Sox10 and phosphate Caspase-3 specifically label oligodendrocyte cells (Stolt et al. 2002) and apoptotic cells (Fernandes-Alnemri et al. 1994; Nicholson et al. 1995), respectively. At these stages, few pCaspase-3 positive cells were detected; more importantly, we failed to detect any Sox10+ cells that were co-labeled with anti-pCaspase-3 in both the control and mutant tissues (Fig. 4C, D; data not shown). Thus, cell death did not appear to contribute to the diminished OPC population in the Shp2 mutants.
Ablation of Shp2 inhibited oligodendrocyte differentiation
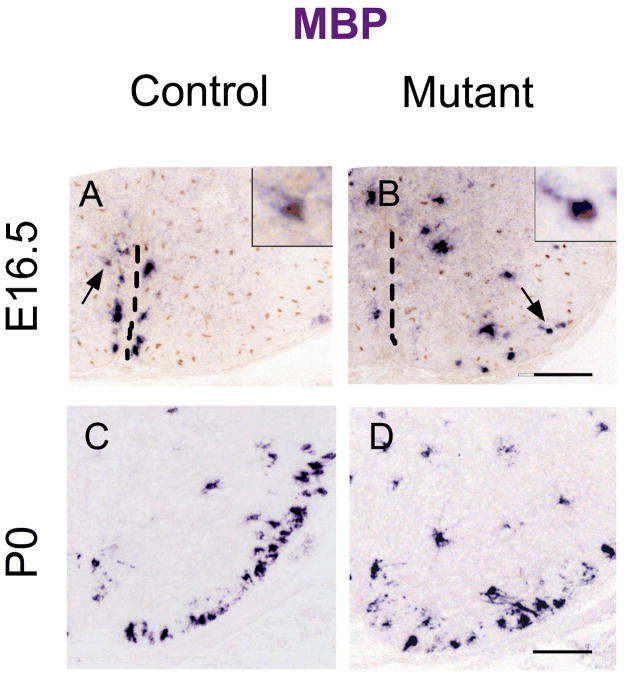
We next investigated the effects of Shp2 mutation on the terminal differentiation of oligodendrocytes by examining the expression of several myelin genes, including myelin basic protein (MBP) and proteolipoprotein (PLP) in the developing spinal cord tissues. At E16.5, a small number of MBP mRNA+ oligodendrocytes started to appear in the control spinal cord immediately flanking the floor plate (Fig 5A). Surprisingly, in the Shp2 mutants, the MBP mRNA+ oligodendrocytes were spread out into the surrounding ventral gray matter and white matter (Fig 5B). This result suggested that the timing of oligodendrocyte differentiation is not delayed in the Shp2 mutant, but the migration of the early differentiated oligodendrocytes was altered at this stage.
Figure 5.
Defective oligodendrocyte migration and differentiation in the Shp2 mutants. A–B. Spinal cord sections of E16.5 embryos were subjected to MBP ISH hybridization followed by anti-Olig2 immunohistochemical staining. The ventral midline was indicated by broken lines, and arrows represented double positive cells shown in insets at the upper right corner. C–D. Spinal cord sections from P0 pups were examined for MBP expression by ISH.
Starting at P0, numerous MBP mRNA + mature oligodendrocytes were observed in the white matter of the control spinal cords; however, the number of MBP mRNA+ cells was markedly reduced in Shp2 mutant tissues (Fig 5C–D). Between P7 to P15, the level of MBP and PLP expression in the white matter remained to be significantly decreased (Fig 6). For instance, in the P7 mutant spinal cord, the number of PLP+ mature oligodendrocytes was reduced by 53%. The impaired oligodendrocyte differentiation was further verified by the APC immunostaining (Fig 6I–K) and by anti-MBP Western blotting in P15 spinal tissues (Fig 6L).
Figure 6.
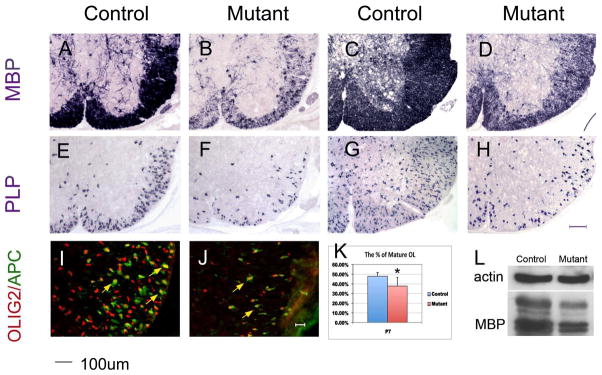
Reduced oligodendrocyte differentiation in Shp2 mutants. Spinal cord sections from P7 (A, B, E, F) and P15 (C, D, G, H) mice were subjected to ISH hybridization with Mbp and Plp probes respectively. (I, J) Spinal cord sections of P7 were simultaneously immunostained with anti-Olig2 and anti-APC antibodies. (K). Percentage of APC+ mature oligodendrocytes among Olig2+ cells in the ventral white matter region (*P<0.05). (L) The Mbp expression level was examined by western blotting. Scale bars, 100 μm in A–H; 20 μm in I and J.
Given that the number of OPCs was also decreased in the mutants, it is possible that Shp2 may not affect the terminal differentiation of OPCs, and the reduced oligodendrocyte differentiation in the mutants may simply reflect the decrease of OPC number. To examine this possibility, we calculated the percentage of oligodendrocytes that underwent terminal differentiation in P7 spinal cords. Double immunostaining with anti-APC (another marker for mature oligodendrocytes) and anti-Olig2 revealed that about 48% and 38% of Olig2+ cells had differentiated into APC+ mature oligodendrocytes in the control and mutant spinal cords, respectively (Fig 6I–K). Thus, a small (10%) but significant decrease in the percentage of mature oligodendrocytes in Olig2+ oligodendrocyte population was detected in the Shp2 mutants.
Ablation of Shp2 leads to reduced axonal myelination in the developing CNS
We next examined whether Shp2 function in oligodendrocytes is required for axonal myelination in the CNS. Spinal cord tissues from P15 control and mutant animals were processed for electron microscopy analysis. In the ventrolateral white matter region at the cortical-spinal tract position, about 78% axons were myelinated in the control tissues, as compared to 33% in the mutants (Fig 7). Therefore, Shp2 mutation resulted in a significant reduction of axonal myelination in parallel to the reduced oligodendrocyte differentiation.
Figure 7.
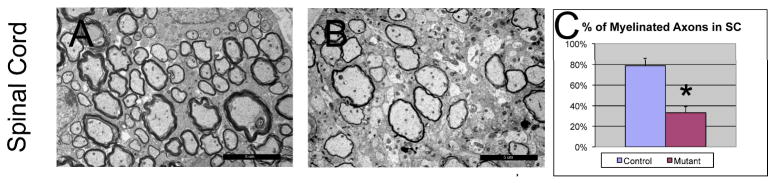
Reduced axonal myelination in the Shp2 mutant spinal cord at P15. The percentage of myelinated axons in the spinal cord is reduced compared to the control (C), *P<0.001. Scale bar, 5 μm.
Discussion
Shp2 functions to promote oligodendrocyte proliferation and differentiation
In this study, we provided the genetic evidence that Shp2 controls the proliferation of OPCs and therefore the size of oligodendrocyte population. In Olig1CreShp2loxp/loxp conditional mutants, fate specification of pMN neural progenitor cells and subsequent motor neuron development were not altered (Fig 1). However, there was a dramatic delay and reduction in the later generation of OPC cells from the pMN domain (Fig 2–3). Throughout embryonic and postnatal stages, the number of OPC cells was considerably smaller in the Shp2 conditional mutant spinal cords. BrdU labeling experiments showed the reduced proliferation rate of OPC cells in the mutant embryonic spinal cord. We failed to detect any OPC cells that underwent apoptosis in both the control and Shp2 mutant spinal tissues. Together, these findings suggest that the reduction of oligodendrocyte population in the Shp2 mutants is likely caused by the combination of delayed OPC generation from the pMN domain and the slower OPC proliferation in the parenchema, rather than by diminished cell survival.
Concomitant with the decrease of OPC population, the number of MBP+/PLP+ mature oligodendrocytes and myelinated axons in early postnatal spinal cords was markedly reduced (Fig 6, 7). However, the percentage of mature oligodendrocytes in the entire oligodendrocyte population in the Shp2 mutants was only slightly reduced as compared to the control, suggesting that Shp2 mutation only had mild effects on oligodendrocyte maturation and myelination (Fig 6I–J, Fig 7). It is possible that the decreased myelinating oligodendrocyte population in Shp2 conditional mutants was mainly caused by decreased OPC population, and to a lesser extent by the impaired differentiation program. Consistent with this notion, in CNPCre/+ Shp2flox/flox mutant spinal cord, ablation of Shp2 resulted in a smaller decrease in OPC population and a milder reduction of oligodendrocyte differentiation and myelin gene expression (unpublished observations).
Shp2 functions downstream of PDGFRα to regulate oligodendrocyte proliferation
Several lines of evidence suggested that the defective oligodendrocyte development of the Shp2 mutants is remarkably similar to that observed in PDGF-A knockout (Fu et al. 2002). First, in both mutant lines, the OPC population is remarkably reduced throughout embryonic and early postnatal stages (Fig 2; Fu et al. 2002). Second, early differentiation of a subset of oligodendrocytes in E16.5 spinal cords was not affected; however, these early-differentiated MBP+ oligodendrocytes were scattered around in the ventral spinal cord in both Shp2 (Fig 4) and PDGF-A null mutants (Fu et al. 2002). At this stage, it is not clear why Shp2 and PDGF-A signaling altered the location or migration, but not the differentiation, of this subpopulation of oligodendrocytes. Third, MBP+/PLP+ mature oligodendrocytes in the white matter at postnatal stages were significantly reduced in both mutants. Together, there findings strongly suggest that Shp2 may function downstream of PDGFRα signaling in regulating OPC proliferation and migration. PDGFRα is one of the earliest receptor tyrosine kinases epxressed in the OPCs since E12.5 (Pringle et al. 1992). Upon stimulation by PDGF-A, PDGFR undergoes autophosphorylation on tyrosine residues (Sultzman et al. 1991). Theorectically, Shp2 can bind to phosphorylated tyrosine residues in PDGFR-α intracellular domain via its SH2 domain, and transmit signals to downstream molecules through its tyrosine phosphatase domain (Feng 1999). At this stage, it is not known what signaling molecules lie downstream of Shp2 in the control of OPC proliferation and differentiation.
The functional relationship between Shp2 and PDGFR appears to be conserved in evolution, as the Drosophila homologue of Shp2 (Csw) transduces signals provided by Torso, a PDGF-like tyrosine kinase receptor (Perkins et al. 1992). Shp2 has also been implicated in positive signaling from PDGFR-β by directly binding to the receptor and becoming tyrosine-phosphorylated (Bennett et al. 1994; Lechleider et al. 1993a; Lechleider et al. 1993b). In addition, Shp2 has recently been shown to mediate neuregulin signaling in the control of Schwann cell differentiation and myelination (Grossmann et al. 2009), suggesting a context-dependent regulation of Shp2 activity in the control of glial development.
Supplementary Material
Acknowledgments
We are very grateful to Drs. Charles Stiles, David Rowitch and Richard Lu for generously providing the Olig1Cre mutant mouse lines and the anti-Olig2 antibody. This work is supported by NIH (NS37717) and by National Multiple Sclerosis Society (RG 3275).
References
- Allard JD, Chang HC, Herbst R, McNeill H, Simon MA. The SH2-containing tyrosine phosphatase corkscrew is required during signaling by sevenless, Ras1 and Raf. Development. 1996;122(4):1137–46. doi: 10.1242/dev.122.4.1137. [DOI] [PubMed] [Google Scholar]
- Barres BA, Schmid R, Sendnter M, Raff MC. Multiple extracellular signals are required for long-term oligodendrocyte survival. Development. 1993;118(1):283–95. doi: 10.1242/dev.118.1.283. [DOI] [PubMed] [Google Scholar]
- Bennett AM, Tang TL, Sugimoto S, Walsh CT, Neel BG. Protein-tyrosine-phosphatase SHPTP2 couples platelet-derived growth factor receptor beta to Ras. Proc Natl Acad Sci U S A. 1994;91(15):7335–9. doi: 10.1073/pnas.91.15.7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogler O, Wren D, Barnett SC, Land H, Noble M. Cooperation between two growth factors promotes extended self-renewal and inhibits differentiation of oligodendrocyte-type-2 astrocyte (O-2A) progenitor cells. Proc Natl Acad Sci U S A. 1990;87(16):6368–72. doi: 10.1073/pnas.87.16.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Qi Y, Hu X, Tan M, Liu Z, Zhang J, Li Q, Sander M, Qiu M. Generation of oligodendrocyte precursor cells from mouse dorsal spinal cord independent of Nkx6 regulation and Shh signaling. Neuron. 2005;45(1):41–53. doi: 10.1016/j.neuron.2004.12.028. [DOI] [PubMed] [Google Scholar]
- Cai J, Zhu Q, Zheng K, Li H, Qi Y, Cao Q, Qiu M. Co-localization of Nkx6.2 and Nkx2.2 homeodomain proteins in differentiated myelinating oligodendrocytes. Glia. 58(4):458–468. doi: 10.1002/glia.20937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calver AR, Hall AC, Yu WP, Walsh FS, Heath JK, Betsholtz C, Richardson WD. Oligodendrocyte population dynamics and the role of PDGF in vivo. Neuron. 1998;20(5):869–82. doi: 10.1016/s0896-6273(00)80469-9. [DOI] [PubMed] [Google Scholar]
- Cao Q, Xu X-M, DeVries WH, Enzmann GU, Ping P, Tsoulfas P, Wood PM, Bunge MB, Whittemore SR. Functional Recovery in Traumatic Spinal Cord Injury after Transplantation of Multineurotrophin-Expressing Glial-Restricted Precursor Cells. J Neurosci. 2005;25(30):6947–6957. doi: 10.1523/JNEUROSCI.1065-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng G-S. Shp-2 Tyrosine Phosphatase: Signaling One Cell or Many. Experimental Cell Research. 1999;253(1):47–54. doi: 10.1006/excr.1999.4668. [DOI] [PubMed] [Google Scholar]
- Feng GS, Hui CC, Pawson T. SH2-containing phosphotyrosine phosphatase as a target of protein-tyrosine kinases. Science. 1993;259(5101):1607–1611. doi: 10.1126/science.8096088. [DOI] [PubMed] [Google Scholar]
- Feng GS, Pawson T. Phosphotyrosine phosphatases with SH2 domains: regulators of signal transduction. Trends Genet. 1994;10(2):54–8. doi: 10.1016/0168-9525(94)90149-x. [DOI] [PubMed] [Google Scholar]
- Fernandes-Alnemri T, Litwack G, Alnemri ES. CPP32, a novel human apoptotic protein with homology to Caenorhabditis elegans cell death protein Ced-3 and mammalian interleukin-1 beta-converting enzyme. Journal of Biological Chemistry. 1994;269(49):30761–30764. [PubMed] [Google Scholar]
- Fruttiger M, Karlsson L, Hall AC, Abramsson A, Calver AR, Bostrom H, Willetts K, Bertold CH, Heath JK, Cothers Betsholtz. Defective oligodendrocyte development and severe hypomyelination in PDGF-A knockout mice. Development. 1999;126(3):457–67. doi: 10.1242/dev.126.3.457. [DOI] [PubMed] [Google Scholar]
- Fu H, Qi Y, Tan M, Cai J, Takebayashi H, Nakafuku M, Richardson W, Qiu M. Dual origin of spinal oligodendrocyte progenitors and evidence for the cooperative role of Olig2 and Nkx2.2 in the control of oligodendrocyte differentiation. Development. 2002;129(3):681–693. doi: 10.1242/dev.129.3.681. [DOI] [PubMed] [Google Scholar]
- Gauthier AS, Furstoss O, Araki T, Chan R, Neel BG, Kaplan DR, Miller FD. Control of CNS cell-fate decisions by SHP-2 and its dysregulation in Noonan syndrome. Neuron. 2007;54(2):245–62. doi: 10.1016/j.neuron.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann KS, Wende H, Paul FE, Cheret C, Garratt AN, Zurborg S, Feinberg K, Besser D, Schulz H, Eothers Peles. The tyrosine phosphatase Shp2 (PTPN11) directs Neuregulin-1/ErbB signaling throughout Schwann cell development. Proceedings of the National Academy of Sciences. 2009;106(39):16704–16709. doi: 10.1073/pnas.0904336106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke Y, Zhang EE, Hagihara K, Wu D, Pang Y, Klein R, Curran T, Ranscht B, Feng G-S. Deletion of Shp2 in the Brain Leads to Defective Proliferation and Differentiation in Neural Stem Cells and Early Postnatal Lethality. Mol Cell Biol. 2007;27(19):6706–6717. doi: 10.1128/MCB.01225-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechleider RJ, Freeman RM, Jr, Neel BG. Tyrosyl phosphorylation and growth factor receptor association of the human corkscrew homologue, SH-PTP2. J Biol Chem. 1993a;268(18):13434–8. [PubMed] [Google Scholar]
- Lechleider RJ, Sugimoto S, Bennett AM, Kashishian AS, Cooper JA, Shoelson SE, Walsh CT, Neel BG. Activation of the SH2-containing phosphotyrosine phosphatase SH-PTP2 by its binding site, phosphotyrosine 1009, on the human platelet-derived growth factor receptor. J Biol Chem. 1993b;268(29):21478–81. [PubMed] [Google Scholar]
- Levison SW, Chuang C, Abramson BJ, Goldman JE. The migrational patterns and developmental fates of glial precursors in the rat subventricular zone are temporally regulated. Development. 1993;119(3):611–22. doi: 10.1242/dev.119.3.611. [DOI] [PubMed] [Google Scholar]
- Lu QR, Sun T, Zhu Z, Ma N, Garcia M, Stiles CD, Rowitch DH. Common Developmental Requirement for Olig Function Indicates a Motor Neuron/Oligodendrocyte Connection. Cell. 2002;109(1):75–86. doi: 10.1016/s0092-8674(02)00678-5. [DOI] [PubMed] [Google Scholar]
- Lu QR, Di Yuk, Alberta JA, Zhu Z, Pawlitzky I, Chan J, McMahon AP, Stiles CD, Rowitch DH. Sonic Hedgehog-Regulated Oligodendrocyte Lineage Genes Encoding bHLH Proteins in the Mammalian Central Nervous System. Neuron. 2000;25(2):317–329. doi: 10.1016/s0896-6273(00)80897-1. [DOI] [PubMed] [Google Scholar]
- Lu X, Chou TB, Williams NG, Roberts T, Perrimon N. Control of cell fate determination by p21ras/Ras1, an essential component of torso signaling in Drosophila. Genes Dev. 1993;7(4):621–32. doi: 10.1101/gad.7.4.621. [DOI] [PubMed] [Google Scholar]
- Mayer M, Bhakoo K, Noble M. Ciliary neurotrophic factor and leukemia inhibitory factor promote the generation, maturation and survival of oligodendrocytes in vitro. Development. 1994;120(1):143–153. doi: 10.1242/dev.120.1.143. [DOI] [PubMed] [Google Scholar]
- McKinnon RD, Matsui T, Dubois-Dalcq M, Aaronson SA. FGF modulates the PDGF-driven pathway of oligodendrocyte development. Neuron. 1990;5(5):603–14. doi: 10.1016/0896-6273(90)90215-2. [DOI] [PubMed] [Google Scholar]
- Miller RH. Regulation of oligodendrocyte development in the vertebrate CNS. Progress in Neurobiology. 2002;67(6):451–467. doi: 10.1016/s0301-0082(02)00058-8. [DOI] [PubMed] [Google Scholar]
- Neel BG, Gu H, Pao L. The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003;28(6):284–93. doi: 10.1016/S0968-0004(03)00091-4. [DOI] [PubMed] [Google Scholar]
- Nicholson DW, Ali A, Thornberry NA, Vaillancourt JP, Ding CK, Gallant M, Gareau Y, Griffin PR, Labelle M, Aothers Lazebnik Y. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376(6535):37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- Nicole S, Andrea G. A single protocol to detect transcripts of various types and expression levels in neural tissue and cultured cells: in situ hybridization using digoxigenin-labelled cRNA probes. Histochemistry and Cell Biology. 1993;V100(6):431–440. doi: 10.1007/BF00267823. [DOI] [PubMed] [Google Scholar]
- Noble M, Barnett SC, Bogler O, Land H, Wolswijk G, Wren D. Control of division and differentiation in oligodendrocyte-type-2 astrocyte progenitor cells. Ciba Found Symp. 1990;150:227–43. doi: 10.1002/9780470513927.ch14. discussion 244-9. [DOI] [PubMed] [Google Scholar]
- Noble M, Murray K, Stroobant P, Waterfield MD, Riddle P. Platelet-derived growth factor promotes division and motility and inhibits premature differentiation of the oligodendrocyte/type-2 astrocyte progenitor cell. Nature. 1988;333(6173):560–2. doi: 10.1038/333560a0. [DOI] [PubMed] [Google Scholar]
- Noll E, Miller RH. Oligodendrocyte precursors originate at the ventral ventricular zone dorsal to the ventral midline region in the embryonic rat spinal cord. Development. 1993;118(2):563–73. doi: 10.1242/dev.118.2.563. [DOI] [PubMed] [Google Scholar]
- Perkins LA, Johnson MR, Melnick MB, Perrimon N. The nonreceptor protein tyrosine phosphatase corkscrew functions in multiple receptor tyrosine kinase pathways in Drosophila. Dev Biol. 1996;180(1):63–81. doi: 10.1006/dbio.1996.0285. [DOI] [PubMed] [Google Scholar]
- Perkins LA, Larsen I, Perrimon N. corkscrew encodes a putative protein tyrosine phosphatase that functions to transduce the terminal signal from the receptor tyrosine kinase torso. Cell. 1992;70(2):225–36. doi: 10.1016/0092-8674(92)90098-w. [DOI] [PubMed] [Google Scholar]
- Pringle NP, Mudhar HS, Collarini EJ, Richardson WD. PDGF receptors in the rat CNS: during late neurogenesis, PDGF alpha-receptor expression appears to be restricted to glial cells of the oligodendrocyte lineage. Development. 1992;115(2):535–51. doi: 10.1242/dev.115.2.535. [DOI] [PubMed] [Google Scholar]
- Raff MC, Lillien LE, Richardson WD, Burne JF, Noble MD. Platelet-derived growth factor from astrocytes drives the clock that times oligodendrocyte development in culture. Nature. 1988;333(6173):562–5. doi: 10.1038/333562a0. [DOI] [PubMed] [Google Scholar]
- Richardson WD, Pringle N, Mosley MJ, Westermark B, Dubois-Dalcq M. A role for platelet-derived growth factor in normal gliogenesis in the central nervous system. Cell. 1988;53(2):309–19. doi: 10.1016/0092-8674(88)90392-3. [DOI] [PubMed] [Google Scholar]
- Richardson WD, Smith HK, Sun T, Pringle NP, Hall A, Woodruff R. Oligodendrocyte lineage and the motor neuron connection. Glia. 2000;29(2):136–142. doi: 10.1002/(sici)1098-1136(20000115)29:2<136::aid-glia6>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Rowitch DH. Glial specification in the vertebrate neural tube. Nat Rev Neurosci. 2004;5(5):409–419. doi: 10.1038/nrn1389. [DOI] [PubMed] [Google Scholar]
- Stolt CC, Rehberg S, Ader M, Lommes P, Riethmacher D, Schachner M, Bartsch U, Wegner M. Terminal differentiation of myelin-forming oligodendrocytes depends on the transcription factor Sox10. Genes and Development. 2002;16(2):165–170. doi: 10.1101/gad.215802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultzman L, Ellis C, Lin LL, Pawson T, Knopf J. Platelet-derived growth factor increases the in vivo activity of phospholipase C-gamma 1 and phospholipase C-gamma 2. Mol Cell Biol. 1991;11(4):2018–25. doi: 10.1128/mcb.11.4.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebayashi H, Yoshida S, Sugimori M, Kosako H, Kominami R, Nakafuku M, Yi Nabeshima. Dynamic expression of basic helix-loop-helix Olig family members: implication of Olig2 in neuron and oligodendrocyte differentiation and identification of a new member, Olig3. Mechanisms of Development. 2000;99(1–2):143–148. doi: 10.1016/s0925-4773(00)00466-4. [DOI] [PubMed] [Google Scholar]
- Vallstedt A, Klos JM, Ericson J. Multiple Dorsoventral Origins of Oligodendrocyte Generation in the Spinal Cord and Hindbrain. 2005;45(1):55–67. doi: 10.1016/j.neuron.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Vos JP, Gard AL, Pfeiffer SE. Regulation of oligodendrocyte cell survival and differentiation by ciliary neurotrophic factor, leukemia inhibitory factor, oncostatin M, and interleukin-6. Perspect Dev Neurobiol. 1996;4(1):39–52. [PubMed] [Google Scholar]
- Zhang EE, Chapeau E, Hagihara K, Feng GS. Neuronal Shp2 tyrosine phosphatase controls energy balance and metabolism. Proc Natl Acad Sci U S A. 2004;101(45):16064–9. doi: 10.1073/pnas.0405041101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Wang S, Anderson DJ. Identification of a Novel Family of Oligodendrocyte Lineage-Specific Basic Helix-Loop-Helix Transcription Factors. Neuron. 2000;25(2):331–343. doi: 10.1016/s0896-6273(00)80898-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.