Abstract
Infection of the nervous system with the human immunodeficiency virus (HIV-1) can lead to cognitive, motor and sensory disorders. HIV-related sensory neuropathy (HIV-SN) mainly contains the HIV infection-related distal sensory polyneuropathy (DSP) and antiretroviral toxic neuropathies (ATN). The main pathological features that characterize DSP and ATN include retrograde (“dying back”) axonal degeneration of long axons in distal regions of legs or arms, loss of unmyelinated fibers, and variable degree of macrophage infiltration in peripheral nerves and dorsal root ganglia (DRG). One of the most common complaints of HIV-DSP is pain. Unfortunately, many conventional agents utilized as pharmacologic therapy for neuropathic pain are not effective for providing satisfactory analgesia in painful HIV-related distal sensory polyneuropathy, because the molecular mechanisms of the painful HIV-SDP are not clear in detail. The HIV envelope glycoprotein, gp120, appears to contribute to this painful neuropathy. Recently, preclinical studies have shown that glia activation in the spinal cord and DRG has become an attractive target for attenuating chronic pain. Cytokines/chemokines have been implicated in a variety of painful neurological diseases and in animal models of HIV-related neuropathic pain. Mitochondria injured by ATN and/or gp120 may be also involved in the development of HIV-neuropathic pain. This review discusses the neurochemical and pharmacological mechanisms of HIV-related neuropathic pain based on the recent advance in the preclinical studies, providing insights into novel pharmacological targets for future therapy.
Keywords: HIV, neuropathic pain, gp120, NRTI, spinal cord, DRG.
1. INTRODUCTION
Typically transient pain (acute pain) that occurs in response to noxious stimuli (nociceptive pain) is early-warning protective, and is mediated by specialized high-threshold primary sensory neurons. Chronic pain, is associated either with tissue damage and inflammation (inflammatory pain) or with lesions to the nervous system (neuropathic pain), characterized by persistent pain. These include, pain experienced in the absence of any obvious peripheral stimulus (spontaneous pain), an increased responsiveness to noxious stimuli (hyperalgesia), and/or pain in response to normally innocuous stimuli (allodynia) [1]. A classification relating to the type of neuropathic disorders, includes mechanical nerve injury (e.g. carpal tunnel syndrome, vertebral disk herniation); metabolic disease (e.g. diabetic polyneuropathy), neurotrophic viral disease (e.g. herpes zoster, human immunodeficiency virus (HIV)), neurotoxicity (e.g. chemotherapy of cancer or tuberculosis), inflammatory and/or immunologic mechanisms (e.g. multiple sclerosis), nervous system focal ischemia (e.g. thalamic syndrome) and multiple neurotransmitter system dysfunctions (e.g. complex regional pain syndrome), etc. [2].
HIV-related neuropathic pain is a debilitating chronic condition that is severe and unrelenting. Despite decades of extensive research, the neuropathological mechanisms remain unknown in detail, hindering our ability to develop effective treatments. This review focuses on current researches on the pathophysiological mechanisms of HIV-neuropathic pain.
2. PERIPHERAL AND CENTRAL SENSITIZATION OF NEUROPATHIC PAIN
In the last 20 years, significant basic research progress has been made in developing and characterizing in vivo experimental models of chronic pain. Two main mechanisms of neuropathic pain, peripheral and central becomes sensitizations, not mutually exclusive, are proposed.
2.1. Peripheral Sensitization
Primary afferent fibers transmit noxious stimuli from the periphery to the central nervous system. In addition, primary afferent fibers have a unique morphology, called pseudo-unipolar, wherein both central and peripheral terminals emanate from a common axonal stalk [3]. Therefore, the majority of proteins synthesized by the dorsal root ganglions (DRG) is distributed to both central and peripheral terminals [3]. Tissue damage or inflammation is often accompanied by the accumulation of endogenous factors released from activated nociceptors or non-neural cells that reside within or infiltrate into the injured area [3-5]. Collectively, these factors represent a wide array of signaling molecules, including neurotransmitters, peptides (substance P, bradykinin), eicosanoids and related lipids (prostaglandins, etc.), neurotrophins, proinflammatory cytokines (interleukin-1β (IL-1β) and IL-6, and tumor necrosis factor α (TNF-α)), and chemokines, as well as extracellular proteases and protons, referred to as the ‘inflammatory soup’ [4]. These factors act directly on the nociceptors by binding to one or more cell surface receptors, including G protein-coupled receptors (GPCR), Transient receptor protein (TRP) channels, acid-sensitive ion channels (ASIC), two-pore potassium channels (K2P), and receptor tyrosine kinases (RTK), as depicted on the peripheral nociceptor terminals [3]. Nerve growth factors (NGF) or proinflammatory cytokines-induced activation of mitogen-activated protein kinases (MAPK) in primary sensory neurons exacerbates hyperalgesia [6, 7]. Transient receptor protein vanilloid 1 (TRPV1) is a key component of the mechanism through which inflammation produces thermal hyperalgesia modulated by components of the inflammatory soup [8]. Some of these inflammatory agents (for example, extracellular protons and lipids) function as direct positive allosteric modulators of the channel, whereas others (bradykinin, ATP, and NGF) bind to their own receptors on primary afferents and modulate TRPV1 through activation of downstream intracellular signaling pathways. These factors result in functional potentiation of target proteins at the peripheral nociceptor terminal, leading to a rapid change in cellular and behavioral sensitivity [9]. This increases the sensitivity and excitability of the nociceptor terminal–a phenomenon known as peripheral sensitization [1, 4], which produces increases in pain sensitivity that is restricted to the site of inflammation.
2.2. Central Sensitization
Central sensitization refers to the process through which a state of hyperexcitability is established in the central nervous system, leading to enhanced processing of nociceptive (painful) messages [10]. Although many mechanisms have been implicated in the central sensitization, there are at least three main aspects involved in the sensitization: glutamatergic neurotransmission/N-Methyl-D-aspartate (NMDA) receptor-mediated hypersensitivity, loss of tonic inhibitory controls (disinhibition), and glial-neuronal interactions [3].
In the spinal dorsal horn, primary afferent C/Aδ fibers release peptide (e.g., substance P/ calcitonin-gene related peptide (CGRP), etc.) and excitatory amino acid (glutamate) products. Acute pain is signaled by the release of glutamate from the central terminals of primary afferent nociceptors, generating excitatory postsynaptic current in second order dorsal horn neurons, which occurs primarily through activation of postsynaptic glutamatergic α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) and kainate subtypes of ionotropic glutamate receptors [3]. Electro-physiologically, glutamatergic AMPA receptor antagonists diminish small afferent-evoked excitation; for glutamate, direct monosynaptic excitation is mediated by non-NMDA receptors (i.e., AMPA receptor) [11]. The NMDA subtype of glutamate channel is silent in normal condition. NMDA antagonists do not appear to reduce monosynaptically mediated afferent-evoked excitation and thus are not believed to be immediately postsynaptic to the primary afferent terminal, though some binding may be on the C-fiber terminal itself. However, in the setting of injury, increased release of neurotransmitters from primary afferent nociceptors sufficiently depolarizes postsynaptic neurons to activate NMDA receptors in second-order neurons [11]. The consequent increase in calcium influx can strengthen synaptic connections between nociceptors and dorsal horn pain transmission neurons, which in turn exacerbate responses to noxious stimuli [3]. A host of downstream signaling pathways and second messenger systems, notably kinases (such as MAPK, protein kinase A, protein kinase C, Phosphoinositide 3-kinase), is involved in the excitability of these neurons [12, 13].
The main type of inhibitory synaptic transmission in the dorsal horn is mediated by γ-aminobutyric acid (GABA) and glycine receptors, which are ligand-gated Cl- channels. Under normal conditions, the inhibitory control on the pain system is very powerful, serving as a ‘gate control’ to maintain the balance between excitatory and inhibitory synaptic inputs [14, 15]. But, in the setting of injury, this inhibition may be lost, resulting in disturbance of the balance leading to abnormal pain sensitivity [16, 17]. Meanwhile, the disinhibition can enable non-nociceptive Aβ afferents to engage the pain transmission circuitry such that normally innocuous stimuli are now perceived as painful [3, 15, 18-21].
Research on glial cells has come of age. The outdated concept that glia were simply the glue that holds the nerve cells together but otherwise have no active role in the brain, has been laid to rest for good [22]. The role of glia in, among other examples, synapse formation, synapse maturation and plasticity, and the rapid conduction of action potentials, as well as their immunological functions in the nervous system, has by now been unequivocally established [22]. Peripheral nerve injury/inflammation promotes release of neurotransmitters and neuropeptides that stimulate glial cells. Microglial activation occurs within minutes but can be long-lasting [23]. Activation of at least 5 major paths including fractalkine, interferon-γ, monocyte chemoattractant protein-1, toll-like receptor 4 (TLR4), and P2X on microglia, is involved in certain neuropathic nociceptive states [24]. Glia release brain-derived neurotrophic factor (BDNF), and a host of cytokines, such as TNFα, IL-1β and IL-6, and other factors (e.g. chemokines), which through their receptors expressed by neurons in the spinal dorsal horn, promotes increased excitability and enhanced pain in response to both noxious (hyperalgesia) and innocuous stimulation (allodynia) [3, 13, 25, 26]. Astrocytes have been recently identified as important components of the tripartite synaptic complex [27]. There is growing evidence that astrocytes regulate synaptic functions of neurons, in part, through the release of gliotransmitters [28, 29].
3. HIV-ASSOCIATED SENSORY NEUROPATHY AND PAIN
Since the first report of HIV/AIDS was published in the United States in 1981, the Centers for Disease Control and Prevention estimates that more than 1.8 million people in the U.S. have been infected with HIV, and more than 1.1 million estimated to be living with the disease today. While the number of new HIV infections was down from its peak in the 1980s, there have been approximately 50,000 new cases occurring every year [30]. Patients with HIV infection have numerous complications including neurological disorders. HIV-associated sensory neuropathies (HIV-SN) are the most common form of peripheral neuropathy, affecting about 30% of adults and children with AIDS [31, 32]. Sensory neuropathies that are a sequel of HIV infection, are known as distal sensory polyneuropathy (DSP), whereas the sensory neuropathies that result from antiretroviral therapy (ART), are known as antiretroviral drug-induced toxic neuropathies (ATN) [33].
The most common complaint of HIV-DSP is pain on the soles; the pain is typically bilateral, of gradual onset, and described as ‘aching’, ‘painful numbness’, or ‘burning’[34]. Patients often have hyperalgesia and allodynia in a stocking and/or glove distribution. The feet are tender to touch, wearing shoes is painful, and the gait becomes ‘antalgic’. In a typical length-dependent fashion, the dysesthesias ascend proximally up the lower extremities over months, and may begin to involve the fingertips at around the same time as they reach the mid-leg level [33, 35]. It is usually most severe on the soles of the feet, and is typically worse at night.
Pathologically, the most common histological feature of both DSP and ATN is characterized by loss of DRG sensory neurons, Wallerian degeneration of the long axons in distal regions, DRG infiltration by HIV-infected macrophages, and a 'dying back' sensory neuropathy [36-40]. Early on, small, unmyelinated sensory fibers are lost, with eventual destruction of the large myelinated fibers as the disease progresses in the patients with HIV. In the periphery and the DRG, there is infiltration of macrophages and other inflammatory cytokines [41]. Clinically, these two forms (HIV-DSP and ATN) of HIV sensory neuropathies are difficult to distinguish.
3.1. Neurochemical Mechanisms of HIV-DSP Neuropathic Pain
HIV-related neuropathic pain is a debilitating chronic condition that is severe and unrelenting. Despite decades of extensive research, the neuropathological mechanisms responsible for the development of this devastating condition remain largely unknown. Aberrant glia-neuron signaling is revealed in the pathogenesis of neuro-AIDS in HIV-1 infected individuals. Infected microglia release products that can be defined as “virotoxins” [42] consisting of toxic viral proteins, and inflammatory “cellular toxins” [43]. While astrocytes contributes less than microglia sources of virotoxins [44], astrocytes can be activated by cellular toxins from microglia. Viral protein gp120 is HIV viral exterior envelope glycoprotein cleaved from gp160. The entry of HIV into cells requires sequential interaction of gp120 with CD4 glycoprotein and chemokine receptors (CXCR4 and/or CCR5 as co-receptors of gp120) on the cell surface [45-48], to induce neurological dysfunction [49-52]. HIV gp120 exerts both direct and indirect neurotoxic effects in the nervous system through the release of proinflammatory factors [53]. A direct role of gp120 in the genesis of the neuropathic pain of DSP has been suggested. In 1996, Eron et al., reported that HIV-infected patients experienced pain at the injection site while they investigated whether immunization of patients who had symptomless HIV-1 infection with an envelope subcomponent vaccine (MNrgp120) to augment immune response, could slow the progression of HIV-1 disease [54]. Intrathecal administration of recombinant gp120 induces robust thermal hyperalgesia and mechanical allodynia [55]. Oh and colleagues have demonstrated that cultured rat DRG neurons express a wide variety of chemokine receptors including C-X-C chemokine receptor 4 (CXCR4), and that gp120 injected into the rat paw induces allodynia, providing evidence that chemokines and gp120 produce painful effects via direct actions on chemokine receptors expressed by nociceptive neurons [56]. Recombinant gp120 transiently delivered epineurally via oxidized cellulose wrapped around the rat sciatic nerve [57], induces mechanical allodynia and thermal hyperalgesia. Since then, these models have been using to investigate the molecular mechanisms of gp120-induced pain and the efficacy of potential therapeutic interventions.
3.1.1. Proinflammatory Cytokines
It is known that proinflammatory cytokines (e.g. TNFα, IL1-β, and IL-6, etc.) play an important role in the development and maintenance of inflammatory and neuropathic pain in preclinical studies [58-62]. Lymphocyte and macrophage infiltration is seen within the DRG of AIDS patients, with concomitant presence of pro-inflammatory cytokines including TNFα, interferon-γ (IFN-γ), IL-1 and IL-6 [38, 41, 63-66]. The increased levels of IL-1β and TNFα in human CSF [67-71] and brain tissue [71-74] have been reported in patients with HIV-1. HIV-1-infected patients have significantly higher plasma levels of TNFα and interleukin-6 [75]. An elevated baseline TNFα level among HIV-1 positive individuals may lead to additional neurodegeneration [76]. It is believed that the nerve injury initiates a cascade of events that lead to the development of chronic pain in patients with HIV [77]. Thus, the early presence of cytokines may be involved in the induction and/or progression of DSP neuropathic pain.
The HIV virus infection is able to increase the production and utilization of several cytokines, such as TNFα, IL-1, or IL-6 [78]. Kitano et al reported the HIV viral production is suppressed in the presence of anti-TNF antibodies in in vitro study [79]. The production of TNFα, a pro-apoptotic cytokine, uses mitochondria as targets [80]. Buch and colleagues have reported that the interplay of TNFα and HIV-1 leads to enhanced expression of the toxic chemokine [81]. Maggirwar and colleagues have showed that HIV protein influences neuronal survival by increasing in TNFα production [82]. Studies show a difference in neuropathogenic manifestations of HIV-1 neuroAIDS between HIV-1 subtypes, clade B- and clade C---infected subjects with clade B being more neuro-pathogenic than clade C; monocytes treated with clade B protein show a significant upregulation of proinflammatory cytokines, TNFα and IL-6, as compared to clade C protein-treated cultures [83].
In in vivo studies, HIV-1 transgenic rats overexpressing gp120, induces reactive gliosis in brain [84]. Intrathecal administration of soluble gp120 induces neuropathic pain and proinflammatory cytokine release in the spinal cord [85]. The gp120-exposed sciatic nerve exhibits pathology, notably axonal swelling and increased TNFα within the nerve trunk [57]. And, intense astrocytic and microglial activation is observed in the spinal cord, and this gliosis persisted for at least 30 days following epineural gp120, in parallel with neuropathic pain behaviors [57]. We and others have reported that application of recombinant gp120 to the sciatic nerve increases TNFα in the DRG and spinal cord [57, 86]. Peripheral gp120 application into the rat sciatic nerve upregulates the expression of spinal TNFα in the mRNA and the protein levels in the microglia and astrocytes at 2 weeks after gp120, and increases TNFα in the L4/5 DRG [86]. Furthermore, intrathecal TNFα siRNA or soluble TNF receptor reduces gp120 application-induced mechanical allodynia, indicating that TNFα in the spinal cord and the DRG are involved in neuropathic pain induced by HIV gp120 [86].
HIV neurotoxicity is mediated through the pro-inflammatory cytokine IL-1β. IL-1β produced by neurons in response to gp120 ligation of CXCR4, acted in an autocrine fashion to sensitize neurons to excitotoxicity [87-89]. The actions of IL-6 in gp120-evoked neuropathic pain states appear to be pro-nociceptive in nature [90]. Watkins and colleagues have demonstrated that blockade of IL-6 abolishes gp120-induced mechanical allodynia and inhibits gp120-mediated increases in TNF, IL-1, and IL-6 mRNA in the dorsal spinal cord, as well as TNFα and IL-1β protein release into the surrounding cerebrospinal fluid [90].
TNFα and IL-1β may act at least partly via MAPK cascades in glia [91-93]. Systemic administration of CNI-1493 (a p38MAPK inhibitor), blocks centrally mediated thermal hyperalgesia and mechanical allodynia induced by intrathecal gp120 (most likely via interfering with proinflammatory cytokine signal transduction) [94].
3.1.2. Chemokines
Chemokines are chemotactic cytokines that were originally discovered as promoters of leukocyte proliferation and mobility. Considering the widespread expression of CXCR4 and other chemokine receptors in the nervous system, CXCR4 is an important factor in the neuro-pathogenesis of HIV/AIDS [95]. In in vitro studies, binding of gp120 to CXCR4 receptors expressed by DRG satellite glial cells, upregulates the release of the Regulated on Activation, Normal T cell Expressed and Secreted (RANTES) chemokine (also known CCL5), which then activates CCR5 receptors expressed by DRG neurons to produce TNFα and subsequent TNFR1-mediated neurotoxicity in an autocrine fashion [37]. On the other hand, gp120 binds to and activates CXCR4 expressed by the DRG neurons in CD-4-independent manner [56, 96] to produce Ca2+-dependent upregulation of chemokine (C-C motif) receptor 2 (CCR2) expression by these neurons, suggesting direct neurotoxic effects of gp120 on neurons [97]. In in vivo studies, unilateral administration of gp120 into sciatic nerve, induces profound tactile hypernociception; monocyte-chemoattracting protein 1 (MCP1) and CCR2 are upregulated by primary sensory neurons in lumbar ganglia by post-operative day (POD) 14. CCR2 receptor antagonist at POD 14 reverses tactile hypernociception in gp120 treated animals [98].
3.1.3. Reactive Oxygen Species (ROS)
Oxidative stress results in activation of a number of complex and interrelated signaling events [99]. One of the pathways of oxidative stress activation is the MAPKs [100]. Increase in intracellular calcium is required for facilitation of neuronal nitric oxide synthase (nNOS) [101], leading to increased nitric oxide (NO). A host of evidence has demonstrated that free radicals have been implicated as mediators of chronic pain [102-107]. HIV-gp120 has been implicated in initiation and/or intensification of ROS and disruption of mitochondrial transmembrane potential; HIV-induced ROS regulates apoptosis signaling through TNFα and its receptors [108]. Watkins and colleagues have reported that intrathecal gp120 induces neuropathic pain and spinal release of NO as well as proinflammatory cytokines, that pretreatment with NO synthase (NOS) inhibitor abolishes gp120-induced mechanical allodynia, and that gp120-induced NO increases proinflammatory cytokines [109]. HIV gp120 induces allodynia by increasing [Ca2+]i, concomitant with activation of prostanoid EP3 and kappa-opioid receptors in the spinal cord [110]. In gp120-induced neuropathic pain model, we found that nitrated superoxide dismutase 2 (SOD2, mainly located at mitochondria) is increased in the spinal cord after peripheral gp120 application [111]. And, the activity of endogenous SOD2 is significantly downregulated by gp120 application; a new mitochondria-targeted superoxide scavenger (Mito-Tempol) significantly reverses mechanical allodynia and the decreased SOD2 activity in the model, suggesting that ROS may be a therapeutic target in the HIV-associated neuropathic pain [111] .
3.1.4. Substance P and its Receptor
The undecapeptide substance P (SP) is the prototype tachykinin and it has been identified in the central and peripheral nervous system, and in the immune system [112]. SP, as a primary sensory neurotransmitter, is found in the smaller, unmyelinated sensory ‘painful’ fibers [113]. SP is released into the dorsal horn of the spinal cord following intense peripheral stimulation, which promotes central hyperexcitability and increased sensitivity to pain [114]. Exogenous substance P, when applied to dorsal horn sensory neurons, has a slow onset and prolonged excitatory action that resembles the pattern of excitation observed after peripheral noxious stimuli [113]. The increased release of SP in the spinal cord may cause the central sensitization and hyperalgesia associated with inflammation [115, 116].
SP receptor (neurokinin-1 receptor, NK-1R)-expressing postsynaptic neurons in the dorsal horn of the spinal cord plays a pivotal role in the generation and maintenance of chronic neuropathic and inflammatory pain [117]. NK-1R is a potential pharmaceutical target [118]. The finding that SP is also secreted by human immune cells and participates in immunoregulation of immune cells may be of importance for the pathogenesis of AIDS [118]. SP plays a critical role in HIV gp120-induced increase in permeability of rat brain endothelium cultures, and this effect of SP on gp120-induced increase in albumin permeability is abrogated by the SP antagonists [118, 119]. Substance P enhances inflammatory cytokine (TNFα, IL-1 and IL-6) production by immune cells such as macrophages through activation of NF-κB [120]. There is a bidirectional relationship between SP and HIV infection of human immune cells [118]. In the studies of preclinical pharmacology, systemic non-peptide NK-1 receptor antagonist (SR140333B) reduces carrageenan-induced heat hyperalgesia in rats [121]. The neurokinin-1 antagonist, RP 67580, is more effective in inhibiting the behavioral response to formalin and the pain-induced activation of c-Fos [122]. But, the failure of inhibitors of NK-1R to exhibit analgesia in human, is likely due to low levels of receptor occupancy and inadequate brain penetration [123].
3.1.5. Gamma-Aminobutyric Acid (GABA)
Gamma-aminobutyric acid (GABA) is synthesized by two glutamic acid decarboxylases: GAD67 (GAD1) and GAD65 (GAD2). Between the two isoforms, GAD67 is responsible for over 90% of basal GABA synthesis and is produced at limiting levels in the brain [124]. GAD67-mediated GABA synthesis and signaling regulate inhibitory synaptic innervation [124]. The loss of GABA inhibition allows the expression of long-lasting synaptic potentiation, and by extension, the development of neuropathic pain in animals [5]. In the hippocampus, TNFα causes an endo-cytosis of GABA-A receptors, lowers surface GABA-A receptors and decreases inhibitory synaptic strength, suggesting that TNFα can regulate neuronal circuit homeostasis in a manner that may exacerbate excitotoxic damage resulting from neuronal insults [125]. We have found that peripheral gp120 application into sciatic nerve lowers the expression of GAD67 in the spinal cord and intrathecal injection of GABA-B agonist baclofen reverses mechanical allodynia induced gp120 into sciatic nerve (our unpublished data).
3.1.6. Other Pharmacological Features in the DSP Neuropathic Pain
Nicotinic acetylcholine receptors (nAchRs) are found on peripheral monocytes, in particular the alpha seven nAchRs (α7AchRs), which when activated suppress the release of the pro-inflammatory cytokines [126, 127]. In spinal cord, α7AchRs are found on microglia. Intrathecal α7AchR agonists (GTS-21 or choline), significantly block and reverse gp120-induced mechanical allodynia, and reduce gp120-induced IL-1β protein and pro-inflammatory cytokine mRNAs within the lumbar spinal cord, supporting that α7AchRs may be a novel target for treating pain in patients with HIV [128]. HIV gp120 shed by the virus can inhibit the appropriate processing of proBDNF into mature BDNF by reducing furin levels in rat primary neurons; proBDNF, in turn, initiates neuronal damage which, in combination with other neurotoxins such as glutamate or TNFα, can lead to apoptosis and neuronal loss[129].
The Wnt proteins are a group of secreted lipid-modified (palmitoylation) signaling proteins. Wnt3a is up-regulated in the spinal dorsal horn of mouse pain models created by intrathecal injection of HIV-gp120 protein, suggesting that Wnt signaling pathways are involved in the nociceptive input induced by HIV-gp120 [130]. Pretreatment with intrathecal cannabilactone CB2R agonist AM1710 prevents bilateral mechanical hypersensitivity induced by intrathecal gp120, and reveals increased DRG IL-1β protein levels from gp120, suggesting that cannabilactone CB2R agonists be emerging as anti-inflammatory agents with pain therapeutic implications [131].
Intrathecal administration of NF-κB inhibitors, pyrrolidinedithiocarbamate (PDTC) or SN50, prior to gp120 partially attenuates gp120-induced allodynia [132]. Systemic p38 MAP kinase inhibitor CNI-1493, blocks intrathecal gp120-induced thermal hyperalgesia and mechanical allodynia [94]. Intrathecal administration of TX14(A), a 14-mer peptide derived from the C terminal region of the saposin C domain of prosaposin both prevents and alleviates intraplantar gp120-induced tactile allodynia, suggesting that the mechanism of action of TX14(A) may include modulation of spinal nociceptive processing [133]. The mechanical hypersensitivity induced by gp120 is reversed by systemic treatment with gabapentin, morphine and the cannabinoid WIN 55,212-2 [134].
Meanwhile, Acharjee and colleagues have reported the effects of a cytotoxic HIV-1 accessory protein, viral protein R (Vpr) on the peripheral nervous system, demonstrating that Vpr causes DRG neuronal damage, likely through cytosolic calcium activation and cytokine perturbation, highlighting Vpr’s contribution to HIV-associated peripheral neuropathy and ensuing neuropathic pain [135].
3.2. Summary
The perivascular macrophages are the primary site of productive HIV infection. Infected macrophages or microglia release viral envelope proteins (gp120), potentially neurotoxic substances such as proinflammatory cytokines (for example, TNFα, IL-1, IL-6), chemokines, and glutamates. These neurotoxic substances stimulate astrocytosis to release similar neurotoxic factors. Many neurons express CXCR4 and CCR5, raising the possibility of direct interaction with gp120. Over activation of CXCR4 activity induces neuronal injury and allows excessive influx of Ca2+, then induce a host of downstream signaling pathways and second messenger systems, notably kinases (such as MAPK, protein kinase A, protein kinase C, phosphoinositide 3-kinase), which is involved in the excitability of these neurons. Based on the mechanisms, it is necessary to develop the effective and novel antagonists, for example, blockers of CXCR4, proinflammatory cytokine neutralizers and kinases inhibitors, ROS scavengers, NK-1R blockers to treat HIV neuropathic pain and neuropathy (Fig. 1).
Fig. (1).
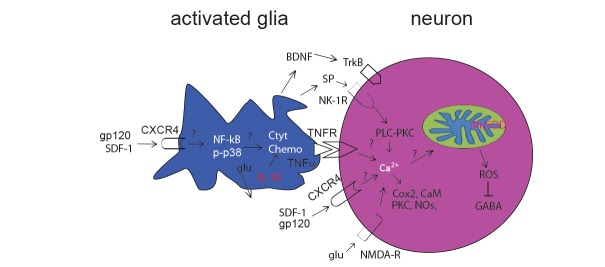
Potential HIV-related neuropathic pain pathway. Peripheral nerve inflammation after HIV infection, promotes the release of neurotransmitters and neuropeptides that stimulate glial cells in the spinal cord. The activated glia induce release of pro-inflammatory factors, such as cytokines, nerve growth factors, chemokines, glutamates, etc., which bind their receptors on the neurons to induce a massive Ca2+ influx into neurons. Ca2+ is rapidly sequestrated by mitochondria. This consequently damages MnSOD activity and increases mitochondrial ROS production, which in turn results in synaptic plasticity of the dorsal horn neurons. It is possible that ROS injures the production of GABA through GAD67 synthesis inhibition. CaM = calcium-calmodulin; Chemo=chemokines; Cyto=cytokines; glu = glutamate; NMDA-R = N-methyl-D-aspartate receptor; NO = nitric oxide.
4. ART-RELATED NEUROPATHIC PAIN
The highly active antiretroviral therapy (HAART) since 1996, dramatically has reduced the morbidity and mortality associated with HIV [136]. HAART usually contains three or more different drugs, such as two nucleoside reverse transcriptase inhibitors (NRTIs) and a protease inhibitor, two NRTIs and a non-nucleoside reverse transcriptase inhibitor or other such combinations. NRTIs decrease plasma viral load, and can result in improvements in immune function (e.g. CD4 lymphocyte count recovery) [137]. Powerful HAART has come tantalizingly close to eradicating the virus from people, driving blood level of HIV so low that standard tests cannot detect it. Therefore, HIV/AIDS have now been transformed from a rapidly progressive disease with high early mortality to a chronic disease. But no one has been cured, because the virus comes roaring back in one who stops taking the drugs. So HAART will be life-long treatment. Although the incidence of most neurological complications of HIV has fallen with HAART, rates of HIV-SN have been rising [138]. Recent estimates of HIV-SN prevalence among cohorts with access to ART range from 20% to 50% [139]. NRTI-associated painful sensory neuropathy affects quality of life in patients with HIV/AIDS.
NRTIs (e.g., stavudine/didehydro-deoxythymidine/d4T, didanosine/2',3'-dideoxyinosine/ddI, and zalcitabine/2',3'-dideoxycytidine/ddC), are neurotoxic and cause a dose-dependent peripheral neuropathy [140]. Somehow, drug toxicity of NRTIs limits the successful treatment in many individuals (see review [137]). The ddC is used particularly for patients who were intolerant of or ineligible for AZT/ddI. The ddC is still widely used in clinics in Africa, Europe and Asia. A major dose-limiting side effect of HIV/AIDS chemo-therapies is a small-fiber painful peripheral neuropathy, mediated by mitochondrial toxicity [141]. Patients receiving HAART develop a distal symmetric small fiber retrograde (‘dying back’) axonal neuropathy with pain [38, 142-151]. However, the detailed mechanism by which these patients with HIV/AIDS experienced pain remains unknown.
4.1. Mechanisms of ART-Related Neuropathic Pain
4.1.1. Mitochondrial Toxicity of NRTI
Mitochondrial DNA (mtDNA) is necessary for many oxidative phosphorylation complex I proteins. The mtDNA depletion causes a deficiency in complex I and an over-utilization of complex II, resulting in elevated superoxide levels [152]. Depletion in mtDNA and increased mtDNA mutations may reduce synthesis of mtDNA-encoded protein subunits required for oxidative phosphorylation [153]. Replication of mtDNA is undertaken by DNApoly-merase-γ, and this enzyme can be inhibited by NRTIs. These agents include the 2’,3’-dideoxy analogues that lack the hydroxyl radical in the 3’ position and are incorporated into DNA but prevent elongation of the DNA strand [154]. Thus, these drugs can induce mtDNA depletion and result in mitochondrial respiratory chain and oxidative phosphorylation deficits. NRTIs exert rapid toxicity by directly inhibiting mitochondrial bioenergetics function [155]. It has been estimated that significant mitochondrial DNA depletion takes place after several days to weeks of explore [156-158]. The resulting reduction in ATP and increased ROS have the potential for further mtDNA damage [159, 160]. The mitochondrial dysfunction induced by ddC alters calcium homeostasis in cultured DRG neurons [161] and in a model of ddC-associated painful peripheral neuropathy [162]. In addition, NRTIs cause direct mitochondrial toxicity through inhibition of the mitochondrial transmembrane potential differential in neurons but not in Schwann cells that are also present in the coculture [163]. Mitochondrial ultrastructural abnormalities have been noted in affected tissues, including peripheral nerves and subcutaneous tissue [151, 164, 165].
4.1.2. Spinal Microgliosis
Glial cells in the CNS are essential for the maintenance of homeostasis. Activated glial cells contribute to immune deregulation and neuroinflammation, which are associated with pain and a variety of neurodegenerative disorders [166]. Spinal microgliosis, as measured by increased CD11b/c immunohistochemical staining and increased numbers of cells expressing CD11b measured by flow cytometry, is evident in the antiretroviral drug ddC or combination of perineural exposure to the HIV-gp120 protein and ddC treatment [167]. Rice and colleagues showed that ddC induced spinal microgliosis using flow cytometric quantification of OX42 (marker of microglia) [167], however, Bennett and colleagues reported that there was no microglia hypertrophy or increased Iba1 (another marker of microglia) staining in the spinal cord in the animals treated with ddC [168].
4.1.3. Proinflammatory Cytokines, Chemokines, and ROS in NRTI-Neuropathy
Levine and colleagues developed an animal model of NRTI-induced neuropathic pain. Systemic administration of ddC, ddI and d4T produces dose-dependent mechanical hypersensitivity and allodynia [162]. The model has been used widely to investigate the molecular mechanisms of NRTI-induced pain and the efficacy of potential therapeutic interventions.
We have reported that systemic ddC induces mechanical allodynia and overexpression of both mRNA and proteins of glial fibrillary acidic protein (GFAP) and TNFα in the spinal dorsal horn, and that TNFα is colocalized with GFAP in the spinal dorsal horn, and with NeuN in the DRG [169]. Knockdown of TNFα with siRNA blocks the mechanical allodynia induced by ddC; intrathecal administration of recombinant TNF soluble receptor, reverses mechanical allodynia induced by ddC, suggesting that TNFα is involved in NRTI-induced neuropathic pain [169].
Systemic ddC induces the expression levels of CXCR4 mRNA in glia and neurons and SDF-1 mRNA in glia increased considerably [170]. Pain hypersensitivity produced by ddC is inhibited by treatment with the CXCR4 antagonist, AMD3100, suggesting that NRTIs produce pain hyper-sensitivity through the upregulation of CXCR4 signaling in the DRG [170].
Oxidative stress, which occurs in nerve tissues of patients undergoing HIV infection, is implicated in cell death of both astrocytes and neurons, and has recently been suggested to play a role in the pathogenesis of neuroAIDS [171]. Microglia produce superoxide and H2O2 upon activation in the CNS; they also produce cytokines which can enhance more production of ROS and NO [172]. Astrocytes equally produce cytokines and NO from iNOS [172]. Evidence suggests that a component of gp120 neurotoxicity may be due to increased oxidative stress [173]. Redox-regulated inflammatory pathways and synergistic proinflammatory stimulation may have significant implications in HIV-infected patients [174]. We have reported that systemic administration of ddC induces neuropathic pain and lowers the activity of endogenous SOD2 in the spinal cord dorsal horn; ROS scavengers significantly reverse mechanical allodynia in the model, suggesting that ROS systems play an important role in the neuropathic pain induced by antiretroviral therapy in patients with HIV/AIDS [175].
4.2. Other Preclinical Features of Pharmacology in ART-Induced Neuropathic Pain
Intradermal or spinal injection of drugs (TMB-8 and Quin-2) that buffer intracellular calcium, significantly attenuates ddC-induced mechanical hypersensitivity, suggesting that [Ca2+]i signaling plays an important role in NRTI-induced mechanical hypersensitivity [162]. Either intrathecal or peripheral TMB-8 alone significantly attenuates ddC-induced hypersensitivity by approximately 50%; furthermore, the combined administration of intradermal plus intrathecal TMB-8 almost completely eliminates ddC-induced hypersensitivity, suggesting that an abnormality in Ca2+ buffering at the peripheral and central terminals of primary afferent nociceptors contributes to ddC-induced painful peripheral neuropathy [162]. Peripheral administration of inhibitors of protein kinase A, protein kinase C, p42/p44-mitogen-activated protein kinase (ERK1/2) and nitric oxide synthase, which have demonstrated anti-hyperalgesic effects in other models of neurophathic pain [13, 176-179], has no effect on ddC-, ddI- and d4T-induced hypersensitivity [162]. Systemic ddC decreases conduction velocity in mechanically-evoked C-fiber activity [180]. Co-morbid conditions may contribute to this dose-limiting effect of HIV/AIDS treatment. Alcohol abuse is one of the most important co-morbid risk factors for peripheral neuropathy in patients with HIV/AIDS [141]; intradermal injection of inhibitors of the mitochondrial electron transport chain, rotenone (complex I) and oligomycin (complex V) into the hind paw, reverses the mechanical allodynia induced by ddC or by low dose of ddC plus ethanol, supporting the clinical impression that alcohol consumption enhances ART neuropathy, and providing evidence for a role of mitochondrial mechanisms underlying this interaction [141]. In addition, mitochondria exist as dynamic interconnected networks that are maintained through a balance of fusion and fission [181], which is associated with many pathological conditions, notably neurodegeneration and aging [182]. Dynamin-regulated protein 1 (Drp1) and Fis1 mediate mitochondrial fission [183, 184]. Intradermal injection of the selective Drp1 inhibitor mdivi-1 into the hind paws of rats produces a dose-dependent inhibition of ddC-induced painful hyperalgesia [185].
The N-type voltage-gated calcium channel (CaV2.2) is a clinically endorsed target in chronic pain treatments. Collapsin response mediator protein 2 (CRMP-2) is a novel modulator of CaV2.2 [186]. By preventing CRMP-2-mediated enhancement of CaV2.2 function, TAT-CBD3 (a peptide of CRMP-2 fused to the transduction domain of TAT protein of HIV) decreases neurotransmitter release from nociceptive DRG neurons, and reverses neuropathic hypersensitivity produced by ddC [187]. ST1-104, which disrupts the interaction between CaV2.2 and CRMP2 interaction, reduces persistent mechanical hypersensitivity induced by systemic administration of ddC [188]. Palmitoylethanolamide (PEA) is efficacious in animal models of neuropathic pain [189]. Systemic administration of L-29 (a PEA analogue) reduces mechanical hyper-sensitivity in ddC-associated hypersensitivity [190]. The mechanical hypersensitivity induced by ddC is reversed by systemic treatment with gabapentin, morphine and the cannabinoid WIN 55,212-2 [191].
Recent report shows that d4T administration to mice results in the increased neuronal activity and BDNF expression in the spinal dorsal horn and hind paws mechanical allodynia that is exacerbated by intrathecal BDNF administration. After d4T, BDNF heterozygous mice are less allodynic than wild-type littermates, which is negated by intrathecal BDNF. Blockage of BDNF-mediated signaling, significantly attenuates the development of mechanical allodynia, and decreases neuronal activity, demonstrating that BDNF in the spinal dorsal horn contributes to the development of NRTI-induced painful peripheral neuropathy and may represent a new therapeutic opportunity [192]. Rice and colleagues have reported that intravenous injections of d4T induces hind paw mechanical hypersensitivity in rats and injury to both the peripheral and central terminals of L5 dorsal root ganglion neurons, that d4T results in increased GFAP immunoreactivity, and that systemic gabapentin and cannabinoid receptor agonist WIN 55,212-2 reverse mechanical hypersensitivity in rats [193].
4.3. Summary
The mechanisms by which NRTIs induce neuropathic pain are not known in detail. NRTIs can alter mtDNA content by inhibiting polymerase gamma, the enzyme responsible for the replication of mtDNA [143, 151, 152, 194]. Mitochondrial DNA is necessary for many oxidative phosphorylation complex I proteins. Mitochondrial DNA depletion causes a deficiency in complex I and an over-utilization of complex II, resulting in elevated superoxide levels [152]. Studies have shown the interaction between ROS and TNFα. NRTIs also directly or indirectly induce other neurotoxic factors, such as chemokines and their receptors, MAPKs or ion channel changes, many of them are related to neuropathic pain. Therefore, understanding the molecular mechanisms is important to design new drugs to treat the NRTIs-related neuropathic pain (Fig. 2).
Fig. (2).
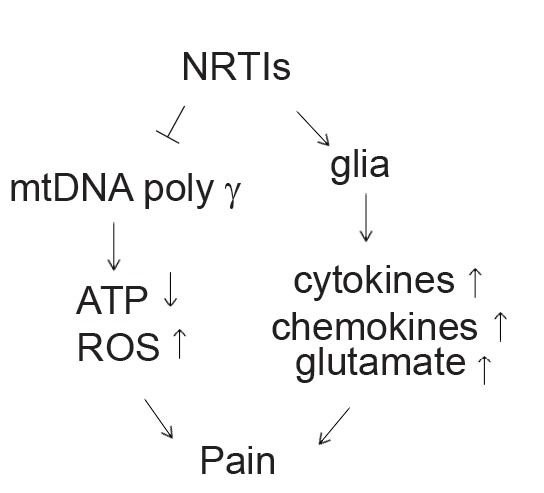
Proposed model for the role of NRTI in the HIV associated neuropathic pain. NRTIs inhibit the transcription of essential enzymes needed for ATP production by inhibiting DNA γ- polymerase. Depletion in mtDNA may reduce synthesis of mtDNAencoded protein subunits required for oxidative phosphorylation, decreasing ATP and increasing mitochondrial oxidative stress. NRTIs also activate glia to release cytokines, chemokines and BDNF to induce neuronal sensitization.
5. MECHANISMS OF PAINFUL NEUROPATHY IN INTERACTION OF HIV INFECTION AND NRTIS
HIV-SN in HIV/AIDS patients is associated with two types of neuropathy that are very similar clinically: distal sensory polyneuropathy, associated with advanced HIV infection per se, and antiretroviral toxic neuropathy, precipitated by the use of antiretroviral drugs in patients at varying stages of HIV infection. The onset of NRTIs-induced neuropathy is typically more acute than the onset of DSP, and pain may be more prominent [33]. In this “double-hit” condition, DRG neurons/sensory axons are damaged or sensitized by viral proteins, including gp120 and proinflammatory factors released from infiltrating HIV-infected macrophages, and are further compromised by antiretroviral drug-induced mitochondrial toxicity. Reductions in mtDNA may be caused by HIV infection alone and precede the use of NRTIs, raising the possibility that HIV directly and/or TNFα released in response to HIV infection during the immune reconstitution may injure mitochondria, potentially making them more vulnerable to the effects of NRTIs [195]. Activity in caspase signaling pathways that ultimately lead to apoptosis, plays a critical role in the generation of neuropathic pain induced by ddC and inflammatory pain induced by TNFα, before death of sensory neurons becomes apparent [196]. Therefore, closely-clinical animal model of HIV-related neuropathy should include the two factors: HIV protein toxicity and NRTIs neurotoxicity.
Keswani and colleagues reported a rodent model of HIV-SN by oral administration of ddI to transgenic mice expressing the HIV gp120 under a GFAP promoter [197]. The neuropathy in these mice is characterized by distal degeneration of unmyelinated sensory axons, similar to the “dying back” pattern of C-fiber loss seen in patients with HIV-SN; gp120 transgenic mice treated with ddI develop mild thermal hyperalgesia [197].
Both perineural HIV-gp120 and systemic ddC produce many features of HIV- and anti-retroviral-related peripheral neuropathy, and that combination of peripheral gp120 and ddC induces an enhanced mechanical allodynia [191]. The combination of gp120 and ddC induces inflammatory response in sciatic nerve and DRG, which is in line with clinic studies indicating that patients with HIV show a certain degree of inflammation involvement in the DRG [38]; combination of gp120 and ddC induces loss of epidermal nerve fibers, which is seen in HIV/AIDS patients [39]. Moreover, a spinal gliosis is apparent at times of peak behavioral sensitivity that is exacerbated in gp120+ddC as compared to either treatment alone. Finally, the hyper-sensitivity to mechanical stimuli is sensitive to systemic treatment with gabapentin, morphine and the cannabinoid WIN 55,212-2. These data therefore merit further investigation for the elucidation of underlying mechanisms and may prove useful for preclinical assessment of drugs for the treatment of HIV-related peripheral neuropathic pain [191].
MCP1 (known as CCL2) and stromal derived factor-1 (SDF1/CXCL12) and their respective receptors, CCR2 and CXCR4, have been implicated in HIV-related neuropathic pain mechanisms including NRTI treatment in rodents [170]. White and colleagues reported that gp120 sciatic nerve injury in combination with ddC at day 14 after gp120 produces pronounced bilateral tactile hypernociception [98]. More importantly, functional MCP1/CCR2 and SDF1/CXCR4 signaling is present in sensory neurons. CXCR4 antagonist AMD3100 effectively reverses the hypernociceptive behavior associated with the gp120+ddC, indicating that the functional upregulation of CCR2 and CXCR4 signaling systems following a combination of gp120 plus NRTI is likely to be of central importance [98].
Our recent studies and others have demonstrated that either gp120 application into sciatic nerve or systemic ddC activates spinal glia using immunohistochemistry and Western blots [86, 134, 169]. Blackbeard and colleagues have reported that gp120+ddC induces spinal microgliosis using flow cytometric quantification or immunohistochemistry [167, 191]. The degree of mechanical hypersensitivity is found to be in line with spinal cord microgliosis in gp120+ddC as determined by CD11b/c cytometry [167]. BDNF in the spinal dorsal horn contributes to the development of NRTI-induced painful peripheral neuropathy [192]. Rice and colleagues, have reported that the truncated isoform of TrkB, a receptor for BDNF, is the most upregulated gene in the gp120+ ddC model using conventional analysis and Gene Set Enrichment Analysis [198].
Alterations in calcium activity may be related to an additive/synergistic effect of a combination of gp120 and ddC exposure. Intradermal or spinal injection of intracellular calcium modulators significantly attenuates ddC-induced mechanical hypersensitivity, suggesting the intracellular calcium dysfunction induced by ddC is likely involved in ddC-associated painfully peripheral neuropathy [162]. HIV gp120 increases [Ca2+]i in populations of the cultured DRG neurons through CXCR4 [56]. The mitochondrial dysfunction induced by ddC alters calcium homeostasis in cultured DRG neurons [161] and in ddC-induced neuropathic pain [162]. HIV gp120 may induce axonal degeneration directly through mitochondrial caspase pathway and indirectly through neuronal apoptosis mediated by the activation of Schwann cells [199]. Such an additive/synergistic effect of the treatments with gp120+ddC in behavioral indices of pain may be related with intracellular calcium mitochondrial dysfunction [98, 191].
In summary, HIV-associated sensory polyneuropathies induced by HIV infection and antiretroviral therapy share clinic symptoms. Animal models of NRTI-induced neuropathy have yielded similar molecular mechanisms compared to that induced by HIV gp120 protein in the neuropathic pain state. Models of combined NRTI and HIV gp120 protein-induced neuropathy have provided similar molecular mechanisms, mainly including the proinflammatory cytokines, chemokine, oxide stress, MAPK and calcium channel changes, many of them are interacted and twisted together and reciprocal relationship in the molecular mechanisms.
CONCLUSION
HIV associated neuropathic pain is a symptom across the entire process of the disease, and remains a common and debilitating symptom frequently reported in clinics. This review sums up the current advance of molecular mechanisms, suggesting that glia activity induced neurochemical factors, such as, proinflammatory cytokines, chemokines, and neurotrophic factor, ROS, calcium channels, and MAP kinases, play important roles in the development of the neuropathic pain. As the mechanisms underlying HIV-related neuropathic pain are better understood, focusing on the molecular targets (e.g., receptor blockers of cytokines and chemokines, scavengers of ROS, etc.) will be valuable for developing novel pharmaceutical approaches to the treatment of HIV-neuropathic pain. Indeed, the precise mechanisms are not fully known, and the preclinical models need to be improved. Therefore, the more detailed molecular mechanisms have yet to be investigated, such as the relationship between neurotoxic factors, regions of molecular targets in the nervous system, and interaction of glia and neurons, which will have the potential to open new pharmaceutical approaches.
ACKNOWLEDGEMENTS
Supported by NIH DA025527, NS066792, and DA026734. Author specially acknowledges the special helps from Drs. David J. Fink and Marina Mata (Neurology, University of Michigan, MI), for their invaluable helps and the supports from Drs. Roy Levitt and Keith Candiotti, and David Lubarsky (Anesthesiology, University of Miami, FL). Author thanks Dr. Stephen Koslow helpful comments.
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
REFERENCES
- 1.Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP do pain and memory share similar mechanisms. Trends Neurosci. 2003;26(12):696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 2.Zimmermann M. Pathobiology of neuropathic pain. Eur. J. Pharmacol. 2001;429(1-3):23–37. doi: 10.1016/s0014-2999(01)01303-6. [DOI] [PubMed] [Google Scholar]
- 3.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139(2):267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413(6852):203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 5.Scholz J, Woolf CJ. Can we conquer pain Nat. Neurosci. 2002;5 Suppl:1062–1067. doi: 10.1038/nn942. [DOI] [PubMed] [Google Scholar]
- 6.Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36(1):57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- 7.Schafers M, Svensson CI, Sommer C, Sorkin LS. Tumor necrosis factor-alpha induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J. Neurosci. 2003;23(7):2517–2521. doi: 10.1523/JNEUROSCI.23-07-02517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21(3):531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 9.Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411(6840):957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- 10.Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306(5944):686–688. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- 11.Yaksh TL, Hua XY, Kalcheva I, Nozaki-Taguchi N, Marsala M. The spinal biology in humans and animals of pain states generated by persistent small afferent input. Proc. Natl. Acad. Sci. U. S. A. 1999;96(14):7680–7686. doi: 10.1073/pnas.96.14.7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J. Pain. 2009;10(9):895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo W, Wang H, Watanabe M, Shimizu K, Zou S, LaGraize SC, Wei F, Dubner R, Ren K. Glial-cytokine-neuronal interactions underlying the mechanisms of persistent pain. J. Neurosci. 2007;27(22):6006–6018. doi: 10.1523/JNEUROSCI.0176-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melzack R, Wall PD. Pain mechanisms a new theory. Science. 1965;150(699):971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 15.Baba H, Ji RR, Kohno T, Moore KA, Ataka T, Wakai A, Okamoto M, Woolf CJ. Removal of GABAergic inhibition facilitates polysynaptic A fiber-mediated excitatory transmission to the superficial spinal dorsal horn. Mol. Cell. Neurosci. 2003;24(3):818–830. doi: 10.1016/s1044-7431(03)00236-7. [DOI] [PubMed] [Google Scholar]
- 16.Hwang JH, Yaksh TL. The effect of spinal GABA receptor agonists on tactile allodynia in a surgically-induced neuropathic pain model in the rat. Pain. 1997;70(1):15–22. doi: 10.1016/s0304-3959(96)03249-6. [DOI] [PubMed] [Google Scholar]
- 17.Malan TP, Mata HP, Porreca F. Spinal GABA(A) and GABA(B) receptor pharmacology in a rat model of neuropathic pain. Anesthesiology. 2002;96(5):1161–1167. doi: 10.1097/00000542-200205000-00020. [DOI] [PubMed] [Google Scholar]
- 18.Sivilotti L, Woolf CJ. The contribution of GABAA and glycine receptors to central sensitization: disinhibition and touch-evoked allodynia in the spinal cord. J. Neurophysiol. 1994;72(1):169–179. doi: 10.1152/jn.1994.72.1.169. [DOI] [PubMed] [Google Scholar]
- 19.LaMotte CC, Kapadia SE. Deafferentation-induced terminal field expansion of myelinated saphenous afferents in the adult rat dorsal horn and the nucleus gracilis following pronase injection of the sciatic nerve. J. Comp. Neurol. 1993;330(1):83–94. doi: 10.1002/cne.903300107. [DOI] [PubMed] [Google Scholar]
- 20.Mannion RJ, Doubell TP, Coggeshall RE, Woolf CJ. Collateral sprouting of uninjured primary afferent A-fibers into the superficial dorsal horn of the adult rat spinal cord after topical capsaicin treatment to the sciatic nerve. J. Neurosci. 1996;16(16):5189–5195. doi: 10.1523/JNEUROSCI.16-16-05189.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baba H, Doubell TP, Woolf CJ. Peripheral inflammation facilitates Abeta fiber-mediated synaptic input to the substantia gelatinosa of the adult rat spinal cord. J. Neurosci. 1999;19(2):859–867. doi: 10.1523/JNEUROSCI.19-02-00859.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stern P. Glia Glee for glia Introduction. Science. 2010;330(6005):773–0. doi: 10.1126/science.330.6005.773. [DOI] [PubMed] [Google Scholar]
- 23.Colton C, Wilcock DM. Assessing activation states in microglia. CNS Neurol. Disord. Drug Targets. 2010;9(2):174–191. doi: 10.2174/187152710791012053. [DOI] [PubMed] [Google Scholar]
- 24.Smith HS. Activated microglia in nociception. Pain Physician. 2010;13(3):295–304. [PubMed] [Google Scholar]
- 25.Woolf CJ, Allchorne A, Safieh-Garabedian B, Poole S. Cytokines nerve growth factor and inflammatory hyperalgesia the contribution of tumour necrosis factor alpha. Br. J. Pharmacol. 1997;121(3):417–424. doi: 10.1038/sj.bjp.0701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LaMotte CC, Kapadia SE, Arsenault K, Wolfe M. Deafferentation-induced expression of GAP-43 NCAM and NILE in the adult rat dorsal horn following pronase injection of the sciatic nerve. Somatosens. Mot. Res. 1995;12(1):71–79. doi: 10.3109/08990229509063143. [DOI] [PubMed] [Google Scholar]
- 27.Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses glia the unacknowledged partner. Trends Neurosci. 1999;22(5):208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- 28.Zhuang Z, Huang J, Cepero ML, Liebl DJ. Eph signaling regulates gliotransmitter release. Commun. Integr. Biol. 2011;4(2):223–226. doi: 10.4161/cib.4.2.14507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halassa MM, Haydon PG. Integrated brain circuits astrocytic networks modulate neuronal activity and behavior. Annu. Rev. Physiol. 2010;72:335–355. doi: 10.1146/annurev-physiol-021909-135843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foundation KF. Fact Sheet The HIV/AIDS Epidemic in the United States. In: http://www.kff.org/hivaids/3029.cfm Ed. 2013 [Google Scholar]
- 31.Schifitto G, McDermott MP, McArthur JC, Marder K, Sacktor N, Epstein L, Kieburtz K. Incidence of and risk factors for HIV-associated distal sensory polyneuropathy. Neurology. 2002;58(12):1764–1768. doi: 10.1212/wnl.58.12.1764. [DOI] [PubMed] [Google Scholar]
- 32.McArthur JH. The reliability and validity of the subjective peripheral neuropathy screen. J. Assoc. Nurses AIDS Care. 1998;9(4):84–94. doi: 10.1016/S1055-3290(98)80048-4. [DOI] [PubMed] [Google Scholar]
- 33.Keswani SC, Pardo CA, Cherry CL, Hoke A, McArthur JC. HIV-associated sensory neuropathies. AIDS. 2002;16(16):2105–2117. doi: 10.1097/00002030-200211080-00002. [DOI] [PubMed] [Google Scholar]
- 34.Cornblath DR, McArthur JC. Predominantly sensory neuropathy in patients with AIDS and AIDS-related complex. Neurology. 1988;38(5):794–796. doi: 10.1212/wnl.38.5.794. [DOI] [PubMed] [Google Scholar]
- 35.Verma S, Estanislao L, Mintz L, Simpson D. Controlling neuropathic pain in HIV. Curr. HIV/AIDS Rep. 2004;1(3):136–141. doi: 10.1007/s11904-004-0020-0. [DOI] [PubMed] [Google Scholar]
- 36.Wulff EA, Wang AK, Simpson DM. HIV-associated peripheral neuropathy epidemiology pathophysiology and treatment. Drugs. 2000;59(6):1251–1260. doi: 10.2165/00003495-200059060-00005. [DOI] [PubMed] [Google Scholar]
- 37.Keswani SC, Polley M, Pardo CA, Griffin JW, McArthur JC, Hoke A. Schwann cell chemokine receptors mediate HIV-1 gp120 toxicity to sensory neurons. Ann. Neurol. 2003;54(3):287–296. doi: 10.1002/ana.10645. [DOI] [PubMed] [Google Scholar]
- 38.Pardo CA, McArthur JC, Griffin JW. HIV neuropathy insights in the pathology of HIV peripheral nerve disease. J. Peripher. Nerv. Syst. 2001;6(1):21–27. doi: 10.1046/j.1529-8027.2001.006001021.x. [DOI] [PubMed] [Google Scholar]
- 39.McCarthy BG, Hsieh ST, Stocks A, Hauer P, Macko C, Cornblath DR, Griffin JW, McArthur JC. Cutaneous innervation in sensory neuropathies: evaluation by skin biopsy. Neurology. 1995;45(10):1848–1855. doi: 10.1212/wnl.45.10.1848. [DOI] [PubMed] [Google Scholar]
- 40.Verma A. Epidemiology and clinical features of HIV-1 associated neuropathies. J. Peripher. Nerv. Syst. 2001;6(1):8–13. doi: 10.1046/j.1529-8027.2001.006001008.x. [DOI] [PubMed] [Google Scholar]
- 41.Wesselingh SL, Glass J, McArthur JC, Griffin JW, Griffin DE. Cytokine dysregulation in HIV-associated neurological disease. Adv. Neuroimmunol. 1994;4(3):199–206. doi: 10.1016/s0960-5428(06)80258-5. [DOI] [PubMed] [Google Scholar]
- 42.Nath A, Hauser KF, Wojna V, Booze RM, Maragos W, Prendergast M, Cass W, Turchan JT. Molecular basis for interactions of HIV and drugs of abuse. J. Acquir. Immune Defic. Syndr. 2002;31 Suppl 2:S62–69. doi: 10.1097/00126334-200210012-00006. [DOI] [PubMed] [Google Scholar]
- 43.Podhaizer EM, Zou S, Fitting S, Samano KL, El-Hage N, Knapp PE, Hauser KF. Morphine and gp120 Toxic Interactions in Striatal Neurons are Dependent on HIV-1 Strain. J. Neuroimmune Pharmacol. 2012;7(4):877–91. doi: 10.1007/s11481-011-9326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kramer-Hammerle S, Rothenaigner I, Wolff H, Bell JE, Brack-Werner R. Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus. Virus Res. 2005;111(2):194–213. doi: 10.1016/j.virusres.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 45.Roth MD, Whittaker KM, Choi R, Tashkin DP, Baldwin GC. Cocaine and sigma-1 receptors modulate HIV infection, chemokine receptors, and the HPA axis in the huPBL-SCID model. J. Leukoc. Biol. 2005;78(6):1198–1203. doi: 10.1189/jlb.0405219. [DOI] [PubMed] [Google Scholar]
- 46.Berman JW, Carson MJ, Chang L, Cox BM, Fox HS, Gonzalez RG, Hanson GR, Hauser KF, Ho WZ, Hong JS, Major EO, Maragos WF, Masliah E, McArthur JC, Miller DB, Nath A, O'Callaghan JP, Persidsky Y, Power C, Rogers TJ, Royal W. 3rd. NeuroAIDS drug abuse and inflammation: building collaborative research activities. J. Neuroimmune Pharmacol. 2006;1(4):351–399. doi: 10.1007/s11481-006-9048-9. [DOI] [PubMed] [Google Scholar]
- 47.Mahajan SD, Schwartz SA, Nair MP. Immunological assays for chemokine detection in in-vitro culture of CNS cells. Biol. Proced. Online. 2003;5:90–102. doi: 10.1251/bpo50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams KC, Burdo TH. HIV and SIV infection the role of cellular restriction and immune responses in viral replication and pathogenesis. APMIS. 2009;117(5-6):400–412. doi: 10.1111/j.1600-0463.2009.02450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gelman BB, Spencer JA, Holzer CE3rd, Soukup VM. Abnormal striatal dopaminergic synapses in National NeuroAIDS Tissue Consortium subjects with HIV encephalitis. J. Neuroimmune Pharmacol. 2006;1(4):410–420. doi: 10.1007/s11481-006-9030-6. [DOI] [PubMed] [Google Scholar]
- 50.Jernigan TL, Gamst AC, Archibald SL, Fennema-Notestine C, Mindt MR, Marcotte TD, Heaton RK, Ellis RJ, Grant I. Effects of methamphetamine dependence and HIV infection on cerebral morphology. Am. J. Psychiatry. 2005;162(8):1461–1472. doi: 10.1176/appi.ajp.162.8.1461. [DOI] [PubMed] [Google Scholar]
- 51.Meeker RB. Feline immunodeficiency virus neuropathogenesis: from cats to calcium. J. Neuroimmune Pharmacol. 2007;2(2):154–170. doi: 10.1007/s11481-006-9045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaul M, Lipton SA. Chemokines and activated macrophages in HIV gp120-induced neuronal apoptosis. Proc. Natl. Acad. Sci. U. S. A. 1999;96(14):8212–8216. doi: 10.1073/pnas.96.14.8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410(6831):988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- 54.Eron JJ, Jr., Ashby MA, Giordano MF, Chernow M, Reiter WM, Deeks SG, Lavelle JP, Conant MA, Yangco BG, Pate PG, Torres RA, Mitsuyasu RT, Twaddell T. Randomised trial of MNrgp120 HIV-1 vaccine in symptomless HIV-1 infection. Lancet. 1996;348(9041):1547–1551. doi: 10.1016/s0140-6736(96)05283-x. [DOI] [PubMed] [Google Scholar]
- 55.Milligan ED, Mehmert KK, Hinde JL, Harvey LO, Martin D, Tracey KJ, Maier SF, Watkins LR. Thermal hyperalgesia and mechanical allodynia produced by intrathecal administration of the human immunodeficiency virus-1 (HIV-1) envelope glycoprotein gp120. Brain Res. 2000;861(1):105–116. doi: 10.1016/s0006-8993(00)02050-3. [DOI] [PubMed] [Google Scholar]
- 56.Oh SB, Tran PB, Gillard SE, Hurley RW, Hammond DL, Miller RJ. Chemokines and glycoprotein120 produce pain hypersensitivity by directly exciting primary nociceptive neurons. J. Neurosci. 2001;21(14):5027–5035. doi: 10.1523/JNEUROSCI.21-14-05027.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Herzberg U, Sagen J. Peripheral nerve exposure to HIV viral envelope protein gp120 induces neuropathic pain and spinal gliosis. J. Neuroimmunol. 2001;116(1):29–39. doi: 10.1016/s0165-5728(01)00288-0. [DOI] [PubMed] [Google Scholar]
- 58.Raghavendra V, Rutkowski MD, DeLeo JA. The role of spinal neuroimmune activation in morphine tolerance/hyperalgesia in neuropathic and sham-operated rats. J. Neurosci. 2002;22(22):9980–9989. doi: 10.1523/JNEUROSCI.22-22-09980.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Watkins LR, Milligan ED, Maier SF. Glial activation a driving force for pathological pain. Trends Neurosci. 2001;24(8):450–455. doi: 10.1016/s0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- 60.DeLeo JA, Tanga FY, Tawfik VL. Neuroimmune activation and neuroinflammation in chronic pain and opioid tolerance/hyperalgesia. Neuroscientist. 2004;10(1):40–52. doi: 10.1177/1073858403259950. [DOI] [PubMed] [Google Scholar]
- 61.Leung L, Cahill CM. TNF-alpha and neuropathic pain--a review. J. Neuroinflammation. 2010;7:27–0. doi: 10.1186/1742-2094-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gao YJ, Zhang L, Ji RR. Spinal injection of TNF-alpha-activated astrocytes produces persistent pain symptom mechanical allodynia by releasing monocyte chemoattractant protein-1. Glia. 2010;58(15):1871–1880. doi: 10.1002/glia.21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nagano I, Shapshak P, Yoshioka M, Xin K, Nakamura S, Bradley WG. Increased NADPH-diaphorase reactivity and cytokine expression in dorsal root ganglia in acquired immunodeficiency syndrome. J. Neurol. Sci. 1996;136(1-2):117–128. doi: 10.1016/0022-510x(95)00317-u. [DOI] [PubMed] [Google Scholar]
- 64.Rizzuto N, Cavallaro T, Monaco S, Morbin M, Bonetti B, Ferrari S, Galiazzo-Rizzuto S, Zanette G, Bertolasi L. Role of HIV in the pathogenesis of distal symmetrical peripheral neuropathy. Acta Neuropathol. 1995;90(3):244–250. doi: 10.1007/BF00296507. [DOI] [PubMed] [Google Scholar]
- 65.Shapshak P, Nagano I, Xin K, Bradley W, McCoy CB, Sun NC, Stewart RV, Yoshioka M, Petito C, Goodkin K, Douyon R, Srivastava AK, Crandall KA. HIV-1 heterogeneity and cytokines. Neuropathogenesis. Adv. Exp. Med. Biol. 1995;373:225–238. doi: 10.1007/978-1-4615-1951-5_31. [DOI] [PubMed] [Google Scholar]
- 66.Yoshioka M, Shapshak P, Srivastava AK, Stewart RV, Nelson SJ, Bradley WG, Berger JR, Rhodes RH, Sun NC, Nakamura S. Expression of HIV-1 and interleukin-6 in lumbosacral dorsal root ganglia of patients with AIDS. Neurology. 1994;44(6):1120–1130. doi: 10.1212/wnl.44.6.1120. [DOI] [PubMed] [Google Scholar]
- 67.Grimaldi LM, Martino GV, Franciotta DM, Brustia R, Castagna A, Pristera R, Lazzarin A. Elevated alpha-tumor necrosis factor levels in spinal fluid from HIV-1-infected patients with central nervous system involvement. Ann. Neurol. 1991;29(1):21–25. doi: 10.1002/ana.410290106. [DOI] [PubMed] [Google Scholar]
- 68.Mastroianni CM, Paoletti F, Valenti C, Vullo V, Jirillo E, Delia S. Tumour necrosis factor (TNF-alpha) and neurological disorders in HIV infection. J. Neurol. Neurosurg. Psychiatry. 1992;55(3):219–221. doi: 10.1136/jnnp.55.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Perrella O, Carrieri PB, Guarnaccia D, Soscia M. Cerebrospinal fluid cytokines in AIDS dementia complex. J. Neurol. 1992;239(7):387–388. doi: 10.1007/BF00812156. [DOI] [PubMed] [Google Scholar]
- 70.Ciardi M, Sharief MK, Thompson EJ, Salotti A, Vullo V, Sorice F, Cirelli A. High cerebrospinal fluid and serum levels of tumor necrosis factor-alpha in asymptomatic HIV-1 seropositive individuals.Correlation with interleukin-2 and soluble IL-2 receptor. J. Neurol. Sci. 1994;125(2):175–179. doi: 10.1016/0022-510x(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 71.Tyor WR, Glass JD, Griffin JW, Becker PS, McArthur JC, Bezman L, Griffin DE. Cytokine expression in the brain during the acquired immunodeficiency syndrome. Ann. Neurol. 1992;31(4):349–360. doi: 10.1002/ana.410310402. [DOI] [PubMed] [Google Scholar]
- 72.Achim CL, Heyes MP, Wiley CA. Quantitation of human immunodeficiency virus immune activation factors, and quinolinic acid in AIDS brains. J. Clin. Invest. 1993;91(6):2769–2775. doi: 10.1172/JCI116518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.An SF, Ciardi A, Giometto B, Scaravilli T, Gray F, Scaravilli F. Investigation on the expression of major histocompatibility complex class II and cytokines and detection of HIV-1 DNA within brains of asymptomatic and symptomatic HIV-1-positive patients. Acta Neuropathol. 1996;91(5):494–503. doi: 10.1007/s004010050457. [DOI] [PubMed] [Google Scholar]
- 74.Vitkovic L, da Cunha A, Tyor WR. Cytokine expression and pathogenesis in AIDS brain. Res. Publ. Assoc. Res. Nerv. Ment. Dis. 1994;72:203–222. [PubMed] [Google Scholar]
- 75.de Larranaga GF, Petroni A, Deluchi G, Alonso BS, Benetucci JA. Viral load and disease progression as responsible for endothelial activation and/or injury in human immunodeficiency virus-1-infected patients. Blood Coagul. Fibrinolysis. 2003;14(1):15–18. doi: 10.1097/00001721-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 76.Kumar M, Kumar AM, Waldrop D, Antoni MH, Eisdorfer C. HIV-1 infection and its impact on the HPA axis, cytokines, and cognition. Stress. 2003;6(3):167–172. doi: 10.1080/10253890310001605376. [DOI] [PubMed] [Google Scholar]
- 77.Dorsey SG, Morton PG. HIV peripheral neuropathy: pathophysiology and clinical implications. AACN Clin. Issues. 2006;17(1):30–36. doi: 10.1097/00044067-200601000-00004. [DOI] [PubMed] [Google Scholar]
- 78.Merrill JE, Chen IS. HIV-1 macrophages, glial cells, and cytokines in AIDS nervous system disease. FASEB J. 1991;5(10):2391–2397. doi: 10.1096/fasebj.5.10.2065887. [DOI] [PubMed] [Google Scholar]
- 79.Kitano K, Rivas CI, Baldwin GC, Vera JC, Golde DW. Tumor necrosis factor-dependent production of human immunodeficiency virus 1 in chronically infected HL-60 cells. Blood. 1993;82(9):2742–2748. [PubMed] [Google Scholar]
- 80.Schulze-Osthoff K, Bakker AC, Vanhaesebroeck B, Beyaert R, Jacob WA, Fiers W. Cytotoxic activity of tumor necrosis factor is mediated by early damage of mitochondrial functions.Evidence for the involvement of mitochondrial radical generation. J. Biol. Chem. 1992;267(8):5317–5323. [PubMed] [Google Scholar]
- 81.Williams R, Dhillon NK, Hegde ST, Yao H, Peng F, Callen S, Chebloune Y, Davis RL, Buch SJ. Proinflammatory cytokines and HIV-1 synergistically enhance CXCL10 expression in human astrocytes. Glia. 2009;57(7):734–743. doi: 10.1002/glia.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sui Z, Sniderhan LF, Schifitto G, Phipps RP, Gelbard HA, Dewhurst S, Maggirwar SB. Functional synergy between CD40 ligand and HIV-1 Tat contributes to inflammation: implications in HIV type 1 dementia. J. Immunol. 2007;178(5):3226–3236. doi: 10.4049/jimmunol.178.5.3226. [DOI] [PubMed] [Google Scholar]
- 83.Gandhi N, Saiyed Z, Thangavel S, Rodriguez J, Rao KV, Nair MP. Differential effects of HIV type 1 clade B and clade C Tat protein on expression of proinflammatory and antiinflammatory cytokines by primary monocytes. AIDS Res. Hum. Retroviruses. 2009;25(7):691–699. doi: 10.1089/aid.2008.0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Reid W, Sadowska M, Denaro F, Rao S, Foulke J, Jr., Hayes N, Jones O, Doodnauth D, Davis H, Sill A, O'Driscoll P, Huso D, Fouts T, Lewis G, Hill M, Kamin-Lewis R, Wei C, Ray P, Gallo RC, Reitz M, Bryant J. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc. Natl. Acad. Sci. U. S. A. 2001;98(16):9271–9276. doi: 10.1073/pnas.161290298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Milligan ED, O'Connor KA, Nguyen KT, Armstrong CB, Twining C, Gaykema RP, Holguin A, Martin D, Maier SF, Watkins LR. Intrathecal HIV-1 envelope glycoprotein gp120 induces enhanced pain states mediated by spinal cord proinflammatory cytokines. J. Neurosci. 2001;21(8):2808–2819. doi: 10.1523/JNEUROSCI.21-08-02808.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zheng W, Ouyang H, Zheng X, Liu S, Mata M, Fink DJ, Hao S. Glial TNFalpha in the spinal cord regulates neuropathic pain induced by HIV gp120 application in rats. Mol. Pain. 2011;7:40–0. doi: 10.1186/1744-8069-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bagetta G, Corasaniti MT, Berliocchi L, Nistico R, Giammarioli AM, Malorni W, Aloe L, Finazzi-Agro A. Involvement of interleukin-1beta in the mechanism of human immunodeficiency virus type 1 (HIV-1) recombinant protein gp120-induced apoptosis in the neocortex of rat. Neuroscience. 1999;89(4):1051–1066. doi: 10.1016/s0306-4522(98)00363-7. [DOI] [PubMed] [Google Scholar]
- 88.Corasaniti MT, Bilotta A, Strongoli MC, Navarra M, Bagetta G, Di Renzo G. HIV-1 coat protein gp120 stimulates interleukin-1beta secretion from human neuroblastoma cells: evidence for a role in the mechanism of cell death. Br. J. Pharmacol. 2001;134(6):1344–1350. doi: 10.1038/sj.bjp.0704382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Corasaniti MT, Piccirilli S, Paoletti A, Nistico R, Stringaro A, Malorni W, Finazzi-Agro A, Bagetta G. Evidence that the HIV-1 coat protein gp120 causes neuronal apoptosis in the neocortex of rat via a mechanism involving CXCR4 chemokine receptor. Neurosci. Lett. 2001;312(2):67–70. doi: 10.1016/s0304-3940(01)02191-7. [DOI] [PubMed] [Google Scholar]
- 90.Schoeniger-Skinner DK, Ledeboer A, Frank MG, Milligan ED, Poole S, Martin D, Maier SF, Watkins LR. Interleukin-6 mediates low-threshold mechanical allodynia induced by intrathecal HIV-1 envelope glycoprotein gp120. Brain Behav. Immun. 2007;21(5):660–667. doi: 10.1016/j.bbi.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bhat NR, Zhang P, Bhat AN. Cytokine induction of inducible nitric oxide synthase in an oligodendrocyte cell line role of p38 mitogen-activated protein inase activation. J. Neurochem. 1999;72(2):472–478. doi: 10.1046/j.1471-4159.1999.0720472.x. [DOI] [PubMed] [Google Scholar]
- 92.Da Silva J, Pierrat B, Mary JL, Lesslauer W. Blockade of p38 mitogen-activated protein kinase pathway inhibits inducible nitric-oxide synthase expression in mouse astrocytes. J. Biol. Chem. 1997;272(45):28373–28380. doi: 10.1074/jbc.272.45.28373. [DOI] [PubMed] [Google Scholar]
- 93.Gorina R, Font-Nieves M, Marquez-Kisinousky L, Santalucia T, Planas AM. Astrocyte TLR4 activation induces a proinflammatory environment through the interplay between MyD88-dependent NFkappaB signaling, MAPK, and Jak1/Stat1 pathways. Glia. 2011;59(2):242–255. doi: 10.1002/glia.21094. [DOI] [PubMed] [Google Scholar]
- 94.Milligan ED, O'Connor KA, Armstrong CB, Hansen MK, Martin D, Tracey KJ, Maier SF, Watkins LR. Systemic administration of CNI-1493 a p38 mitogen-activated protein kinase inhibitor blocks intrathecal human immunodeficiency virus-1 gp120-induced enhanced pain states in rats. J. Pain. 2001;2(6):326–333. doi: 10.1054/jpai.2001.26174. [DOI] [PubMed] [Google Scholar]
- 95.Mocchetti I, Campbell LA, Harry GJ, Avdoshina V. When Human Immunodeficiency Virus Meets Chemokines and Microglia.Neuroprotection or Neurodegeneration? J. Neuroimmune Pharmacol. 2013;8(1):118–31. doi: 10.1007/s11481-012-9353-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Miller RJ, Jung H, Bhangoo SK, White FA. Cytokine and chemokine regulation of sensory neuron function. Handb. Exp. Pharmacol. 2009;(194):417–449. doi: 10.1007/978-3-540-79090-7_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hesselgesser J, Halks-Miller M, DelVecchio V, Peiper SC, Hoxie J, Kolson DL, Taub D, Horuk R. CD4-independent association between HIV-1 gp120 and CXCR4: functional chemokine receptors are expressed in human neurons. Curr. Biol. 1997;7(2):112–121. doi: 10.1016/s0960-9822(06)00055-8. [DOI] [PubMed] [Google Scholar]
- 98.Bhangoo SK, Ripsch MS, Buchanan DJ, Miller RJ, White FA. Increased chemokine signaling in a model of HIV1-associated peripheral neuropathy. Mol. Pain. 2009;5:48–0. doi: 10.1186/1744-8069-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chandra J, Samali A, Orrenius S. Triggering and modulation of apoptosis by oxidative stress. Free Radic. Biol. Med. 2000;29(3-4):323–333. doi: 10.1016/s0891-5849(00)00302-6. [DOI] [PubMed] [Google Scholar]
- 100.Fujisawa T, Takeda K, Ichijo H. ASK family proteins in stress response and disease. Mol. Biotechnol. 2007;37(1):13–18. doi: 10.1007/s12033-007-0053-x. [DOI] [PubMed] [Google Scholar]
- 101.Wang Y, Marsden PA. Nitric oxide synthases gene structure and regulation. Adv. Pharmacol. 1995;34:71–90. doi: 10.1016/s1054-3589(08)61081-9. [DOI] [PubMed] [Google Scholar]
- 102.Crisp T, Minus TO, Coleman ML, Giles JR, Cibula C, Finnerty EP. Aging peripheral nerve injury and nociception effects of the antioxidant 16-desmethyltirilazad. Behav. Brain Res. 2006;166(1):159–165. doi: 10.1016/j.bbr.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 103.Khalil Z, Liu T, Helme RD. Free radicals contribute to the reduction in peripheral vascular responses and the maintenance of thermal hyperalgesia in rats with chronic constriction injury. Pain. 1999;79(1):31–37. doi: 10.1016/S0304-3959(98)00143-2. [DOI] [PubMed] [Google Scholar]
- 104.Liu D, Liu J, Sun D, Wen J. The time course of hydroxyl radical formation following spinal cord injury: the possible role of the iron-catalyzed Haber-Weiss reaction. J. Neurotrauma. 2004;21(6):805–816. doi: 10.1089/0897715041269650. [DOI] [PubMed] [Google Scholar]
- 105.Kim HK, Park SK, Zhou JL, Taglialatela G, Chung K, Coggeshall RE, Chung JM. Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain. 2004;111(1-2):116–124. doi: 10.1016/j.pain.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 106.Mao YF, Yan N, Xu H, Sun JH, Xiong YC, Deng XM. Edaravone a free radical scavenger is effective on neuropathic pain in rats. Brain Res. 2009;1248:68–75. doi: 10.1016/j.brainres.2008.10.073. [DOI] [PubMed] [Google Scholar]
- 107.Schwartz ES, Kim HY, Wang J, Lee I, Klann E, Chung JM, Chung K. Persistent pain is dependent on spinal mitochondrial antioxidant levels. J. Neurosci. 2009;29(1):159–168. doi: 10.1523/JNEUROSCI.3792-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Perl A, Banki K. Genetic and metabolic control of the mitochondrial transmembrane potential and reactive oxygen intermediate production in HIV disease. Antioxid. Redox Signal. 2000;2(3):551–573. doi: 10.1089/15230860050192323. [DOI] [PubMed] [Google Scholar]
- 109.Holguin A, O'Connor KA, Biedenkapp J, Campisi J, Wieseler-Frank J, Milligan ED, Hansen MK, Spataro L, Maksimova E, Bravmann C, Martin D, Fleshner M, Maier SF, Watkins LR. HIV-1 gp120 stimulates proinflammatory cytokine-mediated pain facilitation via activation of nitric oxide synthase-I (nNOS). Pain. 2004;110(3):517–530. doi: 10.1016/j.pain.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 110.Minami T, Matsumura S, Mabuchi T, Kobayashi T, Sugimoto Y, Ushikubi F, Ichikawa A, Narumiya S, Ito S. Functional evidence for interaction between prostaglandin EP3 and kappa-opioid receptor pathways in tactile pain induced by human immunodeficiency virus type-1 (HIV-1) glycoprotein gp120. Neuropharmacology. 2003;45(1):96–105. doi: 10.1016/s0028-3908(03)00133-3. [DOI] [PubMed] [Google Scholar]
- 111.Zheng W, Huang W, Liu S, Levitt RC, Candiotti KA, Hao S. Reactive oxygen species (ROS) plays a role in the neuropathic pain induced by HIV coat protein-gp120 In Society for Neuroscience annual meeting, 341. 5. 13-17 October 2012 New Orleans LA. 2012 [Google Scholar]
- 112.Tuluc F, Lai JP, Kilpatrick LE, Evans DL, Douglas SD. Neurokinin 1 receptor isoforms and the control of innate immunity. Trends Immunol. 2009;30(6):271–276. doi: 10.1016/j.it.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 113.Salt TE, Hill RG. Neurotransmitter candidates of somatosensory primary afferent fibres. Neuroscience. 1983;10(4):1083–1103. doi: 10.1016/0306-4522(83)90101-x. [DOI] [PubMed] [Google Scholar]
- 114.De Felipe C, Herrero JF, O'Brien JA, Palmer JA, Doyle CA, Smith AJ, Laird JM, Belmonte C, Cervero F, Hunt SP. Altered nociception analgesia and aggression in mice lacking the receptor for substance P. Nature. 1998;392(6674):394–397. doi: 10.1038/32904. [DOI] [PubMed] [Google Scholar]
- 115.Neumann S, Doubell TP, Leslie T, Woolf CJ. Inflammatory pain hypersensitivity mediated by phenotypic switch in myelinated primary sensory neurons. Nature. 1996;384(6607):360–364. doi: 10.1038/384360a0. [DOI] [PubMed] [Google Scholar]
- 116.Noguchi K, Ruda MA. Gene regulation in an ascending nociceptive pathway: inflammation-induced increase in preprotachykinin mRNA in rat lamina I spinal projection neurons. J. Neurosci. 1992;12(7):2563–2572. doi: 10.1523/JNEUROSCI.12-07-02563.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nichols ML, Allen BJ, Rogers SD, Ghilardi JR, Honore P, Luger NM, Finke MP, Li J, Lappi DA, Simone DA, Mantyh PW. Transmission of chronic nociception by spinal neurons expressing the substance P receptor. Science. 1999;286(5444):1558–1561. doi: 10.1126/science.286.5444.1558. [DOI] [PubMed] [Google Scholar]
- 118.Ho WZ, Douglas SD. Substance P and neurokinin-1 receptor modulation of HIV. J. Neuroimmunol. 2004;157(1-2):48–55. doi: 10.1016/j.jneuroim.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 119.Annunziata P, Cioni C, Toneatto S, Paccagnini E. HIV-1 gp120 increases the permeability of rat brain endothelium cultures by a mechanism involving substance P. AIDS. 1998;12(18):2377–2385. doi: 10.1097/00002030-199818000-00006. [DOI] [PubMed] [Google Scholar]
- 120.Lai JP, Lai S, Tuluc F, Tansky MF, Kilpatrick LE, Leeman SE, Douglas SD. Differences in the length of the carboxyl terminus mediate functional properties of neurokinin-1 receptor. Proc. Natl. Acad. Sci. U. S. A. 2008;105(34):12605–12610. doi: 10.1073/pnas.0806632105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Teodoro FC, Tronco Junior MF, Zampronio AR, Martini AC, Rae GA, Chichorro JG. Peripheral substance P and neurokinin-1 receptors have a role in inflammatory and neuropathic orofacial pain models. Neuropeptides. 2013;47(3):199–206. doi: 10.1016/j.npep.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 122.Baulmann J, Spitznagel H, Herdegen T, Unger T, Culman J. Tachykinin receptor inhibition and c-Fos expression in the rat brain following formalin-induced pain. Neuroscience. 2000;95(3):813–820. doi: 10.1016/s0306-4522(99)00478-9. [DOI] [PubMed] [Google Scholar]
- 123.Hill R. NK1 (substance P) receptor antagonists--why are they not analgesic in humans. Trends Pharmacol. Sci. 2000;21(7):244–246. doi: 10.1016/s0165-6147(00)01502-9. [DOI] [PubMed] [Google Scholar]
- 124.Chattopadhyaya B, Di Cristo G, Wu CZ, Knott G, Kuhlman S, Fu Y, Palmiter RD, Huang ZJ. GAD67-mediated GABA synthesis and signaling regulate inhibitory synaptic innervation in the visual cortex. Neuron. 2007;54(6):889–903. doi: 10.1016/j.neuron.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Stellwagen D, Beattie EC, Seo JY, Malenka RC. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. J. Neurosci. 2005;25(12):3219–3228. doi: 10.1523/JNEUROSCI.4486-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rosas-Ballina M, Goldstein RS, Gallowitsch-Puerta M, Yang L, Valdes-Ferrer SI, Patel NB, Chavan S, Al-Abed Y, Yang H, Tracey KJ. The selective alpha7 agonist GTS-21 attenuates cytokine production in human whole blood and human monocytes activated by ligands for TLR2 TLR3 TLR4 TLR9, and RAGE. Mol. Med. 2009;15(7-8):195–202. doi: 10.2119/molmed.2009.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Waldburger JM, Boyle DL, Pavlov VA, Tracey KJ, Firestein GS. Acetylcholine regulation of synoviocyte cytokine expression by the alpha7 nicotinic receptor. Arthritis Rheumatism. 2008;58(11):3439–3449. doi: 10.1002/art.23987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Loram LC, Harrison JA, Chao L, Taylor FR, Reddy A, Travis CL, Giffard R, Al-Abed Y, Tracey K, Maier SF, Watkins LR. Intrathecal injection of an alpha seven nicotinic acetylcholine receptor agonist attenuates gp120-induced mechanical allodynia and spinal pro-inflammatory cytokine profiles in rats. Brain Behav. Immun. 2010;24(6):959–967. doi: 10.1016/j.bbi.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bachis A, Avdoshina V, Zecca L, Parsadanian M, Mocchetti I. Human immunodeficiency virus type 1 alters brain-derived neurotrophic factor processing in neurons. J. Neurosci. 2012;32(28):9477–9484. doi: 10.1523/JNEUROSCI.0865-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shi Y, Yuan S, Li B, Wang J, Carlton SM, Chung K, Chung JM, Tang SJ. Regulation of Wnt signaling by nociceptive input in animal models. Mol. Pain. 2012;8(1):47–0. doi: 10.1186/1744-8069-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wilkerson JL, Gentry KR, Dengler EC, Wallace JA, Kerwin AA, Armijo LM, Kuhn MN, Thakur GA, Makriyannis A, Milligan ED. Intrathecal cannabilactone CB(2)R agonist, AM1710, controls pathological pain and restores basal cytokine levels. Pain. 2012;153(5):1091–1106. doi: 10.1016/j.pain.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ledeboer A, Gamanos M, Lai W, Martin D, Maier SF, Watkins LR, Quan N. Involvement of spinal cord nuclear factor kappaB activation in rat models of proinflammatory cytokine-mediated pain facilitation. Eur. J. Neurosci. 2005;22(8):1977–1986. doi: 10.1111/j.1460-9568.2005.04379.x. [DOI] [PubMed] [Google Scholar]
- 133.Jolivalt CG, Dacunha JM, Esch FS, Calcutt NA. Central action of prosaptide TX14(A) against gp120-induced allodynia in rats. Eur. J. Pain. 2008;12(1):76–81. doi: 10.1016/j.ejpain.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 134.Wallace VC, Blackbeard J, Pheby T, Segerdahl AR, Davies M, Hasnie F, Hall S, McMahon SB, Rice AS. Pharmacological behavioural and mechanistic analysis of HIV-1 gp120 induced painful neuropathy. Pain. 2007;133(1-3):47–63. doi: 10.1016/j.pain.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Acharjee S, Noorbakhsh F, Stemkowski PL, Olechowski C, Cohen EA, Ballanyi K, Kerr B, Pardo C, Smith PA, Power C. HIV-1 viral protein R causes peripheral nervous system injury associated with in vivo neuropathic pain. FASEB J. 2010;24(11):4343–4353. doi: 10.1096/fj.10-162313. [DOI] [PubMed] [Google Scholar]
- 136.Mocroft A, Ledergerber B, Katlama C, Kirk O, Reiss P, d'Arminio Monforte A, Knysz B, Dietrich M, Phillips AN, Lundgren JD, Euro Ssg. Decline in the AIDS and death rates in the EuroSIDA study: an observational study. Lancet. 2003;362(9377):22–29. doi: 10.1016/s0140-6736(03)13802-0. [DOI] [PubMed] [Google Scholar]
- 137.Haas DW, Geraghty DE, Andersen J, Mar J, Motsinger AA, D'Aquila RT, Unutmaz D, Benson CA, Ritchie MD, Landay A. Immunogenetics of CD4 lymphocyte count recovery during antiretroviral therapy: An AIDS Clinical Trials Group study. J. Infect. Dis. 2006;194(8):1098–1107. doi: 10.1086/507313. [DOI] [PubMed] [Google Scholar]
- 138.Bacellar H, Munoz A, Miller EN, Cohen BA, Besley D, Selnes OA, Becker JT, McArthur JC. Temporal trends in the incidence of HIV-1-related neurologic diseases: Multicenter AIDS Cohort Study 1985-1992. Neurology. 1994;44(10):1892–1900. doi: 10.1212/wnl.44.10.1892. [DOI] [PubMed] [Google Scholar]
- 139.Phillips TJ, Cherry CL, Cox S, Marshall SJ, Rice AS. Pharmacological Treatment of Painful HIV-Associated Sensory Neuropathy: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. PLoS One. 2010;5(12):e14433–0. doi: 10.1371/journal.pone.0014433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Manji H. Neuropathy in HIV infection. Curr. Opin. Neurol. 2000;13(5):589–592. doi: 10.1097/00019052-200010000-00014. [DOI] [PubMed] [Google Scholar]
- 141.Ferrari LF, Levine JD. Alcohol consumption enhances antiretroviral painful peripheral neuropathy by mitochondrial mechanisms. Eur. J. Neurosci. 2010;32(5):811–818. doi: 10.1111/j.1460-9568.2010.07355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dubinsky RM, Yarchoan R, Dalakas M, Broder S. Reversible axonal neuropathy from the treatment of AIDS and related disorders with 2',3'-dideoxycytidine (ddC). Muscle Nerve. 1989;12(10):856–860. doi: 10.1002/mus.880121012. [DOI] [PubMed] [Google Scholar]
- 143.Dalakas MC. Peripheral neuropathy and antiretroviral drugs. J. Peripher. Nerv. Syst. 2001;6(1):14–20. doi: 10.1046/j.1529-8027.2001.006001014.x. [DOI] [PubMed] [Google Scholar]
- 144.Reliquet V, Mussini JM, Chennebault JM, Lafeuillade A, Raffi F. Peripheral neuropathy during stavudine-didanosine antiretroviral therapy. HIV Med. 2001;2(2):92–96. doi: 10.1046/j.1468-1293.2001.00066.x. [DOI] [PubMed] [Google Scholar]
- 145.Simpson DM. Selected peripheral neuropathies associated with human immunodeficiency virus infection and antiretroviral therapy. J. Neurovirol. 2002;8 Suppl 2:33–41. doi: 10.1080/13550280290167939. [DOI] [PubMed] [Google Scholar]
- 146.Luciano CA, Pardo CA, McArthur JC. Recent developments in the HIV neuropathies. Curr. Opin. Neurol. 2003;16(3):403–409. doi: 10.1097/01.wco.0000073943.19076.98. [DOI] [PubMed] [Google Scholar]
- 147.Berger AR, Arezzo JC, Schaumburg HH, Skowron G, Merigan T, Bozzette S, Richman D, Soo W. 2',3'-dideoxycytidine (ddC) toxic neuropathy: a study of 52 patients. Neurology. 1993;43(2):358–362. doi: 10.1212/wnl.43.2.358. [DOI] [PubMed] [Google Scholar]
- 148.McCarthy WF, Gable J, Lawrence J, Thompson M. A retrospective study to determine if hydroxyurea augmentation of antiretroviral drug regimens that contain ddI and/or d4T increases the risk of developing peripheral neuropathy in HIV-1 infected individuals. Pharmacoepidemiol. Drug Saf. 2000;9(1):49–53. doi: 10.1002/(SICI)1099-1557(200001/02)9:1<49::AID-PDS465>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 149.Arenas-Pinto A, Bhaskaran K, Dunn D, Weller IV. The risk of developing peripheral neuropathy induced by nucleoside reverse transcriptase inhibitors decreases over time: evidence from the Delta trial. Antivir. Ther. 2008;13(2):289–295. [PubMed] [Google Scholar]
- 150.Lopez OL, Becker JT, Dew MA, Caldararo R. Risk modifiers for peripheral sensory neuropathy in HIV infection/ AIDS. Eur. J. Neurol. 2004;11(2):97–102. doi: 10.1046/j.1351-5101.2003.00713.x. [DOI] [PubMed] [Google Scholar]
- 151.Dalakas MC, Semino-Mora C, Leon-Monzon M. Mitochondrial alterations with mitochondrial DNA depletion in the nerves of AIDS patients with peripheral neuropathy induced by 2'3'-dideoxycytidine (ddC). Lab. Invest. 2001;81(11):1537–1544. doi: 10.1038/labinvest.3780367. [DOI] [PubMed] [Google Scholar]
- 152.Lewis W, Day BJ, Copeland WC. Mitochondrial toxicity of NRTI antiviral drugs: an integrated cellular perspective. Nat. Rev. Drug Discov. 2003;2(10):812–822. doi: 10.1038/nrd1201. [DOI] [PubMed] [Google Scholar]
- 153.Foster C, Lyall H. HIV and mitochondrial toxicity in children. J. Antimicrob. Chemother. 2008;61(1):8–12. doi: 10.1093/jac/dkm411. [DOI] [PubMed] [Google Scholar]
- 154.Schapira AH. Mitochondrial disorders. Curr Opin. Neurol. 2000;13(5):527–532. doi: 10.1097/00019052-200010000-00004. [DOI] [PubMed] [Google Scholar]
- 155.Yamaguchi T, Katoh I, Kurata S. Azidothymidine causes functional and structural destruction of mitochondria glutathione deficiency and HIV-1 promoter sensitization. Eur. J. Biochem. 2002;269(11):2782–2788. doi: 10.1046/j.1432-1033.2002.02954.x. [DOI] [PubMed] [Google Scholar]
- 156.Chen CH, Vazquez-Padua M, Cheng YC. Effect of anti-human immunodeficiency virus nucleoside analogs on mitochondrial DNA and its implication for delayed toxicity. Mol. Pharmacol. 1991;39(5):625–628. [PubMed] [Google Scholar]
- 157.Lewis LD, Hamzeh FM, Lietman PS. Ultrastructural changes associated with reduced mitochondrial DNA and impaired mitochondrial function in the presence of 2'3'-dideoxycytidine. Antimicrob. Agents Chemother. 1992;36(9):2061–2065. doi: 10.1128/aac.36.9.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chen CH, Cheng YC. Delayed cytotoxicity and selective loss of mitochondrial DNA in cells treated with the anti-human immunodeficiency virus compound 2',3'-dideoxycytidine. J. Biol. Chem. 1989;264(20):11934–11937. [PubMed] [Google Scholar]
- 159.Lewis W, Copeland WC, Day BJ. Mitochondrial dna depletion oxidative stress and mutation mechanisms of dysfunction from nucleoside reverse transcriptase inhibitors. Lab. Invest. 2001;81(6):777–790. doi: 10.1038/labinvest.3780288. [DOI] [PubMed] [Google Scholar]
- 160.Lewis W, Kohler JJ, Hosseini SH, Haase CP, Copeland WC, Bienstock RJ, Ludaway T, McNaught J, Russ R, Stuart T, Santoianni R. Antiretroviral nucleosides deoxynucleotide carrier and mitochondrial DNA evidence supporting the DNA pol gamma hypothesis. AIDS. 2006;20(5):675–684. doi: 10.1097/01.aids.0000216367.23325.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Werth JL, Zhou B, Nutter LM, Thayer SA. 2',3'-Dideoxycytidine alters calcium buffering in cultured dorsal root ganglion neurons. Mol. Pharmacol. 1994;45(6):1119–1124. [PubMed] [Google Scholar]
- 162.Joseph EK, Chen X, Khasar SG, Levine JD. Novel mechanism of enhanced nociception in a model of AIDS therapy-induced painful peripheral neuropathy in the rat. Pain. 2004;107(1-2):147–158. doi: 10.1016/j.pain.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 163.Keswani SC, Chander B, Hasan C, Griffin JW, McArthur JC, Hoke A. FK506 is neuroprotective in a model of antiretroviral toxic neuropathy. Ann. Neurol. 2003;53(1):57–64. doi: 10.1002/ana.10401. [DOI] [PubMed] [Google Scholar]
- 164.Lewis W, Dalakas MC. Mitochondrial toxicity of antiviral drugs. Nat. Med. 1995;1(5):417–422. doi: 10.1038/nm0595-417. [DOI] [PubMed] [Google Scholar]
- 165.Cherry CL, Gahan ME, McArthur JC, Lewin SR, Hoy JF, Wesselingh SL. Exposure to dideoxynucleosides is reflected in lowered mitochondrial DNA in subcutaneous fat. J. Acquir. Immune Defic. Syndr. 2002;30(3):271–277. doi: 10.1097/00126334-200207010-00002. [DOI] [PubMed] [Google Scholar]
- 166.Lucin KM, Wyss-Coray T. Immune activation in brain aging and neurodegeneration too much or too little. Neuron. 2009;64(1):110–122. doi: 10.1016/j.neuron.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Blackbeard J, Wallace VC, O'Dea KP, Hasnie F, Segerdahl A, Pheby T, Field MJ, Takata M, Rice AS. The correlation between pain-related behaviour and spinal microgliosis in four distinct models of peripheral neuropathy. Eur. J. Pain. 2012;16(10):1357–1367. doi: 10.1002/j.1532-2149.2012.00140.x. [DOI] [PubMed] [Google Scholar]
- 168.Zheng FY, Xiao WH, Bennett GJ. The response of spinal microglia to chemotherapy-evoked painful peripheral neuropathies is distinct from that evoked by traumatic nerve injuries. Neuroscience. 2011;176:447–454. doi: 10.1016/j.neuroscience.2010.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zheng X, Ouyang H, Liu S, Mata M, Fink DJ, Hao S. TNFalpha is involved in neuropathic pain induced by nucleoside reverse transcriptase inhibitor in rats. Brain Behav. Immun. 2011;25(8):1668–1676. doi: 10.1016/j.bbi.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bhangoo SK, Ren D, Miller RJ, Chan DM, Ripsch MS, Weiss C, McGinnis C, White FA. CXCR4 chemokine receptor signaling mediates pain hypersensitivity in association with antiretroviral toxic neuropathy. Brain Behav. Immun. 2007;21(5):581–591. doi: 10.1016/j.bbi.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mollace V, Nottet HS, Clayette P, Turco MC, Muscoli C, Salvemini D, Perno CF. Oxidative stress and neuroAIDS: triggers, modulators and novel antioxidants. Trends Neurosci. 2001;24(7):411–416. doi: 10.1016/s0166-2236(00)01819-1. [DOI] [PubMed] [Google Scholar]
- 172.Halliwell B. Oxidative stress and neurodegeneration: where are we now. J. Neurochem. 2006;97(6):1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- 173.Wallace DR, Dodson S, Nath A, Booze RM. Estrogen attenuates gp120- and tat1-72-induced oxidative stress and prevents loss of dopamine transporter function. Synapse. 2006;59(1):51–60. doi: 10.1002/syn.20214. [DOI] [PubMed] [Google Scholar]
- 174.Flora G, Lee YW, Nath A, Hennig B, Maragos W, Toborek M. Methamphetamine potentiates HIV-1 Tat protein-mediated activation of redox-sensitive pathways in discrete regions of the brain. Exp. Neurol. 2003;179(1):60–70. doi: 10.1006/exnr.2002.8048. [DOI] [PubMed] [Google Scholar]
- 175.Zheng W, Zheng X, Liu S, Hao S. 807.09 12-16 November 2011 Washington DC.: 2011. ROS in the spinal cord is involved in the neuropathic pain induced by antiretroviral drug in rats.Society for Neuroscience annual meting. [Google Scholar]
- 176.Aley KO, Levine JD. Role of protein kinase A in the maintenance of inflammatory pain. J. Neurosci. 1999;19(6):2181–2186. doi: 10.1523/JNEUROSCI.19-06-02181.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Aley KO, Levine JD. Different peripheral mechanisms mediate enhanced nociception in metabolic/toxic and traumatic painful peripheral neuropathies in the rat. Neuroscience. 2002;111(2):389–397. doi: 10.1016/s0306-4522(02)00009-x. [DOI] [PubMed] [Google Scholar]
- 178.Aley KO, McCarter G, Levine JD. Nitric oxide signaling in pain and nociceptor sensitization in the rat. J. Neurosci. 1998;18(17):7008–7014. doi: 10.1523/JNEUROSCI.18-17-07008.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhuang ZY, Gerner P, Woolf CJ, Ji RR. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain. 2005;114(1-2):149–159. doi: 10.1016/j.pain.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 180.Chen X, Levine JD. Mechanically-evoked C-fiber activity in painful alcohol and AIDS therapy neuropathy in the rat. Mol. Pain. 2007;3:5–0. doi: 10.1186/1744-8069-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kashatus DF, Lim KH, Brady DC, Pershing NL, Cox AD, Counter CM. RALA and RALBP1 regulate mitochondrial fission at mitosis. Nat. Cell Biol. 2011;13(9):1108–1115. doi: 10.1038/ncb2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yoon Y, Galloway CA, Jhun BS, Yu T. Mitochondrial dynamics in diabetes. Antioxid. Redox Signal. 2011;14(3):439–457. doi: 10.1089/ars.2010.3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Karbowski M, Youle RJ. Dynamics of mitochondrial morphology in healthy cells and during apoptosis. Cell Death Differ. 2003;10(8):870–880. doi: 10.1038/sj.cdd.4401260. [DOI] [PubMed] [Google Scholar]
- 184.Leinninger GM, Backus C, Sastry AM, Yi YB, Wang CW, Feldman EL. Mitochondria in DRG neurons undergo hyperglycemic mediated injury through Bim, Bax and the fission protein Drp1. Neurobiol. Dis. 2006;23(1):11–22. doi: 10.1016/j.nbd.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 185.Ferrari LF, Chum A, Bogen O, Reichling DB, Levine JD. Role of Drp1 a key mitochondrial fission protein, in neuropathic pain. J. Neurosci. 2011;31(31):11404–11410. doi: 10.1523/JNEUROSCI.2223-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chi XX, Schmutzler BS, Brittain JM, Wang Y, Hingtgen CM, Nicol GD, Khanna R. Regulation of N-type voltage-gated calcium channels (Cav2.) and transmitter release by collapsin response mediator protein-2 (CRMP-2) in sensory neurons. J. Cell Sci. 2009;122(Pt 23):4351–4362. doi: 10.1242/jcs.053280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Brittain JM, Duarte DB, Wilson SM, Zhu W, Ballard C, Johnson PL, Liu N, Xiong W, Ripsch MS, Wang Y, Fehrenbacher JC, Fitz SD, Khanna M, Park CK, Schmutzler BS, Cheon BM, Due MR, Brustovetsky T, Ashpole NM, Hudmon A, Meroueh SO, Hingtgen CM, Brustovetsky N, Ji RR, Hurley JH, Jin X, Shekhar A, Xu XM, Oxford GS, Vasko MR, White FA, Khanna R. Suppression of inflammatory and neuropathic pain by uncoupling CRMP-2 from the presynaptic Ca(2+) channel complex. Nat. Med. 2011;17(7):822–829. doi: 10.1038/nm.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ju W, Li Q, Allette YM, Ripsch MS, White FA, Khanna R. Suppression of pain-related behavior in two distinct rodent models of peripheral neuropathy by a homopolyarginine-conjugated CRMP2 peptide. J. Neurochem. 2013;124(6):869–79. doi: 10.1111/jnc.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Helyes Z, Nemeth J, Than M, Bolcskei K, Pinter E, Szolcsanyi J. Inhibitory effect of anandamide on resiniferatoxin-induced sensory neuropeptide release in vivo and neuropathic hyperalgesia in the rat. Life Sci. 2003;73(18):2345–2353. doi: 10.1016/s0024-3205(03)00651-9. [DOI] [PubMed] [Google Scholar]
- 190.Wallace VC, Segerdahl AR, Lambert DM, Vandevoorde S, Blackbeard J, Pheby T, Hasnie F, Rice AS. The effect of the palmitoylethanolamide analogue, palmitoylallylamide (L-29) on pain behaviour in rodent models of neuropathy. Br. J. Pharmacol. 2007;151(7):1117–1128. doi: 10.1038/sj.bjp.0707326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wallace VC, Blackbeard J, Segerdahl AR, Hasnie F, Pheby T, McMahon SB, Rice AS. Characterization of rodent models of HIV-gp120 and anti-retroviral-associated neuropathic pain. Brain. 2007;130(Pt 10):2688–2702. doi: 10.1093/brain/awm195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Renn CL, Leitch CC, Lessans S, Rhee P, McGuire WC, Smith BA, Traub RJ, Dorsey SG. Brain-derived neurotrophic factor modulates antiretroviral-induced mechanical allodynia in the mouse. J. Neurosci. Res. 2011;89(10):1551–1565. doi: 10.1002/jnr.22685. [DOI] [PubMed] [Google Scholar]
- 193.Huang W, Calvo M, Karu K, Olausen HR, Bathgate G, Okuse K, Bennett DL, Rice AS. A clinically relevant rodent model of the HIV antiretroviral drug stavudine induced painful peripheral neuropathy. Pain. 2013;154(4):560–575. doi: 10.1016/j.pain.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 194.Aerbajinai W, Zhu J, Gao Z, Chin K, Rodgers GP. Thalidomide induces gamma-globin gene expression through increased reactive oxygen species-mediated p38 MAPK signaling and histone H4 acetylation in adult erythropoiesis. Blood. 2007;110(8):2864–2871. doi: 10.1182/blood-2007-01-065201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Pinti M, Salomoni P, Cossarizza A. Anti-HIV drugs and the mitochondria. Biochim. Biophys. Acta. 2006;1757(5-6):700–707. doi: 10.1016/j.bbabio.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 196.Joseph EK, Levine JD. Caspase signalling in neuropathic and inflammatory pain in the rat. Eur. J. Neurosci. 2004;20(11):2896–2902. doi: 10.1111/j.1460-9568.2004.03750.x. [DOI] [PubMed] [Google Scholar]
- 197.Keswani SC, Jack C, Zhou C, Hoke A. Establishment of a rodent model of HIV-associated sensory neuropathy. J. Neurosci. 2006;26(40):10299–10304. doi: 10.1523/JNEUROSCI.3135-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Maratou K, Wallace VC, Hasnie FS, Okuse K, Hosseini R, Jina N, Blackbeard J, Pheby T, Orengo C, Dickenson AH, McMahon SB, Rice AS. Comparison of dorsal root ganglion gene expression in rat models of traumatic and HIV-associated neuropathic pain. Eur. J. Pain. 2009;13(4):387–98. doi: 10.1016/j.ejpain.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Melli G, Keswani SC, Fischer A, Chen W, Hoke A. Spatially distinct and functionally independent mechanisms of axonal degeneration in a model of HIV-associated sensory neuropathy. Brain. 2006;129(Pt 5):1330–1338. doi: 10.1093/brain/awl058. [DOI] [PubMed] [Google Scholar]


