Abstract
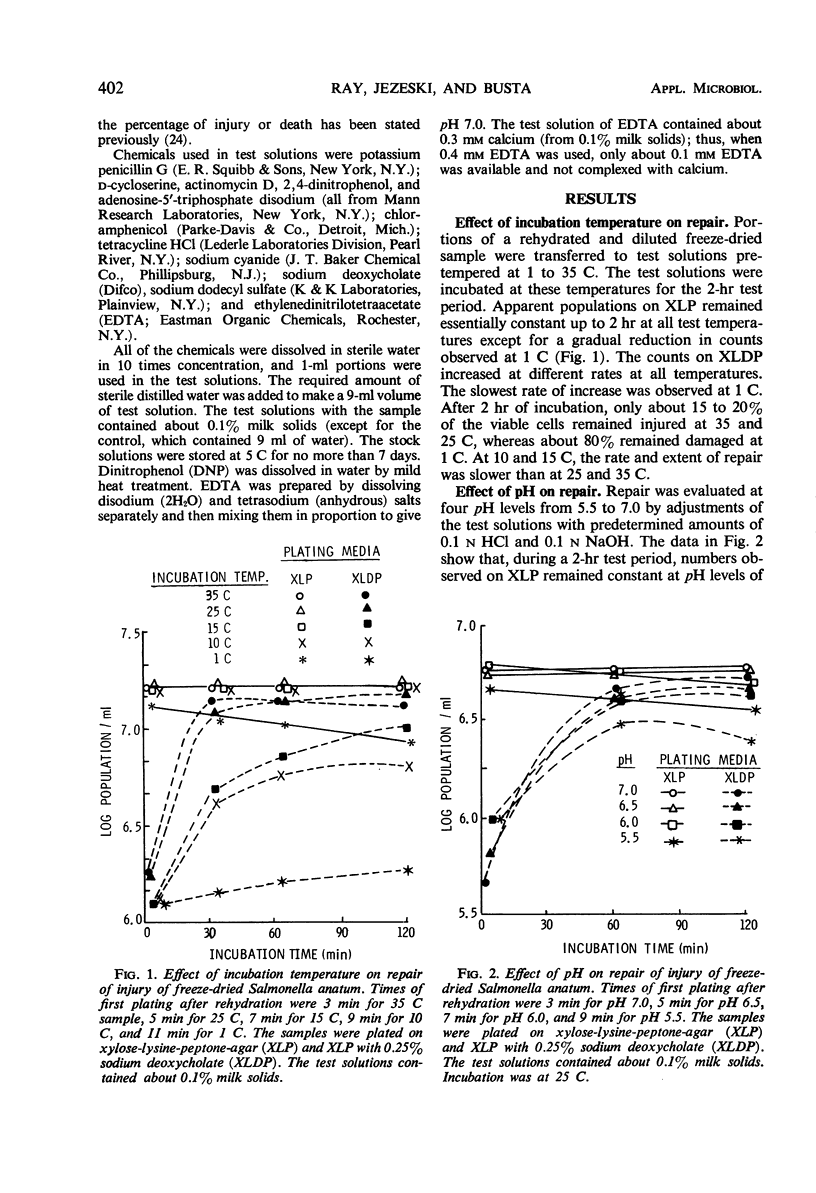
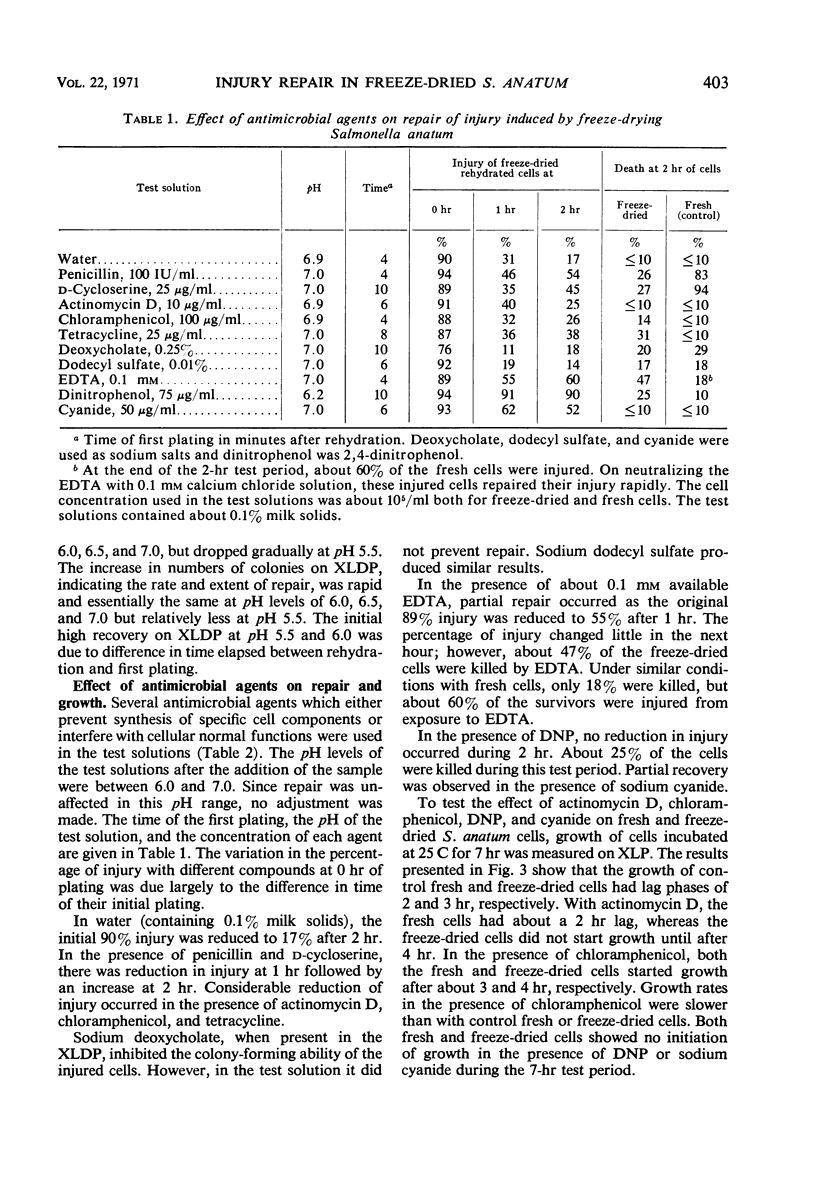
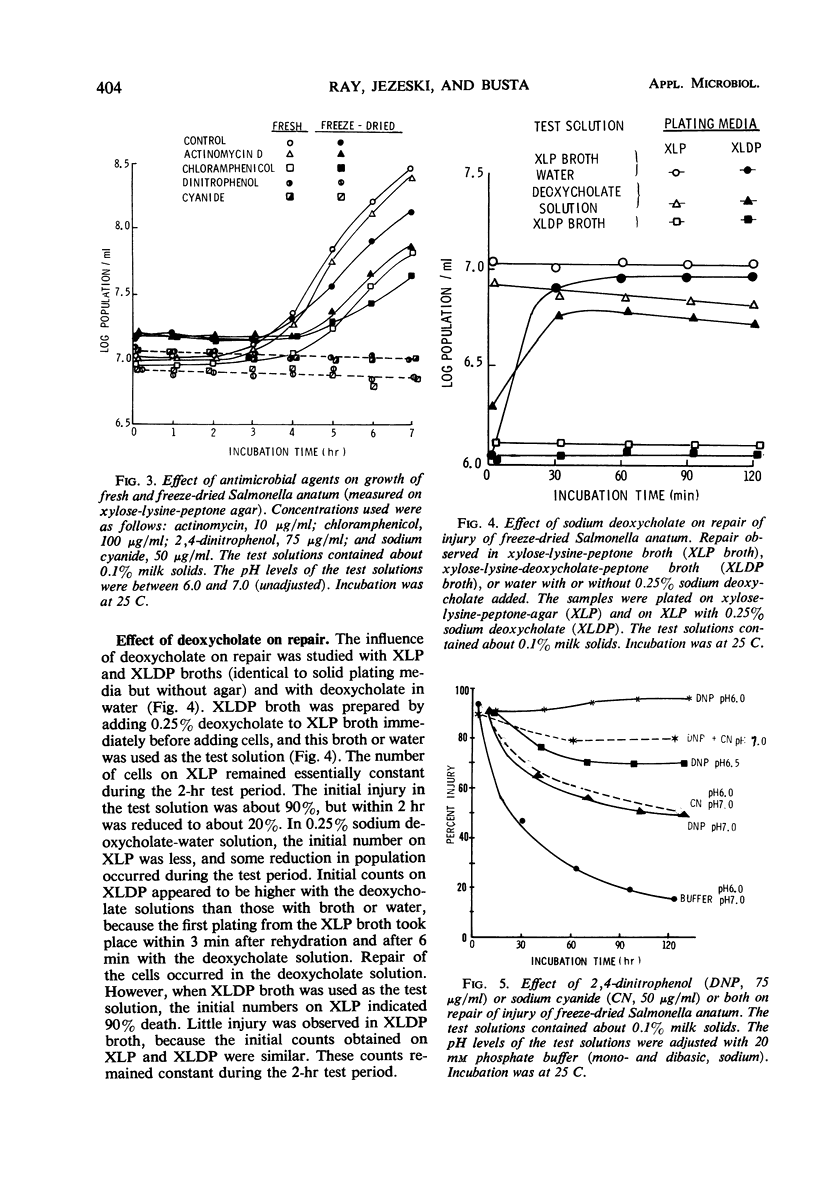
Repair of injury induced by freeze-drying Salmonella anatum in nonfat milk solids occurred rapidly after rehydration. Injury in surviving cells was defined as the inability to form colonies on a plating medium containing deoxycholate. Death was defined as inability to form colonies in the same medium without this selective agent. The rate of repair of injury was reduced by lowering the temperature from 35 C to 10 C and was extremely low at 1 C. Repair was independent of influence of pH between 6.0 and 7.0. Repair did not require synthesis of protein, ribonucleic acid, or cell wall mucopeptide, but did require energy in the form of adenosine triphosphate (ATP) synthesized through oxidative phosphorylation. The requirement for ATP was based on dinitrophenol or cyanide interference with repair. Dinitrophenol activity was pH-dependent; no repair occurred at pH 6.0 and some repair was observed at pH 6.5 and above. Injured cells were extremely sensitive to low concentrations of ethylenedinitrilotetraacetate. This indicated that freeze-drying injury of S. anatum may involve the lipopolysaccharide portion of the cell wall and that repair of this damage requires ATP synthesis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allwood M. C., Russell A. D. Growth and metabolic activities of heat treated Staphylococcus aureus. J Appl Bacteriol. 1969 Mar;32(1):79–85. doi: 10.1111/j.1365-2672.1969.tb02191.x. [DOI] [PubMed] [Google Scholar]
- BRODIE A. F., GRAY C. T. Activation of coupled oxidative phosphorylation in bacterial particulates by a soluble factor (s). Biochim Biophys Acta. 1956 Feb;19(2):384–386. doi: 10.1016/0006-3002(56)90448-6. [DOI] [PubMed] [Google Scholar]
- Bairdparker A. C., Davenport E. The effect of recovery medium on the isolation of Staphylococcus aureus after heat treatment and after the storage of frozen or dried cells. J Appl Bacteriol. 1965 Dec;28(3):390–402. doi: 10.1111/j.1365-2672.1965.tb02169.x. [DOI] [PubMed] [Google Scholar]
- Bretz H. W., Kocka F. E. Resistance to actinomycin D of Escherichia coli after frozen storage. Can J Microbiol. 1967 Jul;13(7):914–917. doi: 10.1139/m67-121. [DOI] [PubMed] [Google Scholar]
- CAVALIERI L. F., NEMCHIN R. G. THE MODE OF INTERACTION OF ACTINOMYCIN D WITH DEOXYRIBONUCLEIC ACID. Biochim Biophys Acta. 1964 Aug 12;87:641–652. doi: 10.1016/0926-6550(64)90282-8. [DOI] [PubMed] [Google Scholar]
- CLARKE P. H., LILLY M. D. A general structure for cell walls of gram-negative bacteria. Nature. 1962 Aug 4;195:517–518. doi: 10.1038/195516b0. [DOI] [PubMed] [Google Scholar]
- GALE E. F. The assimilation of amino-acids by bacteria; action of inhibitors on the accumulation of free glutamic acid in Staphylococcus aureus and Streptococcus faecalis. Biochem J. 1951 Mar;48(3):286–290. doi: 10.1042/bj0480286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. W., Wilkinson S. G. The effect of ethylenediaminetetra-acetic acid on the cell walls of some gram-negative bacteria. J Gen Microbiol. 1965 Jun;39(3):385–399. doi: 10.1099/00221287-39-3-385. [DOI] [PubMed] [Google Scholar]
- Iandolo J. J., Ordal Z. J. Repair of thermal injury of Staphylococcus aureus. J Bacteriol. 1966 Jan;91(1):134–142. doi: 10.1128/jb.91.1.134-142.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEIVE L. A NONSPECIFIC INCREASE IN PERMEABILITY IN ESCHERICHIA COLI PRODUCED BY EDTA. Proc Natl Acad Sci U S A. 1965 Apr;53:745–750. doi: 10.1073/pnas.53.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederberg J. BACTERIAL PROTOPLASTS INDUCED BY PENICILLIN. Proc Natl Acad Sci U S A. 1956 Sep;42(9):574–577. doi: 10.1073/pnas.42.9.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leive L. Studies on the permeability change produced in coliform bacteria by ethylenediaminetetraacetate. J Biol Chem. 1968 May 10;243(9):2373–2380. [PubMed] [Google Scholar]
- MYERS D. K., SLATER E. C. The enzymic hydrolysis of adenosine triphosphate by liver mitochondria. I. Activities at different pH values. Biochem J. 1957 Dec;67(4):558–572. doi: 10.1042/bj0670558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss C. W., Speck M. L. Release of biologically active peptides from Escherichia coli at subzero temperatures. J Bacteriol. 1966 Mar;91(3):1105–1111. doi: 10.1128/jb.91.3.1105-1111.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee P., Bhattacharjee S. B. Recovery of bacteria from damages induced by heat. J Gen Microbiol. 1970 Feb;60(2):233–238. doi: 10.1099/00221287-60-2-233. [DOI] [PubMed] [Google Scholar]
- NEUHAUS F. C., LYNCH J. L. Studies on the inhibition of D-alanyl-D-alanine synthetase by the antibiotic D-cycloserine. Biochem Biophys Res Commun. 1962 Aug 7;8:377–382. doi: 10.1016/0006-291x(62)90011-6. [DOI] [PubMed] [Google Scholar]
- PINCHOT G. B. Phosphorylation coupled to electron transport in cell-free extracts of Alcaligenes faecalis. J Biol Chem. 1953 Nov;205(1):65–74. [PubMed] [Google Scholar]
- PULLMAN M. E., PENEFSKY H. S., DATTA A., RACKER E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. I. Purification and properties of soluble dinitrophenol-stimulated adenosine triphosphatase. J Biol Chem. 1960 Nov;235:3322–3329. [PubMed] [Google Scholar]
- Pierson M. D., Tomlins R. I., Ordal Z. J. Biosynthesis during recovery of heat-injured Salmonella typhimurium. J Bacteriol. 1971 Mar;105(3):1234–1236. doi: 10.1128/jb.105.3.1234-1236.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RENDI R., OCHOA S. Effect of chloramphenicol on protein synthesis in cell-free preparations of Escherichia coli. J Biol Chem. 1962 Dec;237:3711–3713. [PubMed] [Google Scholar]
- Ray B., Jezeski J. J., Busta F. F. Effect of rehydration on recovery, repair, and growth of injured freeze-dried Salmonella anatum. Appl Microbiol. 1971 Aug;22(2):184–189. doi: 10.1128/am.22.2.184-189.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinskey T. J., Silverman G. J. Characterization of injury incurred by Escherichia coli upon freeze-drying. J Bacteriol. 1970 Feb;101(2):429–437. doi: 10.1128/jb.101.2.429-437.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogin S. J., Ordal Z. J. Regeneration of ribosomes and ribosomal ribonucleic acid during repair of thermal injury to Staphylococcus. J Bacteriol. 1967 Oct;94(4):1082–1087. doi: 10.1128/jb.94.4.1082-1087.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlins R. I., Ordal Z. J. Requirements of Salmonella typhimurium for recovery from thermal injury. J Bacteriol. 1971 Feb;105(2):512–518. doi: 10.1128/jb.105.2.512-518.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAGMAN J. Evidence of cytoplasmic membrane injury in the drying of bacteria. J Bacteriol. 1960 Oct;80:558–564. doi: 10.1128/jb.80.4.558-564.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOSILAIT W. D., NASON A., TERRELL A. J. [Pyridine nucleotide-quinone reductase. II. Rôle in electron transport]. J Biol Chem. 1954 Jan;206(1):271–282. [PubMed] [Google Scholar]
- Webb S. J. The effects of oxygen on the possible repair of dehydration damage by Escherichia coli. J Gen Microbiol. 1969 Nov;58(3):317–326. doi: 10.1099/00221287-58-3-317. [DOI] [PubMed] [Google Scholar]
- Wolin M. J. Lysis of Vibrio succinogenes by ethylenediamine-tetraacetic acid or lysozyme. J Bacteriol. 1966 May;91(5):1781–1786. doi: 10.1128/jb.91.5.1781-1786.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]


