Abstract
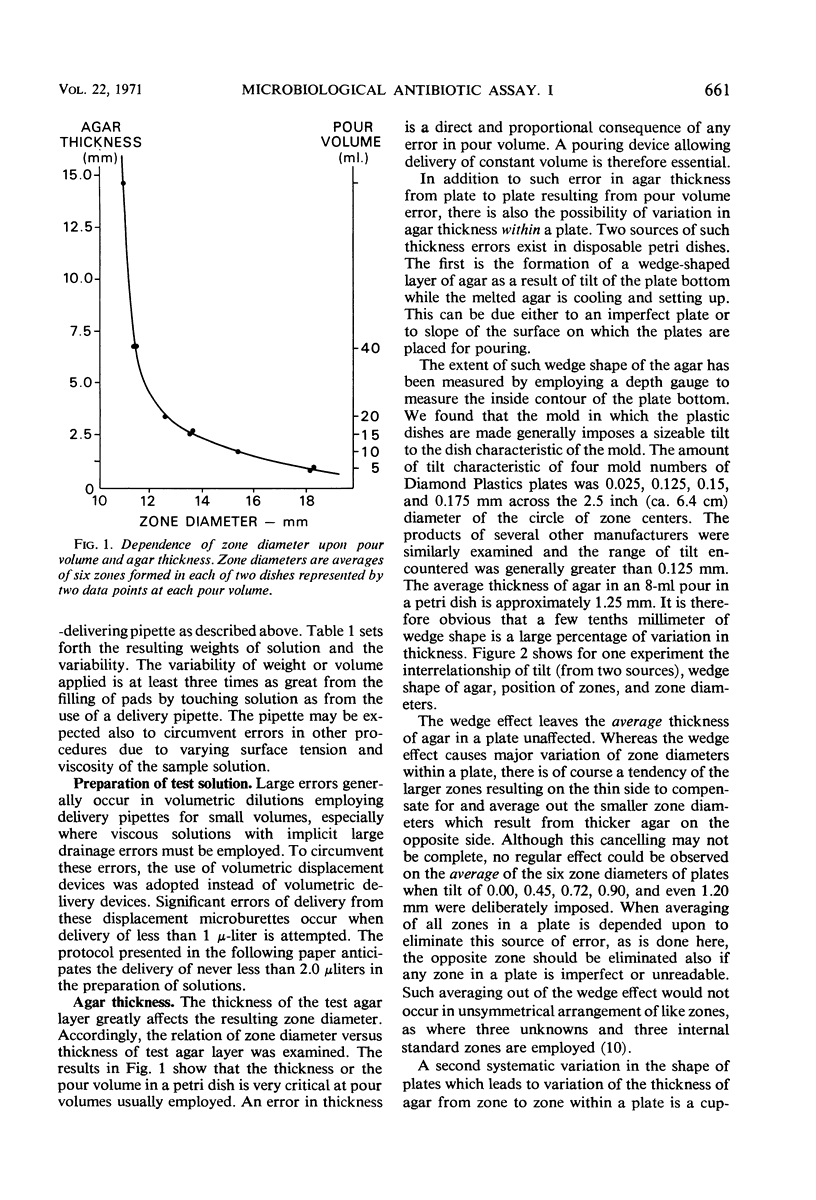
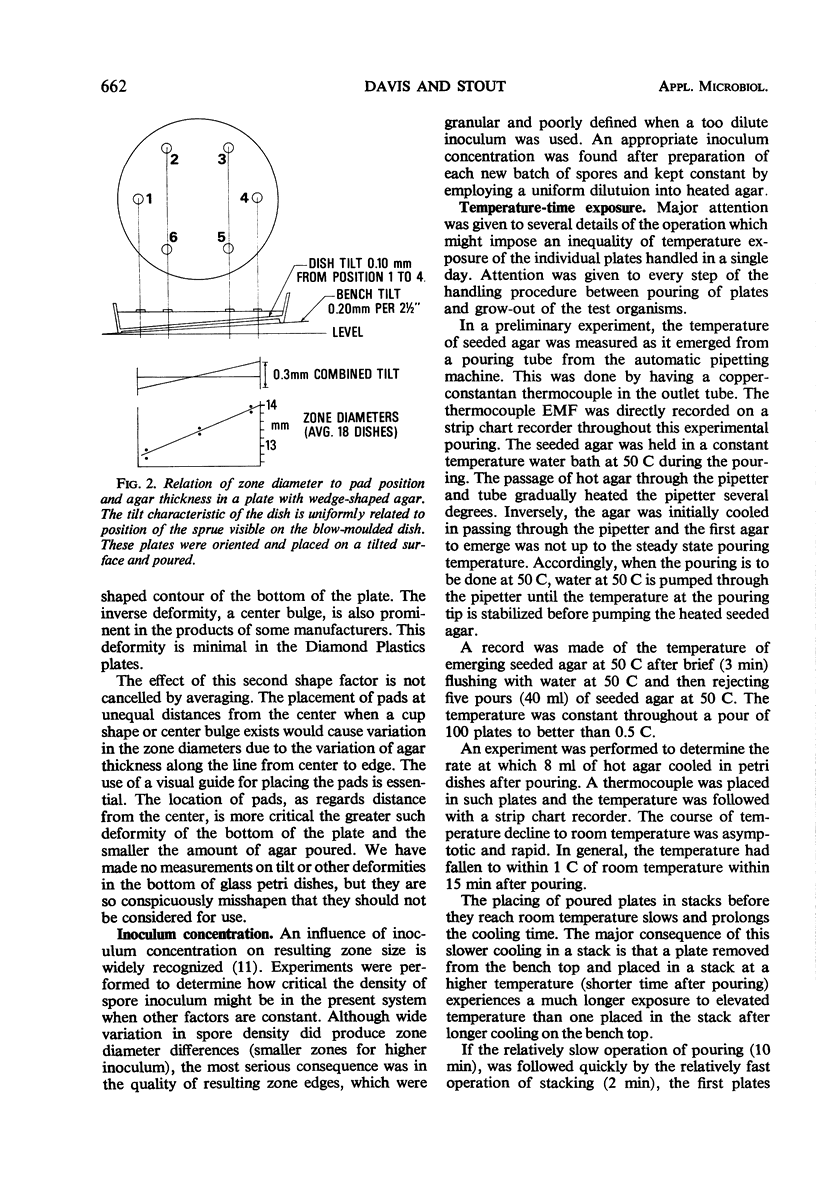
Several factors are investigated that normally cause variation in zone diameters in conventional disc plate diffusion assay procedures. Of these factors the most serious is the unequal exposure of the individual plates at top or bottom of stacks to temperatures above and below room temperature. This unequal temperature exposure is avoided by novel handling and incubation procedures. A major variable, but one which can be controlled, is the varying time interval between pouring seeded agar and the time of applying the pads with antibiotic to the plates. This influence of time of setting and the effects of several other sequential operations are combined into a composite variable. This variable is then accounted for and normalized by interposing “external” reference plates set with a reference solution in the sequence of approximately 100 plates. No “internal” reference zones are employed. Such factors as volume of agar poured, wedge shape of agar in a dish, volumetric errors in dilutions, and timing considerations are studied and discussed. The results of this study form the basis for a test protocol which is presented in a following paper.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COOPER K. E., LINTON A. H. The importance of the temperature during the early hours of incubation of agar plates in assays. J Gen Microbiol. 1952 Aug;7(1-2):8–17. doi: 10.1099/00221287-7-1-2-8. [DOI] [PubMed] [Google Scholar]
- GAVIN J. J. Analytical microbiology. II. The diffusion methods. Appl Microbiol. 1957 Jan;5(1):25–33. doi: 10.1128/am.5.1.25-33.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAVIN J. J. Microbiological process report: analytical microbiology. III. Turbidimetric methods. Appl Microbiol. 1957 Jul;5(4):235–243. doi: 10.1128/am.5.4.235-243.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUMPHREY J. H., LIGHTBOWN J. W. A general theory for plate assay of antibiotics with some practical applications. J Gen Microbiol. 1952 Aug;7(1-2):129–143. doi: 10.1099/00221287-7-1-2-129. [DOI] [PubMed] [Google Scholar]
- Kuzel N. R., Kavanagh F. W. Automated system for analytical microbiology. II. Construction of system and evaluation of antibiotics and vitamins. J Pharm Sci. 1971 May;60(5):767–773. doi: 10.1002/jps.2600600523. [DOI] [PubMed] [Google Scholar]
- Simon H. J., Yin E. J. Microbioassay of antimicrobial agents. Appl Microbiol. 1970 Apr;19(4):573–579. doi: 10.1128/am.19.4.573-579.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMSEN V. F. THE RELATION BETWEEN INOCULUM AND ZONE SIZE IN SENSITIVITY TESTS BY THE AGAR DIFFUSION METHOD: WITH A SPECIAL VIEW TO THE IMPORTANCE OF PREDIFFUSION. Acta Pathol Microbiol Scand. 1964;61:303–316. doi: 10.1111/apm.1964.61.2.303. [DOI] [PubMed] [Google Scholar]


