Abstract
Objective
Memory complaints increase as women transition from premenopausal to postmenopausal stages. We explored the extent to which subjective memory complaints were associated with objective cognitive test performance, affective symptoms, and menopausal symptoms in midlife women with moderate to severe vasomotor symptoms. We predicted that subjective memory complaints would be related to affective symptoms and lower performance on tests of memory and attention.
Methods
Sixty-eight midlife women (mean age = 53; 54% African American) with ≥ 35 hot flashes per week completed the Memory Functioning Questionnaire (MFQ), a battery of objective cognitive tests, a menopausal symptom inventory and mood questionnaires. Linear regression analyses were conducted to examine predictors (symptoms and objective cognitive scores) of ratings on each of four MFQ subscales and a validated, single-item rating of current memory.
Results
Negative affect and delayed verbal memory predicted a single-item rating of current memory. Negative affect and poorer scores on tests of attention and working memory predicted Frequency of Forgetting. Lower positive affect, higher vasomotor symptoms, and increased age predicted lower Retrospective Memory Functioning. Increased age predicted Use of Mnemonics.
Conclusions
These findings strengthen the growing body of evidence that women with memory complaints during the menopausal transition have an accurate appraisal of their memory function and that their complaints also relate to affect and to a lesser extent vasomotor symptoms. Given that cognitive performance was in the normal range, these findings suggest that women can detect subtle changes in memory performance during the transition.
Keywords: Cognition, Memory, Metamemory, Menopause, Hot Flashes, Hormones
Memory complaints are a common symptom of the menopausal transition. Findings from the Study of Women’s Health Across the Nation (SWAN) revealed a significant difference in the frequency of complaints of forgetfulness when comparing the premenopausal stage (31%) to the perimenopausal (41%) and postmenopausal (44%) stages.1 Memory complaints were related to obtaining less than a high school education, being over 44 years old, lacking full-time employment, and having low levels of physical activity.1 In the Seattle Midlife Women’s Health Study, 62% of women noted an undesirable change in memory functioning, and they most often attributed the decline in memory to stress, health issues, and aging.2 A follow-up study revealed that forgetfulness related to hot flashes and difficulty concentrating.3 Despite some differences, memory complaints among midlife women are high cross-culturally; in the Decisions at Menopause Study, over 45% of women living in Massachusetts, Madrid, and Beirut complained of memory loss, compared to 34% of women living in Rabat, Morocco.4
Three studies have investigated the relationship between subjective memory complaints and objective memory performance in women during the menopausal transition. In a study involving a total of 120 pre-, peri-, and postmenopausal women, lower verbal memory and attention performance as well as mood symptoms were associated with subjective complaints (i.e., “Do you have problems with your memory?”);5 however, stage of menopause did not influence this relationship. A second study of 24 perimenopausal women found a relationship between objective memory performance on the Rey Auditory Verbal Learning Test and subjective memory on the Memory Functioning Questionnaire (MFQ).6 A larger follow-up study in 75 perimenopausal women found that depressive symptoms, somatic complaints, sleep, complex attention, and working memory performance were associated with subjective complaints on the MFQ.7 Overall, across studies, subjective memory complaints were associated with mood symptoms as well as objective cognitive test performance; however, it is important to note that the cognitive domains associated with these complaints differed across studies (e.g., attention, verbal memory, and working memory).
In SWAN and other studies, self-reported hot flashes are unrelated to objective cognitive performance;8–10 however, in studies that do not include measures of objective cognitive performance, hot flashes have been shown to be a significant predictor of subjective forgetfulness.11 There are conflicting reports on the association between vasomotor symptoms and subjective memory complaints. In a study of women across the lifespan, vasomotor frequency was related to increased interference from memory problems during daily living and a higher likelihood of spontaneously reporting cognitive problems.5 Furthermore, the intensity of hot flashes was positively associated with the duration of reported memory and attention problems. Conversely, there was not a relationship between subjective memory complaints and self-reported vasomotor symptoms in two study populations of perimenopausal women.6, 7
The aim of the present study was to extend studies of the relationship between objective and subjective memory complaints in midlife women to a sample of women with moderate to severe vasomotor symptoms. To this end, we measured subjective memory complaints and objective memory performance in a sample of 68 midlife women with at least 35 hot flashes per week. We included measures of menopausal symptoms (including vasomotor and sleep symptoms), negative and positive affect, and a comprehensive neuropsychological test battery including measures of episodic memory (i.e., list learning, story recall), working memory, attention, and processing speed. Finally, as a primary outcome measure we used a validated measure of subjective memory which was used previously in a study of perimenopausal women.5 We predicted that lower scores on objective cognitive tests and affective symptoms would predict increased subjective memory complaints. In addition, we predicted that higher vasomotor symptoms would be associated with more subjective memory complaints.
METHODS
Participants
Participants were recruited from a pool of women who were screened for entry into two related studies: 1) a randomized clinical trial comparing the effect of black cohosh, red clover, conjugated equine estrogens plus medroxyprogesterone acetate, and placebo on vasomotor symptoms;12 and 2) an ancillary study examining treatment-related effects on cognitive outcomes.13 Women were recruited into the main clinical trial through internet and bulletin board advertisements and targeted mailings to women residing in the Chicago area. Inclusionary criteria for the clinical trial included: 1) last menstrual period 6 months to 10 years before recruitment; 2) a minimum of 35 hot flashes per week as indicated by diaries completed over a minimum of 2 weeks; and 3) intact uterus and ovaries. Exclusionary criteria included: 4) use of hormone therapy, alternative botanical menopausal therapies, or oral contraceptive use within 6 months of study entry; 5) smoking; 6) contraindications to hormone therapy (e.g., vascular disease, hypertension, abnormal vaginal bleeding, history of blood clots, diabetes, abnormal mammogram); and 7) major systemic illness. Additional exclusionary criteria for the ancillary cognitive study included: 8) diagnosis of an Axis I psychiatric disorder; 9) any medical condition that affects cognitive function (e.g. stroke, traumatic brain injury); 10) use of prescription or over-the-counter medications that affect cognitive function (e.g., antidepressants, gingko biloba); 11) first language other than English; and 12) participation in other clinical trials within 30 days. The data in this report were obtained before treatment initiation in women who volunteered to participate in an ancillary study that examined cognitive outcomes.13
A total of 70 women were recruited; two women were excluded because English was not their first language, leaving a total of 68 women in the final analyses. Table 1 presents the demographic characteristics of the 68 healthy midlife women who participated in the study. The participants ranged in age from 44 to 62 years (mean = 53.0).
Table 1.
Demographic information for participants.
| Variables | Range | M (SD) |
|---|---|---|
| Age | 44–62 | 53.00 (4.3) |
| Education | 12–20 | 15.1 (2.2) |
| Race (%) | ||
| Black | 54.4% | |
| White | 42.6% | |
| Asian | 1.5% | |
| Hispanic | 1.5% | |
| Reproductive Stage (%)a | ||
| Perimenopausal | 21% | |
| Postmenopausal | 79% | |
| Mean Months since LMP a | 42.9 (34.5) | |
| Green Climacteric | ||
| Vasomotor | 0–6 | 4.0 (1.6) |
| Somatic | 0–11 | 2.4 (2.1) |
| Psychological | 0–18 | 8.4 (4.2) |
| Utian Quality of Life Total | 60–102 | 81.9 (10.7) |
| PANAS Positive Affect | 14–46 | 32.3 (7.8) |
| PANAS Negative Affect | 10–33 | 16.4 (5.4) |
| Pittsburgh Sleep Quality Index Total | 1–9 | 4.54 (1.98) |
| Memory Functioning Questionnaire | ||
| Current Memory Rating | 1–7 | 4.1 (1.1) |
| Frequency of Forgetting | 2.50–6.56 | 4.7 (0.9) |
| Seriousness of Forgetting | 1.67–7 | 4.2 (1.2) |
| Retrospective Memory Functioning | 1–6 | 3.1 (1.0) |
| Use of Memory Aids & Mnemonics | 1–6.25 | 3.1 (1.4) |
Note: n = 68.
Values are missing for two women.
Before initiating treatment for the larger clinical trial participants completed the entire cognitive battery in one visit which lasted approximately 1.5 hours. The battery included the Memory Functioning Questionnaire, menopausal symptom inventory, sleep inventory, and affective symptom scales. Demographic information was obtained at the beginning of the baseline visit. Each participant met one-on-one with a trained test administrator who was blinded to study treatment, menopausal stage and the magnitude of subjective memory complaints when administering the battery. The cognitive test battery was modeled after the battery used in the COGnitive complaints in Early meNopause Trial (COGENT), a study comparing the cognitive effects of combination hormone therapy and placebo in recently menopausal women with subjective cognitive complaints.14 The test battery included the following measures:
Subjective Menopausal Symptom, Mood, Sleep and Memory Outcomes
Greene Climacteric Scale (GCS)15
This questionnaire assessed bother of menopausal symptoms occurring “at the moment”. The questionnaire contained 21 items that were scored on a 4-point Likert scale (0 = “not at all” to 3 = “extremely”). Four subcategories were measured, including: psychological symptoms; somatic symptoms; vasomotor symptoms and sexual dysfunction.
Modified Pittsburgh Sleep Quality Index (PSQI)
This questionnaire was modified from the original PSQI16 to measure latency to fall asleep, sleep duration and sleep disturbances. A total sleep score was calculated using a previously published scoring scheme, with higher scores indicating greater sleep disturbance.16
Positive and Negative Affect Scale (PANAS)17
This 21-item self-report questionnaire measured positive and negative mood states. Participants rated each mood state on a five-point Likert scale based on how descriptive each state was of their mood during the previous seven days. Examples of negative affect items included “distressed,” “anxious”, and “hostile.” Examples of positive affect items included “interested,” “excited” and “strong.” Ratings for each item ranged from 1 to 5, with 1 indicating “very slightly” and 5 indicating “extremely.” Outcomes included positive mood and negative mood, with a maximum score of five for each scale.
The Memory Functioning Questionnaire (MFQ)18
This questionnaire assessed subjective memory complaints. Participants rated the frequency with which they experience a series of memory lapses. Higher numbers indicated fewer subjective complaints. Each item was scored on a 1 to 7 Likert scale (1 = severe memory problems; 7 = no problems). The questionnaire yielded four subscales, including: Frequency of Forgetting; Seriousness of Forgetting; Retrospective Functioning; and Mnemonics Usage. Frequency of Forgetting assessed how often participants have memory failures in 18 situations. Seriousness of Forgetting assessed how serious participants consider memory failures in the same 18 situations. Retrospective Functioning assessed current memory functioning in relation to five different points earlier in life (e.g., 5 years ago, 10 years ago, etc.). Mnemonics Usage assessed how often participants use eight common memory aids. Current memory rating was based solely on the first test item, “How would you rate your memory in terms of the kinds of problems that you have?” This single item has been used as a general indicator of memory complaints.19
Objective Cognitive Outcomes
California Verbal Learning Test (CVLT-modified)20
We used a modified version of the CVLT that was used in the Women’s Health Initiative Study of Cognitive Aging (WHISCA) and COGENT.14, 21–23 The outcomes from the modified CVLT included total verbal learning and short- and long-delay free recall. During learning, participants were read a list of sixteen items from four semantic categories (e.g., vegetables) a total of three times, and after each iteration participants were asked to recall as many of those items as possible. An interference list consisting of sixteen words from four different semantic categories was read immediately following the end of the third learning trial. Participants were then asked to recall as many words as possible from the interference list. Following the interference recall, the short-delay recall trial was given wherein participants were prompted to recall as many words as possible from the original target list. Finally, after a 20-minute delay, participants were again prompted to recall as many words from the original word list (long-delay free recall).
Logical Memory Subtest of the Wechsler Memory Scale-Revised (WMS-R/LM-R)24
Participants were read a brief story and were informed that they would be asked to recall this story immediately, and again at a later time (30-minute delay). Outcome measures included standardized scores of story recall accuracy both immediately after presentation and after a 20-minute delay. Total scores ranged from 0 to 25.
Benton Visual Retention Test (BVRT)25
This test assessed short-term figural memory. Participants viewed a line drawing for 10 seconds, and were asked to immediately reproduce the drawing from memory on a blank piece of paper. The complexity of the line drawing increased across each of 10 trials. The outcome was the total number of correctly reproduced drawings.
Modified Card Rotations Test26
This test measured visuospatial skill. Participants were given three minutes to view a series of 20 line drawing geometric figures, each accompanied by eight alternatives which were either a two- or three-dimensional rotation of the target. Participants were instructed to mark targets that represented a 2-dimensional rotation of the target as “same” and mark those which were three-dimensional rotations as “different”. The outcome was the total number of correctly identified responses minus the number of incorrect responses, with a maximum score of 160.
Letter Fluency25
Participants were given one minute to generate as many words as possible that begin with a particular letter. The outcome was the total number of words produced across three trials.
Digit Span Forward and Backward24
This test assessed attention, short-term memory and working memory. For Digit Span Forward, the examiner read a series of number aloud and instructed the participant to repeat the series back to the examiner. For Digit Span Backwards, the participant was again read a string of numbers, and was asked to repeat the string back in reverse order. The outcome measures were the number of correctly completed trials for each part.
Brief Test of Attention - Modified (BTA)27, 28
This test measured auditory attention. During each of 20 trials, the examiner read aloud a list of numbers and letters, which increased in length and therefore difficulty. Participants were instructed to indicate the number of letters or numbers that were presented in each trial. The outcome was the total number of trials correctly completed.
Finding A’s Test29
This test assessed visuoperceptual speed and attention. Participants were given sheets of paper each containing five columns of words, and each of these columns had five words containing the letter ‘A.’ Participants were given two minutes to cross out as many words containing the letter ‘A’ as possible. The outcome was the total number of correctly marked words.
Statistical Analysis
We first checked the distribution of each variable for completeness, outliers, and distribution. Five individuals were missing items on the MFQ, and one individual was missing education, so missing data were re-coded to the statistical mean of that variable. All variables were normally distributed so analyses were conducted on raw scores. To avoid problems of multicollinearity, two composite z-scores were created, one for the CVLT and one for Logical Memory. The composite verbal memory score included the following CVLT measures—total words recalled across Trials 1–3, short- and long-delay free recall. The composite Logical Memory measure included immediate and delayed free recall. For each of these, we first computed z-scores for individual tests and then averaged these z-scores into composite z-scores. Next, we conducted a series of Pearson correlations between each of the MFQ outcomes and objective cognitive measures (CVLT composite, Logical Memory composite, and all other individual cognitive measures), menopausal symptoms (vasomotor, sleep), positive and negative affect, and demographic characteristics (age, education). In subsequent multivariable linear regression analyses, we examined variables associated with the MFQ outcomes at p<0.20. We only analyzed the individual measures comprising the composite scores when significant associations were found between the composite score and MFQ outcomes (these models used same covariates from the overall model). We performed manual forward and backward selection procedures to determine the best fitting and most parsimonious models. We included variables with trends toward significance (p≤0.10) in the final regression models. All p values were two sided and the statistical significance level was set at p<0.05. All analyses were performed using SAS, version 9.2 (SAS Institute, Inc., Cary, NC).
RESULTS
Table 1 shows the demographic characteristics of the sample. Our sample ranged in age between 44 and 62 years. Women were primarily African-American (54.4%), on average 3.6 years from their last menstrual period (LMP), and postmenopausal (79%). All participants had at least twelve years of education. As expected in women who report moderate-to-severe hot flashes, the burden of vasomotor symptoms was high when compared with other samples of midlife women.14, 30 Positive and negative affect was similar to reported normative data in a large sample of adults (n = 1,003).31 Table 2 presents the mean scores on the neuropsychological tasks. Cognitive scores were all within normal limits and were similar to scores in the COGENT trial, which used a similar cognitive battery in younger postmenopausal women.14
Table 2.
Mean (SD) scores for primary cognitive outcomes in midlife women with moderate-to-severe hot flashes.
| Range | Mean score (SD) | |
|---|---|---|
| Verbal Learning and Memory | ||
| CVLT Total Learing | 15–42 | 29.94 (6.07) |
| CVLT Short-Delay Free Recall | 3–15 | 9.50 (2.8) |
| CVLT Long-Delay Free Recall | 3–16 | 9.99 (3.0) |
| Logical Memory Immediate Recall | 6–21 | 13.57 (3.7) |
| Logical Memory Immediate Delayed Recall | 4–21 | 12.91 (3.8) |
| Working Memory | ||
| Digit Span Backward Correct | 2–14 | 6.41 (2.3) |
| Visual Memory | ||
| BVRT Total Score | 0–10 | 6.07 (2.3) |
| Visual Abililty | ||
| Card Rotation Total Correct | −7–117 | 63.61 (28.7) |
| Attention | ||
| BTA Total Score | 4–10 | 8.12 (1.6) |
| Finding A’s Total Correct | 7–56 | 29.54 (8.3) |
| Digit Span Forward Correct | 5–13 | 8.35 (2.0) |
| Verbal Fluency | ||
| Verbal Fluency Total | 13–88 | 42.99 (13.2) |
Notes n = 68. CVLT = California Verbal learning Task; BVRT = Benton Visual Retention Test; BTA = Brief Test of Attention.
Table 3 shows the variables that predicted Current Memory rating and scores on the four MFQ subscales. Less negative affective symptoms and better objective memory performance on the CVLT composite predicted fewer complaints about Current Memory Functioning (Figure 1). Better objective memory performance on the long delay trial of the CVLT significantly predicted fewer complaints about Current Memory Functioning (β=0.24, p=0.04) whereas trends were observed for total learning across Trials 1–3 (β=0.20, p=0.09) and short delay trial (β=0.22, p=0.055). Poorer performance on the Digit Span forward and higher endorsement of negative affect significantly predicted more frequent forgetting on the Frequency of Forgetting scale whereas a trend was observed for Digit Span backward. Lower positive affect, more vasomotor symptoms, and increased age predicted worse Retrospective Functioning. Finally, older age predicted greater use of Mnemonics. There were no significant predictors on the Seriousness of Forgetting Scale and self-reported sleep quality did not related to any subscale in the MFQ.
Table 3.
Predictors of subjective memory functioning by subscale of the Memory Functioning Questionnaire (MFQ) in midlife women with moderate-to-severe hot flashes.
| Subjective Outcomes on MFQ Subscale |
Predictors | B | SE | β | Model R2 |
|---|---|---|---|---|---|
| Current Memory Rating | Intercept | 4.07 | 0.12 | 0.14 | |
| PANAS Negative* | −0.05 | 0.02 | −0.25 | ||
| CVLT Composite* | 0.26 | 0.13 | 0.23 | ||
| Frequency of Forgetting | Intercept | 5.85 | 0.34 | 0.25 | |
| PANAS Negative* | −0.04 | 0.02 | −0.22 | ||
| Digit Span Forward* | 0.11 | 0.05 | 0.25 | ||
| Digit Span BackwardT | 0.09 | 0.05 | 0.24 | ||
| Seriousness of Forgetting | - | - | - | - | - |
| Retrospective Functioning | Intercept | 3.41 | 0.17 | 0.19 | |
| Age* | −0.05 | 0.03 | −0.24 | ||
| PANAS Positive** | 0.04 | 0.01 | 0.34 | ||
| GCS Vasomotor* | 0.13 | 0.07 | 0.23 | ||
| Mnemonics Usage | Intercept | 3.08 | 0.16 | 0.07 | |
| Age* | −0.09 | 0.04 | −0.26 |
Note. n = 68.
p < .001,
p < .05,
p > .05 and p < 0.10.
PANAS=Positive and Negative Affect Schedule, CVLT = California Verbal Learning Test, GCS= Greene Climacteric Scale. Higher scores on Current Memory Functioning, Frequency of Forgetting Seriousness of Forgetting, Retrospective Functioning and Mnemonics Usage relate to fewer problems or complaints within that domain. High values on the PANAS signify more mood states being endorsed. Higher scores on CVLT and Digit Span signify better performance. Higher values on the GCS signify more bothersome symptoms.
Figure 1.
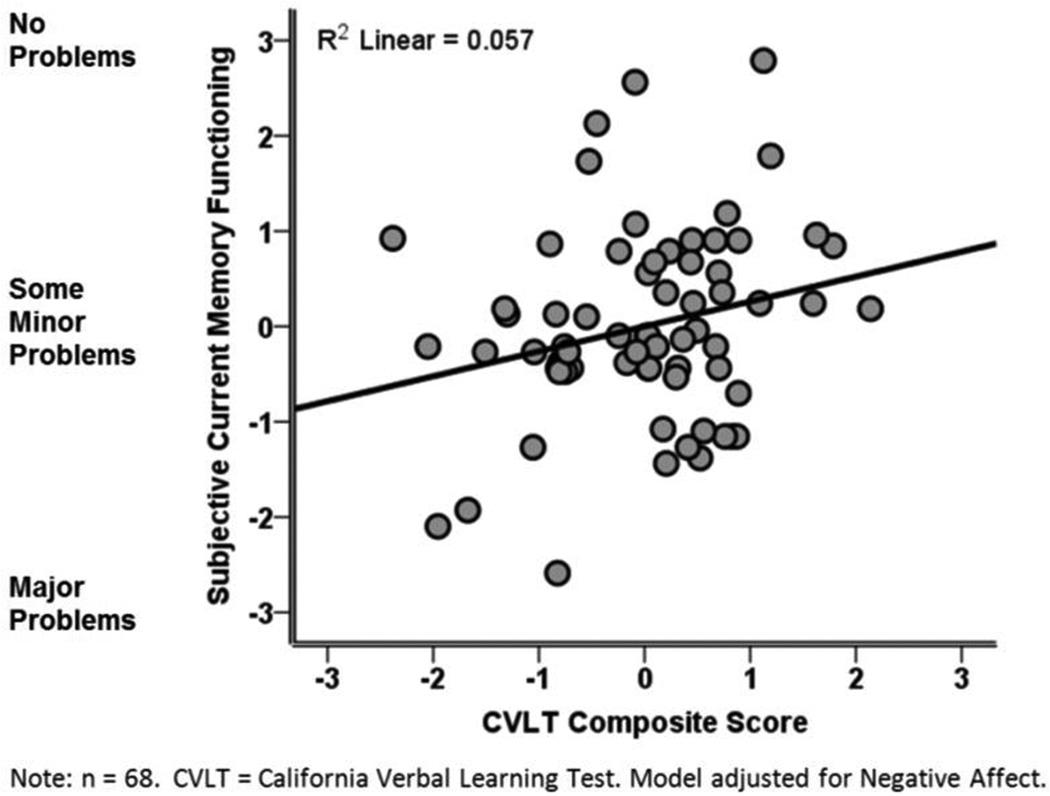
Poorer performance on the CVLT composite is associated with more severe subjective memory complaints in midlife women with moderate-to-severe hot flashes.
DISCUSSION
To our knowledge, the present study is the first to examine the relationship between subjective and objective memory complaints in a sample of women with moderate-to-severe hot flashes. Overall, the present study provides further validation that objective memory performance predicts the magnitude of subjective memory complaints in midlife women. Better subjective memory on the single-item, “How would you rate your memory in terms of the kinds of problems that you have?”19 related to better negative affect and better performance on an objective measure of delayed verbal memory. The other objective cognitive outcome that related to subjective memory was Digit Span Forward, which predicted Frequency of Forgetting. Affect was broadly predictive of subjective complaints. Hot flashes predicted worse scores on the Retrospective Memory Function Subscale, which assessed the extent to which women felt that their memory had declined from an earlier point in life. Older chronological age predicted increased use of mnemonic aids. Cognitive test scores for this sample were within normal limits, so these relationships reflect detection of cognitive variations within the range of normal cognitive test performance, not the detection of any clinical significant impairment in performance.
Our general pattern of findings is quite similar to findings from Weber et al.,7 who examined MFQ subscale scores in relation to similar predictor variables. Our sample included 68 midlife women (54% African American; mean age = 53 y; mean education = 15 y; 100% endorsing 35 or more hot flashes per week), and Weber et al.’s was based on 75 perimenopausal women (91% white; mean age = 49 y; mean education 16 y; 50% endorsing at least some hot flashes).7 Similar to the present study, Weber et al. found that more complaints of Frequency of Forgetting related to poorer attention and increased depressive symptoms, whereas more complaints about Seriousness of Forgetting related only to increased depressive symptoms.7 We found that global memory complaints related to both negative affect and objective cognitive performance as measured on a delayed verbal memory test. Weber et al. used a summary variable computed across scales as an index of overall subjective memory. As in the present study, they found that overall subjective memory complaints related to both affect and objective cognitive performance as measured by working memory. The consistency of findings from these two studies helps to validate women’s subjective memory complaints during the menopausal transition.
Higher self-reported vasomotor symptoms were associated with more retrospective memory functioning complaints, suggesting women with more vasomotor symptoms experienced cognitive complaints longer than women with fewer vasomotor symptoms. These results parallel previous research showing that intensity of vasomotor symptoms was associated with longer duration of memory and attention complaints.5 On the other hand, two studies of perimenopausal women found no relationship between vasomotor symptoms and subjective memory complaints.6, 7 A number of studies have examined the relationship between objective memory performance and hot flashes but find no association, even in large samples of women studied in the SWAN.8–10, 32 The SWAN dichotomized self-reported hot flashes into symptomatic versus asymptomatic women.32 It may be that relationships between vasomotor symptoms and memory complaints are most evident in highly symptomatic women and when vasomotor symptoms are examined as continuous variables.
Within the context of the broader cognitive literature, these results in midlife women are in agreement with some studies in older adults of both sexes which find that MFQ scores relate to both depressive symptoms and objective cognitive performance19, 33, 34 but contrast with other studies.35, 36 A review of studies comparing subjective and objective cognitive performance in adults of both sexes concluded that subjective memory complaints are not reliably related to cognitive impairment but are consistently related to depressive symptoms.37 The review also concluded that current cognitive complaints were more strongly related to future cognitive impairment and dementia than to current cognitive impairment.37
The clinical significance of memory complaints at midlife has yet to be determined, particularly in women transitioning through the menopause. Within this study population, objective cognitive performance was within expected ranges, and did not suggest clinical impairment; however recent neuroimaging studies in healthy middle aged men and women have demonstrated that individuals with cognitive complaints show altered patterns of brain activation during performance of cognitive tests of working memory when compared with those who have few to no complaints.38 This alteration in brain function was evident in reduced activation in the frontal cortex and was interpreted as indicative of brain vulnerability. A recent neuroimaging study showed that brain activation in the frontal cortex differed across pre-, peri-, and postmenopausal groups of women who performed an in-scanner verbal encoding task, and these differences remained after controlling for age.39
CONCLUSIONS
Better insights into the clinical significance of midlife memory complaints to later cognitive aging will be gained from continued longitudinal investigation of subjective and objective cognitive function as women age reproductively and chronologically. The largest study of objective cognitive performance across the menopausal transition indicates that processing speed and memory decrease during the perimenopause but return to expected levels during the postmenopause.32, 40 By contrast, subjective complaints increase with reproductive aging, with 40–65% of women endorsing some negative changes in their memory.1,2 It is known that depressive symptoms increase as women transition through menopause;41–44 notably, objective cognitive changes are maintained after accounting for depressive and anxiety symptoms.32 Understanding the impact of perimenopausal depressive symptoms on risk for cognitive dysfunction later in life is important because midlife depressive symptoms have been associated with the development of dementia later in life.45, 46
The present study has several limitations. First, although the study provides new insights into memory in women with moderate-to-severe vasomotor symptoms, the generalizability of the findings may be limited because the sample is a convenience sample and is comprised primarily of African-American women. Second, the study is cross-sectional and therefore does not address how the relationship between subjective and objective memory complaints might change with age or menopausal stage. Third, the study did not include a direct, clinical measure of depressive symptoms. Fourth, sleep and vasomotor symptoms were measured by self-report rather than with objective measures.
The strengths of this study include the use of a comprehensive and well-validated measure of subjective memory function, a comprehensive neuropsychological battery, and a new focus on women with moderate-to-severe vasomotor symptoms. Overall, this investigation strengthens the growing body of evidence that women who have memory complaints across the menopausal transition are accurately identifying objective memory changes within the normal range of performance and that mood symptoms influence their judgment of the frequency and seriousness of those complaints.
Acknowledgments
Financial Support: This research was supported by NIH/NCCAM grants K01AT002321-01 and R21AT001868-01 to P. Maki and by 5P50AT000155-01 grant to Norman Farnsworth (PI) for the NIH/NCCAM Botanical Dietary Supplements for Women's Health, with S. Geller as PI of the clinical trial in the Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: Pauline Maki has received consulting fees from Noven Pharmaceuticals. Lee Shulman has received speaking fees and honoraria from Noven Pharmaceuticals.
LITERATURE CITED
- 1.Gold EB, Sternfeld B, Kelsey JL, et al. Relation of demographic and lifestyle factors to symptoms in a multi-racial/ethnic population of women 40–55 years of age. American journal of epidemiology. 2000;152(5):463–473. doi: 10.1093/aje/152.5.463. [DOI] [PubMed] [Google Scholar]
- 2.Woods NF, Mitchell ES, Adams C. Menopause. 4. Vol. 7. New York, NY: 2000. Memory functioning among midlife women: observations from the Seattle Midlife Women's Health Study; pp. 257–265. [PubMed] [Google Scholar]
- 3.Woods NF, Smith-DiJulio K, Percival DB, Tao EY, Mariella A, Mitchell S. Menopause. 2. Vol. 15. New York, NY: 2008. Depressed mood during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women's Health Study; pp. 223–232. [DOI] [PubMed] [Google Scholar]
- 4.Obermeyer CM, Sievert LL. Menopause. 4. Vol. 14. New York, NY: 2007. Cross-cultural comparisons: midlife, aging, and menopause; pp. 663–667. [DOI] [PubMed] [Google Scholar]
- 5.Schaafsma M, Homewood J, Taylor A. Subjective cognitive complaints at menopause associated with declines in performance of verbal memory and attentional processes. Climacteric. 2009:1–15. doi: 10.3109/13697130903009187. [DOI] [PubMed] [Google Scholar]
- 6.Weber M, Mapstone M. Menopause. 4. Vol. 16. New York, NY: 2009. Memory complaints and memory performance in the menopausal transition; pp. 694–700. [DOI] [PubMed] [Google Scholar]
- 7.Weber MT, Mapstone M, Staskiewicz J, Maki PM. Menopause. 7. Vol. 19. New York, NY: 2012. Reconciling subjective memory complaints with objective memory performance in the menopausal transition; pp. 735–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ford N, Slade P, Butler G. An absence of evidence linking perceived memory problems to the menopause. The British journal of general practice : the journal of the Royal College of General Practitioners. 2004;54(503):434–438. [PMC free article] [PubMed] [Google Scholar]
- 9.LeBlanc ES, Neiss MB, Carello PE, Samuels MH, Janowsky JS. Menopause. 2. Vol. 14. New York, NY: 2007. Hot flashes and estrogen therapy do not influence cognition in early menopausal women; pp. 191–202. [Randomized Controlled Trial Research Support, N.I.H., Extramural]. [DOI] [PubMed] [Google Scholar]
- 10.Polo-Kantola P, Erkkola R. Sleep and the menopause. The journal of the British Menopause Society. 2004;10(4):145–150. doi: 10.1258/1362180042721076. [Research Support, Non-U.S. Gov't Review]. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell ES, Woods NF. Cognitive symptoms during the menopausal transition and early postmenopause. Climacteric. 2011;14(2):252–261. doi: 10.3109/13697137.2010.516848. [Research Support, N.I. H., Extramural]. [DOI] [PubMed] [Google Scholar]
- 12.Geller SE, Shulman LP, van Breemen RB, et al. Menopause. 6. Vol. 16. New York, NY: 2009. Safety and efficacy of black cohosh and red clover for the management of vasomotor symptoms: a randomized controlled trial; pp. 1156–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maki PM, Rubin LH, Fornelli D, et al. Menopause. 6. Vol. 16. New York, NY: 2009. Effects of botanicals and combined hormone therapy on cognition in postmenopausal women; pp. 1167–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maki PM, Gast MJ, Vieweg AJ, Burriss SW, Yaffe K. Hormone therapy in menopausal women with cognitive complaints: a randomized, double-blind trial. Neurology. 2007;69(13):1322–1330. doi: 10.1212/01.wnl.0000277275.42504.93. [Randomized Controlled Trial Research Support, Non-U.S. Gov't]. [DOI] [PubMed] [Google Scholar]
- 15.Greene J. Constructing a standard climacteric scale. Maturitas. 1998;29 doi: 10.1016/s0378-5122(98)00025-5. [DOI] [PubMed] [Google Scholar]
- 16.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 17.Watson D, Clark L, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of personality and social psychology. 1988;54:1062–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 18.Gilewski MJ, Zelinski EM. Memory Functioning Questionnaire (MFQ) Psychopharmacology bulletin. 1988;24(4):665–670. [PubMed] [Google Scholar]
- 19.Smith GE, Petersen RC, Ivnik RJ, Malec JF, Tangalos EG. Subjective memory complaints, psychological distress, and longitudinal change in objective memory performance. Psychol Aging. 1996;11(2):272–279. doi: 10.1037//0882-7974.11.2.272. [DOI] [PubMed] [Google Scholar]
- 20.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test - Research Edition. New York: The Psychological Corporation; 1987. [Google Scholar]
- 21.Resnick SM, Coker LH, Maki PM, Rapp SR, Espeland MA, Shumaker SA. The Women's Health Initiative Study of Cognitive Aging (WHISCA): a randomized clinical trial of the effects of hormone therapy on age-associated cognitive decline. Clinical trials (London, England) 2004;1(5):440–450. doi: 10.1191/1740774504cn040oa. [DOI] [PubMed] [Google Scholar]
- 22.Resnick SM, Maki PM, Rapp SR, et al. Effects of combination estrogen plus progestin hormone treatment on cognition and affect. The Journal of clinical endocrinology and metabolism. 2006;91(5):1802–1810. doi: 10.1210/jc.2005-2097. [Randomized Controlled Trial Research Support, N.I.H., Extramural Research Support, N.I.H., Intramural Research Support, Non-U.S. Gov't]. [DOI] [PubMed] [Google Scholar]
- 23.Resnick SM, Espeland MA, An Y, et al. Effects of conjugated equine estrogens on cognition and affect in postmenopausal women with prior hysterectomy. The Journal of clinical endocrinology and metabolism. 2009;94(11):4152–4161. doi: 10.1210/jc.2009-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wechsler D. Wechsler Adult Intelligence Scale - Revised. New York: Psychological Corporation; 1981. [Google Scholar]
- 25.Benton AL. Differential behavioral effects in frontal lobe disease. Neuropsychologia. 1968;6:53–60. [Google Scholar]
- 26.Wilson J, DeFries J, McLearn G, Vandenberg S, Johnson R, Rashad M. Cognitive abilities: use of family data as a control to assess sex and age differences in two ethnic groups. International Journal of Aging and Human Development. 1975;6:261–276. doi: 10.2190/BBJP-XKUG-C6EW-KYB7. [DOI] [PubMed] [Google Scholar]
- 27.Schretlen D, Bobholz JH, Brandt J. Development and psychometric properties of the Brief Test of Attention. Clinical Neuropsychologist. 1996;10:80–89. [Google Scholar]
- 28.Schretlen D, Brandt J, Bobholz JH. Validation of the Brief Test of Attention in patients with Huntington's disease. Clinical Neuropsychologist. 1996;10:90–95. [Google Scholar]
- 29.Ekstrom RB, French JW, Harman HH. Manual for Kit of Factor-Referenced Cognitive Tests. Princeton NJ: Educational Testing Service; 1976. [Google Scholar]
- 30.Ziaei S, Moghasemi M, Faghihzadeh S. Comparative effects of conventional hormone replacement therapy and tibolone on climacteric symptoms and sexual dysfunction in postmenopausal women. Climacteric. 2010;13(2):147–156. doi: 10.1080/13697130903009195. [Comparative Study Randomized Controlled Trial]. [DOI] [PubMed] [Google Scholar]
- 31.Crawford JR, Henry JD. The positive and negative affect schedule (PANAS): construct validity, measurement properties and normative data in a large non-clinical sample. The British journal of clinical psychology / the British Psychological Society. 2004;43(Pt 3):245–265. doi: 10.1348/0144665031752934. [Validation Studies]. [DOI] [PubMed] [Google Scholar]
- 32.Greendale GA, Wight RG, Huang MH, et al. Menopause-associated symptoms and cognitive performance: results from the study of women's health across the nation. American journal of epidemiology. 2010;171(11):1214–1224. doi: 10.1093/aje/kwq067. [Research Support, N.I.H., Extramural]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johansson B, Allen-Burge R, Zarit SH. Self-reports on memory functioning in a longitudinal study of the oldest old: relation to current, prospective, and retrospective performance. J Gerontol B Psychol Sci Soc Sci. 1997;52(3):P139–P146. doi: 10.1093/geronb/52b.3.p139. [DOI] [PubMed] [Google Scholar]
- 34.Grut M, Jorm AF, Fratiglioni L, Forsell Y, Viitanen M, Winblad B. Memory complaints of elderly people in a population survey: variation according to dementia stage and depression. J Am Geriatr Soc. 1993;41(12):1295–1300. doi: 10.1111/j.1532-5415.1993.tb06478.x. [DOI] [PubMed] [Google Scholar]
- 35.Zelinski EM, Gilewski MJ, Anthony-Bergstone CR. Memory Functioning Questionnaire: concurrent validity with memory performance and self-reported memory failures. Psychol Aging. 1990;5(3):388–399. doi: 10.1037/0882-7974.5.3.388. [DOI] [PubMed] [Google Scholar]
- 36.Cook S, Marsiske M. Subjective memory beliefs and cognitive performance in normal and mildly impaired older adults. Aging Ment Health. 2006;10(4):413–423. doi: 10.1080/13607860600638487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reid LM, Maclullich AM. Subjective memory complaints and cognitive impairment in older people. Dementia and geriatric cognitive disorders. 2006;22(5–6):471–485. doi: 10.1159/000096295. [DOI] [PubMed] [Google Scholar]
- 38.Haley AP, Eagan DE, Gonzales MM, Biney FO, Cooper RA. Functional magnetic resonance imaging of working memory reveals frontal hypoactivation in middle-aged adults with cognitive complaints. J Int Neuropsychol Soc. 2011;17(5):915–924. doi: 10.1017/S1355617711000956. [DOI] [PubMed] [Google Scholar]
- 39.Berent-Spillson A, Persad CC, Love T, et al. Hormonal Environment Affects Cognition Independent of Age during the Menopause Transition. The Journal of clinical endocrinology and metabolism. 2012 doi: 10.1210/jc.2012-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greendale GA, Huang MH, Wight RG, et al. Effects of the menopause transition and hormone use on cognitive performance in midlife women. Neurology. 2009;72(21):1850–1857. doi: 10.1212/WNL.0b013e3181a71193. [Research Support, N.I.H., Extramural]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cohen LS, Soares CN, Vitonis AF, Otto MW, Harlow BL. Risk for new onset of depression during the menopausal transition: the Harvard study of moods and cycles. Archives of general psychiatry. 2006;63(4):385–390. doi: 10.1001/archpsyc.63.4.385. [DOI] [PubMed] [Google Scholar]
- 42.Freeman EW, Sammel MD, Lin H, Nelson DB. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Archives of general psychiatry. 2006;63(4):375–382. doi: 10.1001/archpsyc.63.4.375. [DOI] [PubMed] [Google Scholar]
- 43.Schmidt PJ, Murphy JH, Haq N, Rubinow DR, Danaceau MA. Stressful life events, personal losses, and perimenopause-related depression. Arch Womens Ment Health. 2004;7(1):19–26. doi: 10.1007/s00737-003-0036-2. [DOI] [PubMed] [Google Scholar]
- 44.Bromberger JT, Matthews KA, Schott LL, et al. Depressive symptoms during the menopausal transition: the Study of Women's Health Across the Nation (SWAN) Journal of affective disorders. 2007;103(1–3):267–272. doi: 10.1016/j.jad.2007.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bolla KI, Lindgren KN, Bonaccorsy C, Bleecker ML. Memory complaints in older adults. Fact or fiction? Arch Neurol. 1991;48(1):61–64. doi: 10.1001/archneur.1991.00530130069022. [DOI] [PubMed] [Google Scholar]
- 46.Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Archives of general psychiatry. 2006;63(5):530–538. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]


