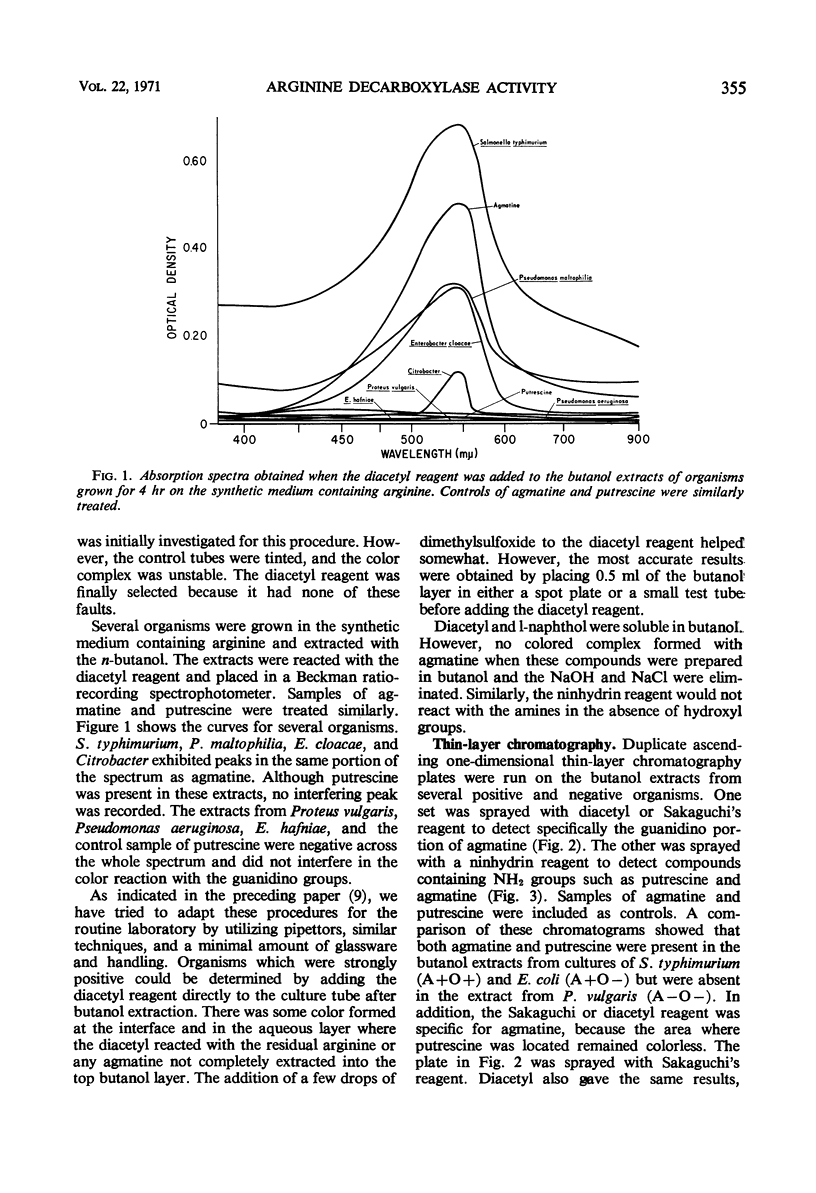
Abstract
A rapid biochemical method for the determination of arginine decarboxylase (EC 4.1.1.19) activity has been developed for use in the routine clinical microbiology laboratory and correlated with similar procedures for ornithine and lysine decarboxylase (EC 4.1.1.18) systems. It is based on the detection of agmatine, the amine end product formed during growth on a synthetic medium containing arginine as the key amino acid. A modified diacetyl reagent is used to detect this amine after a differential butanol extraction of the cultures. This procedure can be used to detect this amine after a 1- to 4-hr incubation period (with the use of an initial concentrated inoculum) or with an overnight culture. Thus, both an indirect measurement based on the alkalinization of the medium and a lengthy incubation period were avoided. Parameters for optimal enzyme activity and the pertinent enzyme systems involved in arginine and agmatine catabolism are discussed in detail.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blethen S. L., Boeker E. A., Snell E. E. Argenine decarboxylase from Escherichia coli. I. Purification and specificity for substrates and coenzyme. J Biol Chem. 1968 Apr 25;243(8):1671–1677. [PubMed] [Google Scholar]
- CARLQUIST P. R. A biochemical test for separating paracolon groups. J Bacteriol. 1956 Mar;71(3):339–341. doi: 10.1128/jb.71.3.339-341.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt M. C., Lockhart B. M., Perry K. Rapid methods for determining decarboxylase activity: ornithine and lysine decarboxylases. Appl Microbiol. 1971 Sep;22(3):344–349. doi: 10.1128/am.22.3.344-349.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MELNYKOVYCH G., SNELL E. E. Nutritional requirements for the formation of arginine decarboxylase in Escherichia coli. J Bacteriol. 1958 Nov;76(5):518–523. doi: 10.1128/jb.76.5.518-523.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris D. R., Pardee A. B. Multiple pathways of putrescine biosynthesis in Escherichia coli. J Biol Chem. 1966 Jul 10;241(13):3129–3135. [PubMed] [Google Scholar]
- MØLLER V. Simplified tests for some amino acid decarboxylases and for the arginine dihydrolase system. Acta Pathol Microbiol Scand. 1955;36(2):158–172. doi: 10.1111/j.1699-0463.1955.tb04583.x. [DOI] [PubMed] [Google Scholar]
- Richard C. Techniques rapides de recherche des lysine-décarboxylase, ornithine-décarboxylase et argining-dihydrolase dans les genres Pseudomonas, Alcaligenes et Moraxella. Ann Inst Pasteur (Paris) 1968 Apr;114(4):425–430. [PubMed] [Google Scholar]
- SHERRIS J. C., SHOESMITH J. G., PARKER M. T., BRECKON D. Tests for the rapid breakdown of arginine by bacteria: their use in the identification of pseudomonads. J Gen Microbiol. 1959 Oct;21:389–396. doi: 10.1099/00221287-21-2-389. [DOI] [PubMed] [Google Scholar]
- SLADE H. D., SLAMP W. C. The formation of arginine dihydrolase by streptococci and some properties of the enzyme system. J Bacteriol. 1952 Oct;64(4):455–466. doi: 10.1128/jb.64.4.455-466.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEEL K. J., MIDGLEY J. Decarboxylase and other reactions of some gram-negative rods. J Gen Microbiol. 1962 Sep;29:171–178. doi: 10.1099/00221287-29-1-171. [DOI] [PubMed] [Google Scholar]
- Tabor H., Tabor C. W. Formation of 1,4-diaminobutane and of spermidine by an ornithine auxotroph of Escherichia coli grown on limiting ornithine or arginine. J Biol Chem. 1969 May 10;244(9):2286–2292. [PubMed] [Google Scholar]
- Traub W. H., Raymond E. A., Linehan J. Identification of Enterobacteriaceae in the clinical microbiology laboratory. Appl Microbiol. 1970 Sep;20(3):303–308. doi: 10.1128/am.20.3.303-308.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]