Abstract
Introduction
Heart failure (HF) is a heterogeneous symptomatic disorder. The goal of this study was to identify and link common profiles of physical and psychological symptoms to 1-year event-free survival in adults with moderate to advanced HF.
Methods
Multiple valid, reliable, and domain-specific measures were used to assess physical and psychological symptoms. Latent class mixture modeling was used to identify distinct symptom profiles. Associations between observed symptom profiles and 1-year event-free survival were quantified using Cox proportional hazards modeling.
Results
The mean age (n=202) was 57±13 years, 50% were male, and 60% had class III/IV HF. Three distinct profiles, mild (41.7%), moderate (30.2%), and severe (28.1%), were identified that captured a gradient of both physical and psychological symptom burden (p<0.001 for all comparisons). Controlling for the Seattle HF Score, adults with the “moderate” symptom profile were 82% more likely (hazard ratio 1.82 (95% confidence interval 1.07–3.11), p=0.028), and adults with the “severe” symptom profile were more than twice as likely (hazard ratio 2.06 (95% confidence interval 1.21–3.52), p=0.001) to have a clinical event within one year than patients with the “mild” symptom profile.
Conclusions
Profiling patterns among physical and psychological symptoms identifies HF patient subgroups with significantly worse 1-year event-free survival independent of prognostication based on objective clinical HF data.
Introduction
Heart failure (HF) is the fastest growing cardiovascular disorder in the U.S. and the most common reason for hospitalization among older adults.1–3 Approximately one out of every seven adults with HF has symptoms at rest or with minimal exertion despite medical therapy4–6 and endures severe symptom burden and poor health-related quality-of-life.7–9 As the prevalence of HF increases,10 so will the number of adults living with daily symptoms who have poor quality-of-life and/or suffer premature death.
It is widely recognized that HF is a complex and heterogeneous disorder.11, 12 Similarly, the occurrence and type of symptoms vary among patients with HF.13, 14 Beyond the hallmark physical signs and symptoms of HF, such as edema and dyspnea, adults with HF also experience sleep disturbances15, 16 and significant psychological symptoms, such as depression, anxiety, and hostility.17–19 Yet, little is known about associations among physical and psychological symptoms in HF, particularly in adults with moderate to advanced HF. Moreover, we are bereft of insight into which patterns of physical and psychological symptoms are associated with unfavorable event-free survival, particularly as most risk prediction models included demographics and objective indices of HF severity and treatment only.20–22
Accordingly, the aims of this study were to 1) identify common profiles among multiple domains of physical and psychological symptoms, and 2) quantify the relationship between observed symptom profiles and 1-year event free survival. We hypothesize that distinct profiles among physical and psychological symptoms could be identified and would be associated with a gradient of clinical-event risk in adults with moderate to advanced HF. Further, we hypothesized that observed symptom profiles would provide complementary and additive information to demographic and clinical characteristics in predicting event-free survival.
Methods
Theoretical Framework
One framework for understanding psychological symptoms in HF involves considering them as consequences of physical symptoms (i.e. secondary symptoms).23, 24 We hypothesized that because physical symptoms (such as shortness of breath and daytime sleepiness) and psychological symptoms (such as depression and anxiety) have common pathophysiological determinants in HF they should occur concomitantly. That is, there are established links between neurohormonal activation and both physical symptoms25, 26 and psychological symptoms.27, 28 There are recognized links between platelet dysfunction and physical29 as well as psychological symptoms.30, 31 There are links between endothelial dysfunction and physical symptoms32, 33 and psychological symptoms.34 Finally there are established links between inflammation and both physical35 and psychological symptoms36 in patients with HF. Accordingly, our approach involved identifying patterns among both physical and psychological symptoms in adults with moderate to advanced HF.
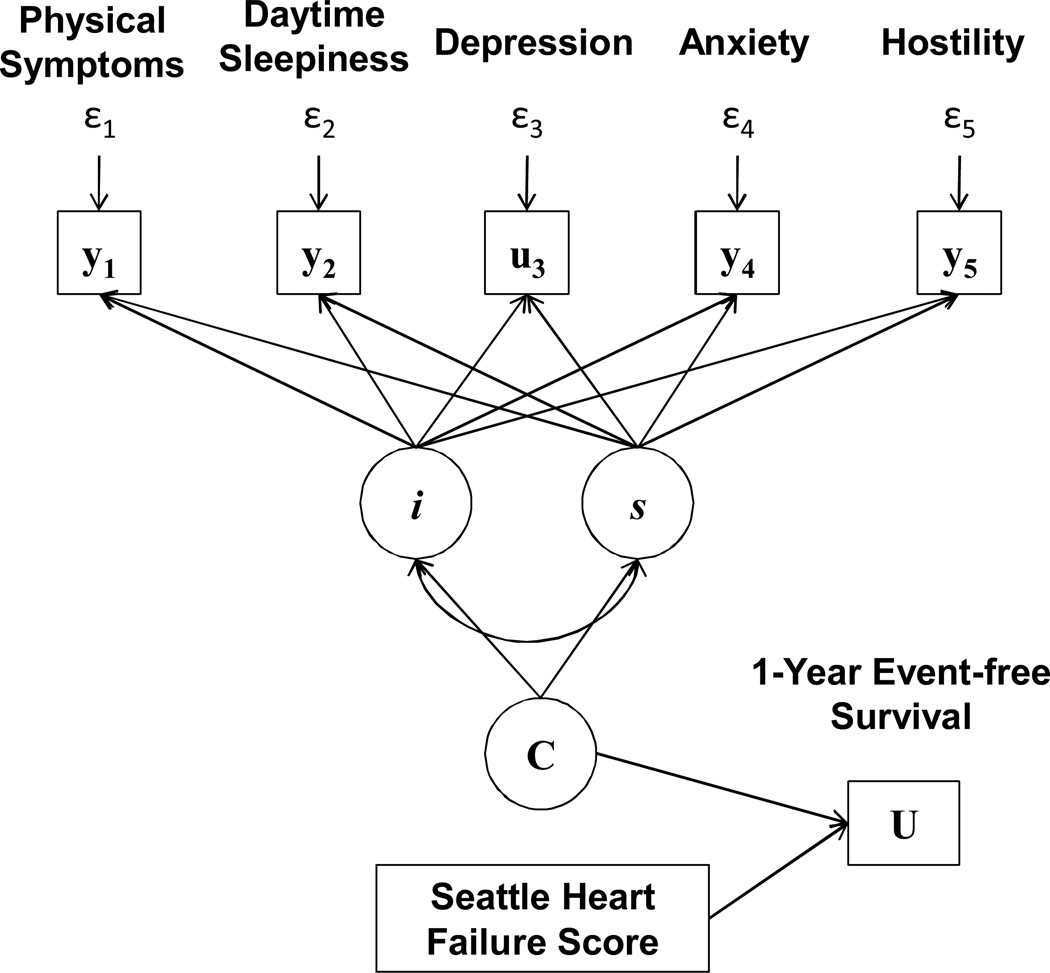
Our model of physical and psychological symptoms in HF (Figure 1) was informed by several tenets of Lenz’s Theory of Unpleasant Symptoms.37, 38 Specifically, we operationalized what Lenz termed ‘interactions among symptoms’ by identifying latent profiles (C) based on the intercepts (i) and slope (s) estimates of multiple continuous (y) and categorical (u) physical and psychological symptom measures. Moreover, and in an assessment of clinical utility, we linked observed symptom profiles to indices of what Lenz called ‘performance.’37, 38 In our case, we quantified associations between observed symptom profiles and 1-year clinical event-free survival (U), and adjusted these associations for the influence of a commonly-used HF risk prediction score (the Seattle HF Score (SHFS)).
Figure 1. Model of Physical and Psychological Symptoms in Heart Failure.
Note: Latent class mixture model identifying latent symptom profiles (C) based on the intercepts (i) and slope (s) estimates of continuous (y) and categorical (u) physical and psychological symptom measures, and predicting 1-year clinical event-free survival (U) controlling for the influence of Seattle Heart Failure Score.
Study Design
This paper addresses a primary aim of a completed prospective cohort study investigating gender differences in physical and psychological symptoms among adults with moderate to advanced HF. Key aspects of the study design include a 1:1 female to male enrollment, and a sampling frame of patients with current heart failure symptoms. Formal inclusion criteria included; 1) being willing and able to provide informed consent, 2) being 21 years of age or greater, 3) having the ability to read and comprehend 5th grade English, 4) experiencing current HF symptoms (New York Heart Association (NYHA) functional classification of II-IV), 5) being on optimal HF treatment or having HF treatment optimized in the opinion of the treating HF cardiologist, and 6) receiving health services locally or by a referral practice. Patients were deemed ineligible if they had a diagnosis of major cognitive impairment (e.g. Alzheimer’s disease) in the medical record, had a major and uncorrected visual impairment, or were unable to complete the study requirements including completing questionnaires written in English. Patients also were excluded if they had previously received heart transplantation or ventricular assist device implantation.
All patients were recruited through a single HF outpatient clinic in the Pacific Northwest that evaluates for and offers advanced HF therapies (e.g. ventricular assist devices and heart transplantation) between 2010 and 2012. Potential participants were approached for study participation immediately following a HF clinic visit. Written informed consent and HIPAA authorization were obtained from all interested participants by study staff not directly involved in patient care; the study was reviewed and approved by the appropriate academic institutional review board. Study participants completed a survey comprised of socio-demographic questions and physical and psychological symptoms measures in the clinic or returned the survey by mail. Review of the participant’s electronic medical record occurred at enrollment and one year following enrollment. Using the extensive referral network and linked electronic medical records and follow-up telephone calls to participants, details on clinical events that occurred during the year following symptom assessment were recorded. There was a 4.2% refusal rate for study participation and a 92% survey completion rate for recruited patients; results in this paper include only patients who completed the symptom survey at enrollment.
Measurement
Socio-demographics were assessed using a questionnaire that inquires about gender, age, marital/partnership status, ethnicity/race and employment. NYHA functional classification was assessed on the day of enrollment by attending HF cardiologists immediately prior to patient enrollment. Clinical and treatment characteristics, including last known left ventricular ejection fraction (LVEF), were collected during an in-depth review of participants’ electronic medical record. Comorbidities were assessed during the electronic medical record review with the Charlson Comorbidity Index.39 A list of 19 comorbid diseases were weighted and characterized as representing low (1–2), medium (3–4), and high (5 or more) comorbid burden. Symptom measures, described below, were chosen specifically to mitigate item overlap and because of the established and solid psychometric properties and frequent use in the study of HF.
Physical Symptoms
Acute symptom distress was measured using the 18-item Heart Failure Somatic Perception Scale (HFSPS).40 Based on the theory of unpleasant symptoms, the HFSPS asks how much the participant was bothered by 18 common physical HF symptoms. The six response options range from 0 (I did not have this symptom) to 5 (extremely bothersome). Theta reliability of the original HFSPS was 0.71–0.78.41 Scores are calculated by summing responses; higher values on the HFSPS indicate worse physical symptom distress. The HFSPS was chosen over other HF symptoms measures9, 42 because of the favorable and established psychometric properties and because it is solely a measure of physical (and not psychological) symptoms.
Daytime Sleepiness
Daytime sleepiness was measured using the 8-item Epworth Sleepiness Scale (ESS).43 The ESS asks respondents to rate how likely they would be to doze off or fall asleep in 8 situations by choosing response options that range from 0 (would never doze) to 3 (high chance). Scores are calculated by summing responses and higher ESS scores indicate worse daytime sleepiness; a score of ≥ 10 indicates significant daytime sleepiness.43
Depression
Depression was measured using the 9-Item Patient Health Questionnaire (PHQ9).44 The PHQ9 scores each of the 9 related DSM-IV criteria providing four response options ranging from 0 (not at all) to 3 (nearly every day); scores are calculated by summing responses. The PHQ9 has 88% sensitivity and specificity for major depression (score ≥ 10), which was the cutoff for depression used in this analysis.44
Anxiety and Hostility
Anxiety and hostility were measured using the 11-item Brief Symptom Inventory (BSI).45 The BSI asks about respondents’ feelings and provides five response options ranging from 0 (not at all) to 4 (extremely). Anxiety (5 items) and hostility (6 items) subscale scores (ranging from 0 to 4) are calculated by adding the ratings and dividing the total by the number of items in the subscale, with higher scores indicating higher distress.
Clinical Events
Time to first all-cause mortality, hospitalization, emergency room admission, ventricular assist device implantation, and heart transplantation was assessed as a cumulative endpoint during the 365 days following enrollment. Clinical events and associated dates were extracted from the electronic medical record and/or assessed by contacting participants by telephone to inquire about events that may have occurred outside of the healthcare system and network of medical records.
Statistical Analysis
All analyses were performed using Stata/MP version 11.0 (StataCorp, College Station, TX) except where noted. Means and standard deviations (SD) and proportions were used to describe the sample. Cronbach’s alpha was calculated as an index of internal consistency of symptom measures. Pearson’s correlations were used to quantify linear associations between symptom measures; Bonferroni adjustments were applied to correct for multiple comparisons.
Latent class mixture modeling was used to identify distinct and common symptom profiles among categories of depression (PHQ9 ≥ 10 vs. <10), and continuous measures of acute symptom distress (HFSPS score), daytime sleepiness (ESS score), anxiety (BSI anxiety score), and hostility (BSI hostility score) (performed with Mplus v.6, Los Angeles, CA). Latent class mixture modeling was chosen over deterministic alternatives to account for the mix of categorical and continuous indicators and to effectively quantify uncertainty in profile membership. Our approach to model specification was based on procedures explicated by Ram and colleagues.46 The Lo-Mendell-Rubin adjusted likelihood ratio test,47 convergence (model entropy near 1.0), the proportion of sample in each profile (not less than 5%), and posterior probabilities (average probability of belonging in “most likely” profile near 1.0) were used to compare alternative models (e.g. k vs. k-1 profiles).48, 49 Differences in symptoms among profiles were quantified using analysis of variance or χ2 tests.
Cox proportional hazards modeling was used in the analysis of time to first event. Hazard Ratios (HR) with 95% Confidence Intervals (CI) were calculated to quantify the influence of symptom profiles in explaining 1-year event-risk. The proportional hazards assumption was justified based on Schoenfeld residuals; the hazard function was constant over time. Model fit was assessed using the overall model χ2 and by calculating Harrell’s C statistic. To account for the influence of many other factors, the influence of symptom profiles on event-free survival was adjusted for the SHFS. The SHFS was calculated based on the original model developed by Levy and colleagues.22 In brief, demographic (i.e. age, gender) objective clinical indices (i.e. ischemic etiology, NYHA functional class, left ventricular ejection fraction, systolic blood pressure, hemoglobin, % lymphocyte count, uric acid, sodium, cholesterol) and HF treatment (i.e. beta blocker, angiotensin converting enzyme inhibitor, allopurinol, diuretic dose, statin use, and device therapy) were multiplied by respective slope coefficients22 to generate a single composite risk-prediction score that in this sample ranged from −0.16 to 3.34. Both unadjusted and adjusted estimates of the influence of observed symptom profile membership on 1-year event-free survival are presented. With a sample of 202, power of .80, alpha of 0.05, and 50% event rate, the minimal detectable HR assessed a priori was 1.50.
Results
The sample (n=202) was predominantly male, Caucasian, and in middle adulthood (Table 1). Most participants were married and approximately 60% had NYHA class III or IV HF. The average LVEF was 28%; the median time from echocardiography until enrollment was 63 days. The average wedge pressure was approximately 19mm/Hg; the median time from right heart catheterization to enrollment was 9 days. Given the size of the standard deviations relative to mean values, there was considerable heterogeneity in all symptom measures. Cronbach’s alpha of the symptom measures ranged from 0.80 (BSI hostility items) to 0.90 (HFSPS acute symptom distress).
Table 1.
Characteristics of the Sample (N=202)
| Patient Characteristics: | Mean±SD or n (%) |
|---|---|
| Age (years) | 56.9±13.3 |
| Female | 101 (50.0) |
| Caucasian | 173 (85.6) |
| Body Mass Index (kg/m2) | 30.7±7.4 |
| Retired or on Disability due to Heart Failure | 92 (45.5) |
| Charlson Comorbidity Category: | |
| Score of 1 or 2 (low) | 124 (61.4) |
| Score of 3 or 4 (medium) | 66 (32.7) |
| Score of 5 or more (high) | 12 (5.9) |
| Heart Failure Characteristics: | |
| Left Ventricular Ejection Fraction (%) | 28.6±12.4 |
| NYHA Functional Class: | |
| Class II | 81 (40.1) |
| Class III | 113 (55.9) |
| Class IV | 8 (4.0) |
| Serum sodium (mEq/L) | 137.5±3.2 |
| Serum hematocrit (%) | 38.3±5.9 |
| Serum BUN-to-creatinine ratio (mg/dL:1) | 20.3±10.3 |
| Prescribed a β-blocker (%) | 183 (90.6) |
| Prescribed an ACE or ARB (%) | 162 (80.2) |
| Prescribed an aldosterone antagonist (%) | 86 (42.6) |
| Last Known Cardiac Index (L/min/m2) | 2.1±0.5 |
| Last Known PCWP (mm/Hg) | 18.9±8.8 |
| Symptoms: | |
| HFSPS Score | 24.3±16.0 |
| ESS Score | 8.2±4.9 |
| Significant Daytime Sleepiness | 69 (34.2) |
| PHQ9 Score | 6.9±5.8 |
| Moderate or Greater Depression | 53 (26.2) |
| BSI Anxiety Score | 0.52±0.59 |
| BSI Hostility Score | 0.41±0.53 |
| Event Risk Prediction: | |
| Seattle Heart Failure Score | 1.8±0.74 |
Abbreviations: ACE = angiotensin converting enzyme; ARB = angiotensin receptor blocker, BSI = Brief Symptom Inventory; BUN = blood urea nitrogen; ESS = Epworth Sleepiness Scale; HFSPS = Heart Failure Somatic Perception Scale; NYHA = New York Heart Association; PCWP = pulmonary capillary wedge pressure; PHQ9 = Patient Health Questionnaire 9 Items, SD = standard deviation.
Physical and Psychological Symptom Profiling
There were moderate to strong linear associations among all symptom measures (Table 2) indicating that physical and psychological symptoms are not independent. Non-parametric correlations (Spearman’s rho) were comparable with similar levels of statistical significance (data not shown). Three distinct physical and psychological symptom profiles were identified (model entropy = 0.962; Lo-Mendell-Rubin test = 191.98, p=0.001). There was a graded increase in the severity of all symptom measures across the three profiles (Table 3). Moreover, there were significant differences in the proportions of patients meeting criteria for categories of depression and for excessive daytime sleepiness by profile. We labeled the three profiles according to overall symptom severity as “mild,” “moderate,” and “severe.” The posterior probabilities for belonging in the most likely profile were 0.989, 0.999, and 0.997, respectively, for the severe, moderate, and mild profiles indicating very limited uncertainty in symptom profile membership.
Table 2.
Linear Associations among Symptom Indices (n=202)
| HFSPS | ESS | PHQ9 | BSI Anxiety | |
|---|---|---|---|---|
| ESS | 0.300† | - | - | - |
| PHQ9 | 0.531† | 0.489† | - | - |
| BSI Anxiety | 0.513† | 0.271† | 0.656† | - |
| BSI Hostility | 0.387† | 0.278† | 0.662† | 0.650† |
p < 0.001 with Bonferroni correction for multiple comparisons
Abbreviations: BSI = Brief Symptom Inventory; ESS = Epworth Sleepiness Scale; HFSPS = Heart Failure Somatic Perception Scale; PHQ9 = Patient Health Questionnaire 9 Items.
Table 3.
Physical and Psychological Symptoms by Profile (N=202)
| Mild (41.7%) |
Moderate (30.2%) |
Severe (28.1%) |
F or χ2 | |
|---|---|---|---|---|
| HFSPS | 9.5±4.7 | 24.6±5.0 | 45.3±10.1 | 456.0† |
| ESS | 6.2±4.3 | 9.5±5.0 | 9.7±4.9 | 12.0† |
| Excessive Sleepiness | 18.8% | 43.1% | 42.6% | 12.3† |
| PHQ9 | 3.1±3.1 | 8.6±5.2 | 10.5±6.4 | 42.7† |
| ≥ Mild Depression | 5.0% | 34.5% | 48.2% | 34.3† |
| BSI Anxiety | 0.23±0.34 | 0.58±0.50 | 0.85±0.66 | 26.9† |
| BSI Hostility | 0.17±0.24 | 0.54±0.60 | 0.61±0.59 | 62.1† |
Note: results are presented in means ± standard deviations
p<0.001 for all comparisons by analysis of variance or χ2
Abbreviations: BSI = Brief Symptom Inventory; ESS = Epworth Sleepiness Scale; HFSPS = Heart Failure Somatic Perception Scale; PHQ9 = Patient Health Questionnaire 9 Items
Symptom Profiles and 1-Year Event-Free Survival
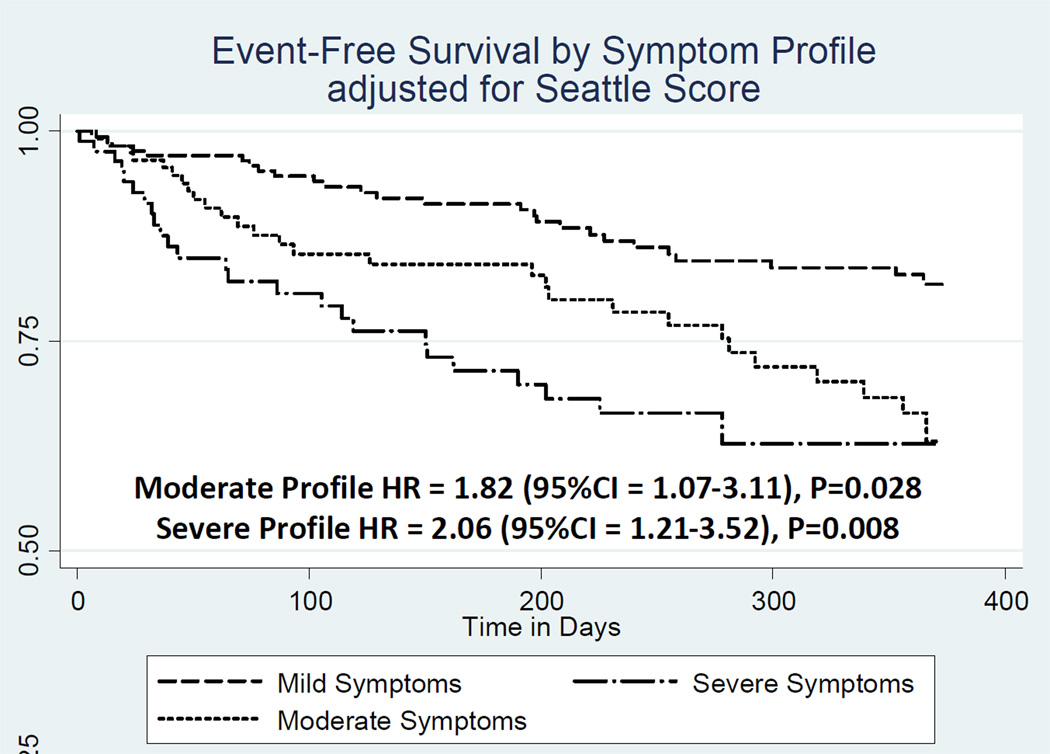
More than half of the sample (56.5%) had a clinical event during a mean follow-up time of 240±141 days until first event. Individual symptom measures were not associated with differences and in combination did not generate a model with statistically significance in predicting clinical event risk (Table 4). Both symptom profile membership and the SHFS independently predicted 1-year event-free survival (Table 5). Adjusting for the SHFS, patients in the moderate symptom profile were 82% more likely (p=0.023), and patients in the severe symptom profile were more than twice as likely (p=0.004) to have a clinical event within one year compared with patients in the mild symptom profile (Table 5; Figure 2).
Table 4.
Symptom Measures and 1-Year Event-Free Survival (N=202)
| Hazard Ratio (95%CI), p-value | |
|---|---|
| HFSPS Score | 1.01 (0.99–1.03); p=0.092 |
| ESS Score | 0.99 (0.93–1.04); p=0.650 |
| PHQ9 | 1.02 (0.96–1.09); p=0.576 |
| BSI Anxiety Score | 0.90 (0.49–1.64); p=0.721 |
| BSI Hostility Score | 1.05 (0.54–2.03); p=0.889 |
| Model χ2 | 6.08, p=0.299 |
| Harrell’s C | 0.590 |
Abbreviations: BSI = Brief Symptom Inventory; CI = Confidence Interval; ESS = Epworth Sleepiness Scale; HFSPS = Heart Failure Somatic Perception Scale; PHQ9 = Patient Health Questionnaire 9 Items.
Table 5.
Symptom Profiles and 1-Year Event-Free Survival (N=202)
| Symptom Profiles | Seattle Heart Failure Score |
Symptom Profiles + Seattle Heart Failure Score |
|
|---|---|---|---|
| HR (95% CI), p-value | HR (95% CI), p-value | HR (95% CI), p-value | |
| Moderate Symptom Profile† | 1.86 (1.09–3.18); p=0.023 | - | 1.82 (1.07–3.11); p=0.028 |
| Severe Symptom Profile† | 2.18 (1.28–3.70); p=0.004 | - | 2.06 (1.21–3.52); p=0.001 |
| Seattle Heart Failure Score | - | 1.65 (1.24–2.19); p=0.001 | 1.62 (1.21–2.18); p=0.001 |
| Model χ2 | 9.66, p=0.008 | 12.29, p<0.001 | 20.25, p<0.001 |
| Harrell’s C | 0.590 | 0.617 | 0.654 |
the mild symptoms profile is the referent group.
Abbreviations: CI = confidence interval; HR = hazard ratio.
Figure 2. Heart Failure Symptom Profiles and 1-Year Event-Free Survival.
Note: Composite risk of first event (all-cause mortality, hospitalization, emergency room admission, ventricular assist device implantation, or heart transplantation), compared with the mild symptom profile.
Abbreviations: CI = confidence interval; HR = adjusted hazards ratio; Seattle Score = Seattle Heart Failure Score.
Discussion
In this prospective cohort study of 202 adults with symptomatic HF, we found strong associations among all measures of symptoms, indicating that physical and psychological symptoms are not independent in HF. Moreover, we identified three common and distinct profiles that capture a clinically-intuitive gradient of both physical and psychological symptom burden. This is the first study to identify unique patterns among both physical and psychological symptoms using multiple, reliable, valid, and symptom-specific measures in HF. Importantly, adjusting for the SHFS the observed symptom profiles were associated with large differences in 1-year event-free survival. Hence, these results serve as preliminary evidence that profiles among physical and psychological symptoms provide additive and complementary information to a commonly-used HF risk prediction model that is based largely on objective clinical data.
Patterns among physical symptoms in HF have been identified previously. For example, Song and colleagues50 used a single measure of symptoms (Memorial Symptom Assessment Scale-HF) to identify a physical symptom cluster centered on dyspnea and another centered on lack of energy and difficulty sleeping in a South Korean sample. Importantly, Song and colleagues indicated that the omission of psychological symptoms was a limitation to their HF symptom clustering approach. Hertzog and colleagues51 also used a single measure of symptoms (investigator developed) to identify three physical symptom profiles. Of particular note, the frequency of depression was not statistically different across the three physical symptom clusters.51 Our results provide evidence that physical symptoms should not be considered independent from psychological symptoms in HF. Jurgens et al.52 and Lee and colleagues53 identified symptom clusters using selected Minnesota Living with Heart Failure Questionnaire items. A benefit to that approach is the ability to incorporate psychological factors into symptom clustering. A limitation of extracting symptoms from inventories like the Minnesota Questionnaire or Memorial Assessment, however, is that single items don’t necessarily reflect the symptom construct of interest and can interject measurement bias.54, 55 Our study builds upon these prior findings by including both physical and psychological symptoms into patient profiling, and by our use of measures that were designed specifically for the reliable and valid measurement thereof, not by extracting single items from a symptom inventory.
Associations between HF symptom profiles and event-free survival have also been published previously. Specifically, Song and colleagues50 identified two physical symptom clusters that were predictive of a gradient in clinical event-risk, and Lee and colleagues53 identified and emotional/cognitive symptom cluster in which total symptom distress was predictive of, and a physical symptom cluster in which symptom distress was not independently associated with, event-free survival. Song and colleagues50 adjusted the relationship between symptom clusters and event-free survival for several clinical factors, and Lee and colleagues53 controlled for five demographic, anthropometric, and clinical factors. Our approach builds upon the findings of our colleagues in that we adjusted our estimates of event hazard for a well-known risk prediction composite score that has been validated in many HF populations and is used clinically for prognostication.56–62 Thus, our findings provide preliminary evidence that physical and psychological symptoms profiles, and not the individual symptom measures themselves, provide independent and additive information about event-risk than commonly used prognostication methods.
Clinical Implications and Directions for Future Research
In understanding the interdependence of physical and psychological symptoms, clinicians may both anticipate and allocate additional resources, such as social work or palliative care services, more effectively. The most clinically relevant application of our findings comes from imagining three hypothetical patients with identical Seattle scores, meaning that they are assessed as having the same prognostication based on common demographic and clinical HF metrics and treatment. The first patient has mild physical and mild psychological symptoms that would likely fall under our threshold for much clinical concern. The second patient has moderate physical and psychological symptoms and is 80% more likely than the first to have a clinical event requiring hospitalization, advanced therapies or worse in the following year. It is likely that case that the validated measures used in this study are not necessary to detect such symptomatology. Instead, this profile is clinically intuitive and may be detected during routine assessment and physical examination. The third patient has severe physical and psychological symptoms and is twice as likely as the first to have a significant clinical event in the coming year. This profile likely reflects the archetypal HF patient that raises our clinical suspicion without the objective data to validate our concern and otherwise indicate a patient at high risk for clinical events.
Given the complexity of HF and the treatment thereof, symptom profiling may facilitate a reasonable balance between individualized and standardized care to improve survival. That is, fitting the severe symptom profile would likely trigger more intensive medical titration or earlier initiation of advanced therapies, prompt a tailored assessment of barriers to effective self-care, and result in greater resource allocation to reduce symptom burden and prevent unnecessary hospitalization. In contrast, fitting the mild symptom profile would likely delay consideration of advanced therapies and treatment would be more standardized and tailored to optimize self-care.
Future research is needed to: a) validate profiling of physical and psychological symptoms, b) quantify relationships between symptom profiles and additional outcomes such as self-care behaviors, c) identify determinants of symptom profile membership, and d) determine the stability of symptoms profiles over time. Moser and colleagues63 recently argued that symptom variability predicts event-free survival in HF and not symptom severity. Thus, identifying trajectories of change in physical and psychological stymptoms over time seems like the best next step in evaluating the utility of HF symptom profiling. Additional research is also needed to test interventions that tailor disease management strategies according to physical and psychological symptom profiling.
Strengths and Limitations
The approach chosen for this study has several strengths. First, there are no prior studies that use multiple and domain-specific measures of both physical and psychological symptoms as the basis of symptom profiling in HF. Second, latent class mixture modeling was used to identify common and distinct profiles to effectively handle continuous and categorical measures and quantify uncertainty in profile membership. In our case, there was extremely limited uncertainty in symptom profile membership. Third, it is rare to study a group of HF patients that is representative of the even gender distribution of the HF population; 50% of our sample was female by design, Finally, our estimates of the relationship between symptom profiles and event-free survival were adjusted to reflect the influence of other demographic, clinical, and treatment factors known to contribute the risk of clinical events.
Beyond limitations that are common among cross-sectional studies, several potential limitations to this study must also be acknowledged. First, the temporal relationship between physical and psychological symptoms cannot be quantified using this analytic approach. Future longitudinal studies may refute or confirm the nature of physical and psychological symptoms in HF over time. Second, we did not seek to identify determinants of membership in a particular symptom profile over the others; this will be the focus of our future work and the work of others. Finally, this research was designed to study adults with symptomatic HF. The relatively young age, low comorbid burden, and moderate to advanced functional class of this sample may make these findings difficult to compare with results of other HF studies.
Conclusion
Physical and psychological symptoms occur concomitantly among adults with moderate to advanced HF. Three physical and psychological symptom profiles captured a gradient of symptom severity and 1-year event-free survival. Physical and psychological symptom profiles may be useful in identifying adults with HF who are at the greatest risk of poor clinical outcomes, should be the focus of additional clinical research, and may serve as the target of future tailored interventions.
What is New?
Physical and psychological symptoms occur concomitantly in heart failure and should not be considered independently.
Profiles of physical and psychological symptoms in heart failure capture a gradient of symptom burden and 1-year event-free survival controlling for the Seattle Heart Failure Score.
Symptom profiling may be useful in identifying adults with HF who are at the greatest risk of poor patient-oriented and clinical outcomes.
Acknowledgment
This work was supported by the Office of Research on Women’s Health and the National Institute of Child Health and Human Development through the Oregon BIRCWH program (HD043488-08). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Office of Research on Women’s Health, the National Institute of Child Health and Human Development, or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Christopher S. Lee, Oregon Health & Science University School of Nursing, Portland, OR.
Jill M. Gelow, Oregon Health & Science University School of Medicine, Portland, OR.
Quin E. Denfeld, Oregon Health & Science University School of Nursing, Portland, OR.
James O. Mudd, Oregon Health & Science University School of Medicine, Portland, OR.
Donna Burgess, Oregon Health & Science University Hospital, Portland, OR.
Jennifer K. Green, Oregon Health & Science University School of Nursing, Portland, OR.
Shirin O. Hiatt, Oregon Health & Science University School of Nursing, Portland, OR.
Corrine Y. Jurgens, Stony Brook University, Stony Brook, NY.
References
- 1.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ. Forecasting the future of cardiovascular disease in the united states: A policy statement from the american heart association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 2.Ross JS, Chen J, Lin Z, Bueno H, Curtis JP, Keenan PS, Normand SL, Schreiner G, Spertus JA, Vidan MT, Wang Y, Krumholz HM. Recent national trends in readmission rates after heart failure hospitalization. Circ Heart Fail. 2010;3:97–103. doi: 10.1161/CIRCHEARTFAILURE.109.885210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the medicare fee-for-service program. The New England Journal of Medicine. 2009;360:1418. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 4.Nohria A, Lewis E, Stevenson LW. Medical management of advanced heart failure. JAMA. 2002;287:628–640. doi: 10.1001/jama.287.5.628. [DOI] [PubMed] [Google Scholar]
- 5.Metra M, Ponikowski P, Dickstein K, McMurray JJ, Gavazzi A, Bergh CH, Fraser AG, Jaarsma T, Pitsis A, Mohacsi P, Bohm M, Anker S, Dargie H, Brutsaert D, Komajda M. Advanced chronic heart failure: A position statement from the study group on advanced heart failure of the heart failure association of the european society of cardiology. Eur J Heart Fail. 2007;9:684–694. doi: 10.1016/j.ejheart.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Russell SD, Miller LW, Pagani FD. Advanced heart failure: A call to action. Congest Heart Fail. 2008;14:316–321. doi: 10.1111/j.1751-7133.2008.00022.x. [DOI] [PubMed] [Google Scholar]
- 7.Schwarz KA, Elman CS. Identification of factors predictive of hospital readmissions for patients with heart failure. Heart Lung. 2003;32:88–99. doi: 10.1067/mhl.2003.15. [DOI] [PubMed] [Google Scholar]
- 8.Westlake C, Dracup K, Fonarow G, Hamilton M. Depression in patients with heart failure. J Card Fail. 2005;11:30–35. doi: 10.1016/j.cardfail.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Zambroski CH, Moser DK, Bhat G, Ziegler C. Impact of symptom prevalence and symptom burden on quality of life in patients with heart failure. Eur J Cardiovasc Nurs. 2005;4:198–206. doi: 10.1016/j.ejcnurse.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2012 update: A report from the american heart association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jessup M, Abraham WT, Casey DE, Feldman AM, Francis GS, Ganiats TG, Konstam MA, Mancini DM, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 focused update: Accf/aha guidelines for the diagnosis and management of heart failure in adults: A report of the american college of cardiology foundation/american heart association task force on practice guidelines: Developed in collaboration with the international society for heart and lung transplantation. Circulation. 2009;119:1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 12.Lindenfeld J, Albert NM, Boehmer JP, Collins SP, Ezekowitz JA, Givertz MM, Katz SD, Klapholz M, Moser DK, Rogers JG, Starling RC, Stevenson WG, Tang WH, Teerlink JR, Walsh MN. Hfsa 2010 comprehensive heart failure practice guideline. J Card Fail. 2010;16:e1–e194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Webel AR, Frazier SK, Lennie TA, Moser DK. Daily variability in dyspnea, edema and body weight in heart failure patients. Eur J Cardiovasc Nurs. 2006;6:60–65. doi: 10.1016/j.ejcnurse.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Hauptman PJ, Masoudi FA, Weintraub WS, Pina I, Jones PG, Spertus JA. Variability in the clinical status of patients with advanced heart failure. J Card Fail. 2004;10:397–402. doi: 10.1016/j.cardfail.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Sharma B, Owens R, Malhotra A. Sleep in congestive heart failure. Med Clin North Am. 2010;94:447–464. doi: 10.1016/j.mcna.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riegel B, Moelter ST, Ratcliffe SJ, Pressler SJ, De Geest S, Potashnik S, Fleck D, Sha D, Sayers SL, Weintraub WS, Weaver TE, Goldberg LR. Excessive daytime sleepiness is associated with poor medication adherence in adults with heart failure. J Card Fail. 2011;17:340–348. doi: 10.1016/j.cardfail.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48:1527–1537. doi: 10.1016/j.jacc.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 18.Konstam V, Moser DK, De Jong MJ. Depression and anxiety in heart failure. J Card Fail. 2005;11:455–463. doi: 10.1016/j.cardfail.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 19.Moser DK, Dracup K, Evangelista LS, Zambroski CH, Lennie TA, Chung ML, Doering LV, Westlake C, Heo S. Comparison of prevalence of symptoms of depression, anxiety, and hostility in elderly patients with heart failure, myocardial infarction, and a coronary artery bypass graft. Heart Lung. 39:378–385. doi: 10.1016/j.hrtlng.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pocock SJ, Ariti CA, McMurray JJ, Maggioni A, Kober L, Squire IB, Swedberg K, Dobson J, Poppe KK, Whalley GA, Doughty RN. Predicting survival in heart failure: A risk score based on 39 372 patients from 30 studies. Eur Heart J. 2012 doi: 10.1093/eurheartj/ehs337. [DOI] [PubMed] [Google Scholar]
- 21.Zugck C, Kruger C, Kell R, Korber S, Schellberg D, Kubler W, Haass M. Risk stratification in middle-aged patients with congestive heart failure: Prospective comparison of the heart failure survival score (hfss) and a simplified two-variable model. Eur J Heart Fail. 2001;3:577–585. doi: 10.1016/s1388-9842(01)00167-2. [DOI] [PubMed] [Google Scholar]
- 22.Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, Anand I, Maggioni A, Burton P, Sullivan MD, Pitt B, Poole-Wilson PA, Mann DL, Packer M. The seattle heart failure model: Prediction of survival in heart failure. Circulation. 2006;113:1424–1433. doi: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]
- 23.Song EK, Moser DK, Lennie TA. Relationship of depressive symptoms to the impact of physical symptoms on functional status in women with heart failure. Am J Crit Care. 2009;18:348–356. doi: 10.4037/ajcc2009450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Jong MJ, Chung ML, Wu JR, Riegel B, Rayens MK, Moser DK. Linkages between anxiety and outcomes in heart failure. Heart Lung. 2011;40:393–404. doi: 10.1016/j.hrtlng.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davila DF, Nunez TJ, Odreman R, de Davila CA. Mechanisms of neurohormonal activation in chronic congestive heart failure: Pathophysiology and therapeutic implications. Int J Cardiol. 2005;101:343–346. doi: 10.1016/j.ijcard.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 26.Gheorghiade M, Shin DD, Thomas TO, Brandimarte F, Fonarow GC, Abraham WT. Congestion is an important diagnostic and therapeutic target in heart failure. Rev Cardiovasc Med. 2006;7(Suppl 1):S12–S24. [PubMed] [Google Scholar]
- 27.Parissis JT, Nikolaou M, Farmakis D, Bistola V, Paraskevaidis IA, Adamopoulos S, Filippatos G, Kremastinos DT. Clinical and prognostic implications of self-rating depression scales and plasma b-type natriuretic peptide in hospitalised patients with chronic heart failure. Heart. 2008;94:585–589. doi: 10.1136/hrt.2007.117390. [DOI] [PubMed] [Google Scholar]
- 28.Grippo AJ, Johnson AK. Stress, depression and cardiovascular dysregulation: A review of neurobiological mechanisms and the integration of research from preclinical disease models. Stress. 2009;12:1–21. doi: 10.1080/10253890802046281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gibbs CR, Blann AD, Watson RD, Lip GY. Abnormalities of hemorheological, endothelial, and platelet function in patients with chronic heart failure in sinus rhythm: Heart Failure Symptom Profiles 26 Effects of angiotensin-converting enzyme inhibitor and beta-blocker therapy. Circulation. 2001;103:1746–1751. doi: 10.1161/01.cir.103.13.1746. [DOI] [PubMed] [Google Scholar]
- 30.Kent LK, Shapiro PA. Depression and related psychological factors in heart disease. Harv Rev Psychiatry. 2009;17:377–388. doi: 10.3109/10673220903463333. [DOI] [PubMed] [Google Scholar]
- 31.York KM, Hassan M, Sheps DS. Psychobiology of depression/distress in congestive heart failure. Heart Fail Rev. 2009;14:35–50. doi: 10.1007/s10741-008-9091-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fischer D, Rossa S, Landmesser U, Spiekermann S, Engberding N, Hornig B, Drexler H. Endothelial dysfunction in patients with chronic heart failure is independently associated with increased incidence of hospitalization, cardiac transplantation, or death. Eur Heart J. 2005;26:65–69. doi: 10.1093/eurheartj/ehi001. [DOI] [PubMed] [Google Scholar]
- 33.Katz SD, Hryniewicz K, Hriljac I, Balidemaj K, Dimayuga C, Hudaihed A, Yasskiy A. Vascular endothelial dysfunction and mortality risk in patients with chronic heart failure. Circulation. 2005;111:310–314. doi: 10.1161/01.CIR.0000153349.77489.CF. [DOI] [PubMed] [Google Scholar]
- 34.Pizzi C, Manzoli L, Mancini S, Costa GM. Analysis of potential predictors of depression among coronary heart disease risk factors including heart rate variability, markers of inflammation, and endothelial function. Eur Heart J. 2008;29:1110–1117. doi: 10.1093/eurheartj/ehn137. [DOI] [PubMed] [Google Scholar]
- 35.Alonso-Martinez JL, Llorente-Diez B, Echegaray-Agara M, Olaz-Preciado F, Urbieta-Echezarreta M, Gonzalez-Arencibia C. C-reactive protein as a predictor of improvement and readmission in heart failure. Eur J Heart Fail. 2002;4:331–336. doi: 10.1016/s1388-9842(02)00021-1. [DOI] [PubMed] [Google Scholar]
- 36.Johansson P, Lesman-Leegte I, Svensson E, Voors A, van Veldhuisen DJ, Jaarsma T. Depressive symptoms and inflammation in patients hospitalized for heart failure. Am Heart J. 2011;161:1053–1059. doi: 10.1016/j.ahj.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 37.Lenz ER, Suppe F, Gift AG, Pugh LC, Milligan RA. Collaborative development of middle-range nursing theories: Toward a theory of unpleasant symptoms. ANS Adv Nurs Sci. 1995;17:1–13. doi: 10.1097/00012272-199503000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Lenz ER, Pugh LC, Milligan RA, Gift A, Suppe F. The middle-range theory of unpleasant symptoms: An update. ANS Adv Nurs Sci. 1997;19:14–27. doi: 10.1097/00012272-199703000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 40.Jurgens CY. Somatic awareness, uncertainty, and delay in care-seeking in acute heart failure. Res Nurs Health. 2006;29:74–86. doi: 10.1002/nur.20118. [DOI] [PubMed] [Google Scholar]
- 41.Jurgens CY, Fain JA, Riegel B. Psychometric testing of the heart failure somatic awareness scale. J Cardiovasc Nurs. 2006;21:95–102. doi: 10.1097/00005082-200603000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Rector TS, Kubo SH, Cohn JN. Validity of the minnesota living with heart failure questionnaire as a measure of therapeutic response to enalapril or placebo. Am J Cardiol. 1993;71:1106–1107. doi: 10.1016/0002-9149(93)90582-w. [DOI] [PubMed] [Google Scholar]
- 43.Johns MW. A new method for measuring daytime sleepiness: The epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 44.Kroenke K, Spitzer RL, Williams JB. The phq-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Derogatis LR, Melisaratos N. The brief symptom inventory: An introductory report. Psychological Medicine. 1983;13:595–605. [PubMed] [Google Scholar]
- 46.Ram N, Grimm KJ. Methods and measures: Growth mixture modeling: A method for identifying differences in longitudinal change among unobserved groups. International Journal of Behavioral Development. 2009;33:565–576. doi: 10.1177/0165025409343765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lo Y, Mendell NR, Rubin DB. Testing the number of components in a normal mixture. Biometrika. 2001;88:767–778. [Google Scholar]
- 48.Jung T, Wickrama KA. An introduction to latent class growth analysis and growth mixture modeling. Social and Personality Compass. 2008;2:302–317. [Google Scholar]
- 49.Nylund KL, Asparouhov T, Muthen B. Deciding on the number of classes in latent class analysis and growth mixture modeling: A monte carlo simulation study. Struct Equ Modeling. 2007;14:535–569. [Google Scholar]
- 50.Song EK, Moser DK, Rayens MK, Lennie TA. Symptom clusters predict event-free survival in patients with heart failure. J Cardiovasc Nurs. 2010;25:284–291. doi: 10.1097/JCN.0b013e3181cfbcbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hertzog MA, Pozehl B, Duncan K. Cluster analysis of symptom occurrence to identify subgroups of heart failure patients: A pilot study. J Cardiovasc Nurs. 2010;25:273–283. doi: 10.1097/JCN.0b013e3181cfbb6c. [DOI] [PubMed] [Google Scholar]
- 52.Jurgens CY, Moser DK, Armola R, Carlson B, Sethares K, Riegel B. Symptom clusters of heart failure. Res Nurs Health. 2009;32:551–560. doi: 10.1002/nur.20343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee KS, Song EK, Lennie TA, Frazier SK, Chung ML, Heo S, Wu JR, Rayens MK, Riegel B, Moser DK. Symptom clusters in men and women with heart failure and their impact on cardiac event-free survival. J Cardiovasc Nurs. 2010;25:263–272. doi: 10.1097/JCN.0b013e3181cfbb88. [DOI] [PubMed] [Google Scholar]
- 54.Kim HJ, Abraham I, Malone PS. Analytical methods and issues for symptom cluster research in oncology. Curr Opin Support Palliat Care. 2012 doi: 10.1097/SPC.0b013e32835bf28b. [DOI] [PubMed] [Google Scholar]
- 55.Barsevick AM, Whitmer K, Nail LM, Beck SL, Dudley WN. Symptom cluster research: Conceptual, design, measurement, and analysis issues. J Pain Symptom Manage. 2006;31:85–95. doi: 10.1016/j.jpainsymman.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 56.Goda A, Williams P, Mancini D, Lund LH. Selecting patients for heart transplantation: Comparison of the heart failure survival score (hfss) and the seattle heart failure model (shfm) J Heart Lung Transplant. 2011;30:1236–1243. doi: 10.1016/j.healun.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 57.Benbarkat H, Addetia K, Eisenberg MJ, Sheppard R, Filion KB, Michel C. Application of the seattle heart failure model in patients >80 years of age enrolled in a tertiary care heart failure clinic. Am J Cardiol. 2012;110:1663–1666. doi: 10.1016/j.amjcard.2012.07.034. [DOI] [PubMed] [Google Scholar]
- 58.John R, Eckman P. Utility of the seattle heart failure model in stratification of heart failure patients for ventricular assist device therapy. ASAIO J. 2012;58:91–92. doi: 10.1097/MAT.0b013e318249144f. [DOI] [PubMed] [Google Scholar]
- 59.Perrotta L, Ricciardi G, Pieragnoli P, Chiostri M, Pontecorboli G, De Santo T, Bellocci F, Vitulano N, Emdin M, Mascioli G, Ricceri I, Porciani MC, Michelucci A, Padeletti L. Application of the seattle heart failure model in patients on cardiac resynchronization therapy. Pacing Clin Electrophysiol. 2012;35:88–94. doi: 10.1111/j.1540-8159.2011.03258.x. [DOI] [PubMed] [Google Scholar]
- 60.Gorodeski EZ, Chu EC, Chow CH, Levy WC, Hsich E, Starling RC. Application of the seattle heart failure model in ambulatory patients presented to an advanced heart failure therapeutics committee. Circ Heart Fail. 2010;3:706–714. doi: 10.1161/CIRCHEARTFAILURE.110.944280. [DOI] [PubMed] [Google Scholar]
- 61.Ketchum ES, Moorman AJ, Fishbein DP, Mokadam NA, Verrier ED, Aldea GS, Andrus S, Kenyon KW, Levy WC. Predictive value of the seattle heart failure model in patients undergoing left ventricular assist device placement. J Heart Lung Transplant. 2010;29:1021–1025. doi: 10.1016/j.healun.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 62.Kalogeropoulos AP, Georgiopoulou VV, Giamouzis G, Smith AL, Agha SA, Waheed S, Laskar S, Puskas J, Dunbar S, Vega D, Levy WC, Butler J. Utility of the seattle heart failure model in patients with advanced heart failure. J Am Coll Cardiol. 2009;53:334–342. doi: 10.1016/j.jacc.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 63.Moser DK, Frazier SK, Worrall-Carter L, Biddle MJ, Chung ML, Lee KS, Lennie TA. Symptom variability, not severity, predicts rehospitalization and mortality in patients with heart failure. Eur J Cardiovasc Nurs. 2011;10:124–129. doi: 10.1016/j.ejcnurse.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]