Abstract
Epidemiological evidence supports a link between sleep loss and obesity. However, the detrimental impact of sleep deprivation on central brain mechanisms governing appetitive food desire remains unknown. Here we report that sleep deprivation significantly decreases activity in appetitive evaluation regions within the human frontal cortex and insula cortex during food desirability choices, combined with a converse amplification of activity within the amygdala. Moreover, this bi-directional change in the profile of brain activity is further associated with a significant increase in the desire for weight-gain promoting high-calorie foods following sleep deprivation, the extent of which is predicted by the subjective severity of sleep loss across participants. These findings provide an explanatory brain mechanism by which insufficient sleep may lead to the development/maintenance of obesity through diminished activity in higher-order cortical evaluation regions, combined with excess subcortical responsivity in the amygdala, resulting in selection of foods most capable of triggering weight-gain.
Introduction
Mounting epidemiological data implicates sleep loss as a risk factor for obesity in both children and adults worldwide1. Moreover, sleep deprivation alters appetite-regulating hormones and increases caloric intake2,3. Given the continued decline in sleep duration in industrialized nations, mirrored by the steep rise in obesity in these same populations1, understanding the association between sleep loss and weight gain has become of paramount concern for global public health.
Despite such population-level as well as peripheral body evidence, the central brain mechanisms explaining the impact of sleep deprivation on appetitive food desire that can lead to weight-gain remain unknown. Discovering such sleep-dependent neural dysfunction may represent a critical component to understanding the link between sleep loss and obesity2. It would further contribute to a central nervous system explanation for the failure to appropriately regulate dietary intake and thus develop or maintain obesity under conditions of insufficient sleep. Using a food-desire task in combination with human functional MRI (fMRI), here we sought to characterize the impact of sleep loss on the brain mechanisms governing appetitive food desire.
The study focused a priori on a discreet set of well-characterized cortical and subcortical regions of interest (ROIs) known to be instrumental in appetitive desire and food stimulus evaluation4. At the cortical level, the anterior insula cortex, lateral orbital frontal cortex and anterior cingulate cortex, all have well established roles in signaling stimulus value across contexts, including appetitive choices, and in integrating food features that govern preferences (e.g., the odor and flavor of food)5,6. Moreover, disrupted functional activity within frontal cortex, including these anterior cortical regions, is widely considered to be one hallmark of sleep loss7. At the subcortical level, both the amygdala and the ventral striatum have been strongly implicated in governing the motivation to eat4. The amygdala has consistently demonstrated responsivity to food stimuli, especially when the salience of food stimuli are high8. Activity in the ventral striatum in response to foods accurately predicts immediate food intake9, binge eating10 as well as real world weight gain11. Moreover, previous work has demonstrated that activity in the amygdala and striatum in other (non-appetitive) affective tasks is elevated following sleep loss12,13.
Building on this established literature, the current study sought to test two non-mutually exclusive hypotheses regarding the central brain mechanisms that may lead to weight-promoting food choices following sleep loss One hypothesis is that failure to recruit cortical regions necessary for optimal evaluation of food stimuli (the anterior cingulate, the lateral orbitofrontal cortex and the anterior insula)leads to improper food choice selection (i.e. choosing items with greater weight-gain potential). A second hypothesis is that excessive reactivity in two subcortical regions known to signal food salience and promote eating behavior (the amygdala and the ventral striatum)may exaggerate food salience and motivated consumption for appetitive food stimuli, also leading to weight-gain potential. The findings reported here demonstrate not only reduced recruitment of all three key cortical regions necessary for food stimulus evaluation, but also amplified subcortical amygdala (yet not ventral striatal) reactivity under sleep deprivation. Such changes offer a novel explanatory brain mechanism by which insufficient sleep may lead to altered food choices and thus the development or maintenance of obesity.
Results
Neural responses to food desire under sleep deprivation
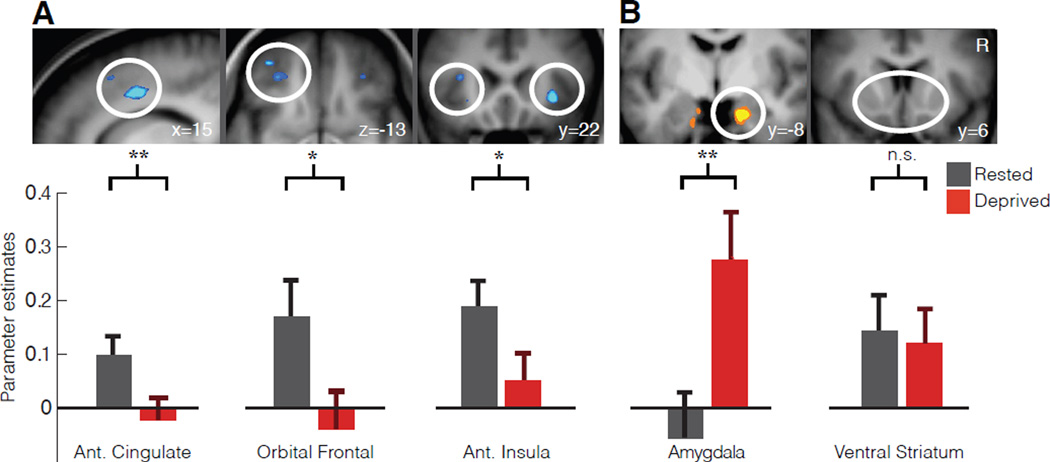
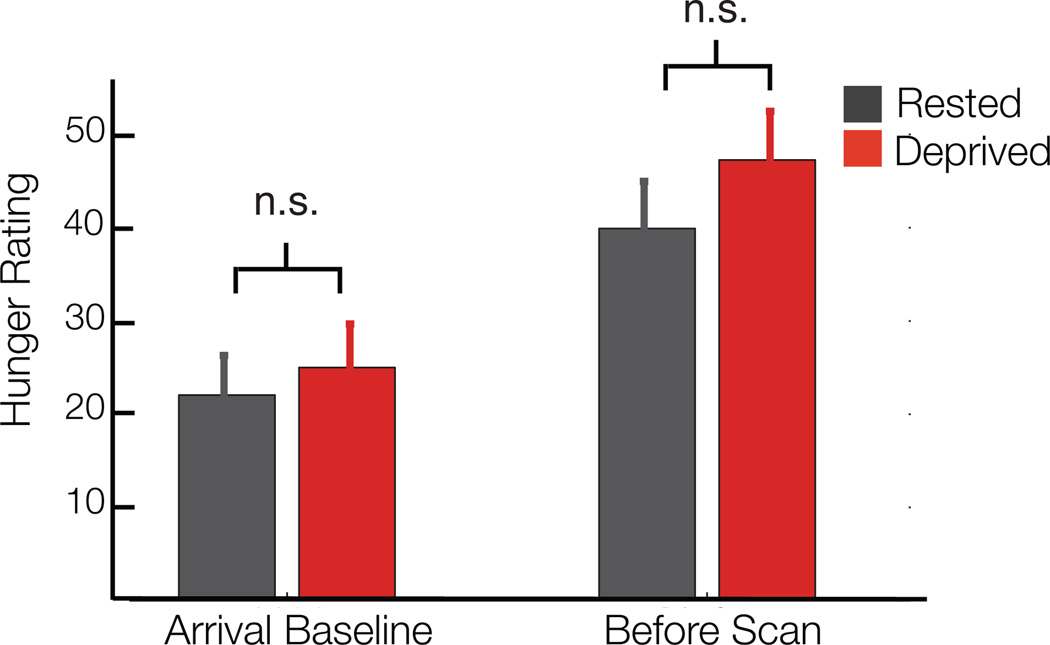
Compared to the sleep rested state, sleep deprivation significantly reduced activity in all three cortical regions of interest —the anterior cingulate cortex (T=3.87; p=0.0008), lateral orbital frontal cortex (T=2.08; p=0.0491) and anterior insula cortex (T=2.63; p=0.0154) —as food desire progressively increased (Fig 1A) confirmed by t-tests of averaged parameter estimates at 5mm spheres placed around literature based sites (see Methods). Note that this significance threshold is p< 0.05 for each region, however, if all five regions of interest are considered as a family of independent tests and correcting for multiple tests we find that lateral orbital frontal cortex and anterior insula no longer survive this more stringent statistical threshold while the anterior cingulate remains significant. It should be noted that this approach makes an assumption of independence of these regions, which may not be the case considering their collective function in appetitive processing. When considering the subcortical regions of interest, the amygdala responsivity to the desirability of food items was significantly increased (T=3.08; p=0.0055) following sleep deprivation, compared to the sleep rested state (Fig 1B). In contrast, this profile of amplified subcortical activity was not observed in ventral striatum (T=−0.28; p=0.7852), showing no significant difference in responsivity between the sleep deprivation and rested conditions. Here again the amygdala survives correction for five comparisons if these regions are taken as a family of independent tests. Additionally, all ROIs demonstrating significance when comparing the average activity described above also express clusters of significant activity within these ROIs that survive familywise error (FWE) rate correction for multiple comparisons (p<0.05; Table 1). Taken together, these findings indicate that sleep deprivation diminished activity in an established set of cortical appetitive evaluation regions as food desire progressively increased, yet triggered a converse increase in subcortical amygdala reactivity known to signal food salience in the context of appetitive choice8. Importantly, self reported hunger levels were no different between the sleep rested and sleep deprived conditions (p=0.28; see Fig 2), indicating that differences in brain activity could not be explained on the basis of hunger differences alone.
Fig. 1. Neural consequences of sleep deprivation on food desirability.
Sleep deprivation lead to marked decreases in the anterior cingulate, left lateral orbital frontal cortex and anterior insula reactivity to food desirability (A). In addition, sleep deprivation lead to a significant increase in amygdala reactivity to food desirability but no significant difference in ventral striatum reactivity (B). All parameter estimates are from a GLM with a parametric contrast of individual “want” ratings from twenty-three participants. Whole brain analysis (above) thresholded at p<0.005 for display purposes for sleep deprivation increases (B) and decreases (A). Region of interest analysis (below) are mean parameter estimates with standard errors of the mean extracted from 5mm spheres centered at foci taken form previous literature (See methods; circles indicate general areas of interest not specific foci; * indicates p<0.05 uncorrected for paired t-tests across 23 participants and ** indicates p<0.05 with Bonferroni correction for five regions of interest). For completeness, and since this is the first study to our knowledge to assess neural responses to food desire after sleep loss, Table 2 reports whole brain activation differences between sleep rested and deprived conditions (p<0.001 uncorrected using voxel-wise paired t-tests). Error bars are s.d.
Table 1.
Small Volume corrections analysis
| Region | T | Cluster Size |
X | Y | Z | Corrected p-value |
|---|---|---|---|---|---|---|
| Sleep Rested > Sleep Deprived | ||||||
| R Anterior cingulate | 4.37 | 20 | 16 | 2 | 40 | 0.006 |
| L Orbital Frontal | 3.65 | 1 | −34 | 48 | −14 | 0.026 |
| R/L Insula | 4.21 | 6 | 32 | 22 | −4 | 0.017 |
| Sleep Deprived > Sleep Rested | ||||||
| R Amygdala | 4.39 | 27 | 18 | −8 | −26 | 0.006 |
Small volume correction analysis for a priori regions of interest taken as 8mm spheres centered at literature based ROIs (Sleep Rested < > Sleep Deprived) of the parametric contrast of want ratings (i.e. regions correlated with increasing food desire and differing by condition). The ROIs are the same as reported in the main manuscript (see Methods). Cluster size is in voxels; voxel size is 2 mm3.
Fig. 2. Self reported hunger levels.
Collected using a visual analog scale with a 10cm line, y-axis is in millimeters. There were no significant differences between sleep rested and sleep deprived sessions either at arrival or before the scan session. However, hunger levels were significantly greater before the scan compared to arrival in both groups (p < .05; paired t-tests across 23 participants). Error bars are s.d.
Behavioral changes in food desire under sleep deprivation
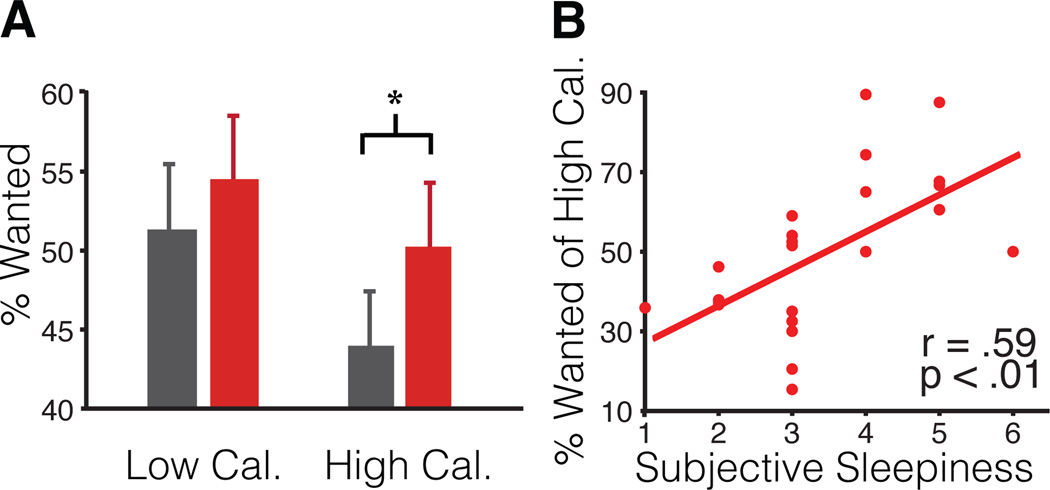
Complimenting these changes in brain responsivity, we further examined whether sleep deprivation triggered an increased desirability for food items that carried the greatest weight-gain promoting potential i.e. high-calorie food items. Relative to the sleep rested state, sleep deprivation resulted in a significant increase in the proportion of “wanted” food items carrying high-caloric content (T=2.21, p=0.04). In contrast, no corresponding differences between the sleep rested and deprived states were observed for low calorie items (T=1.15, p=0.26; Fig 3A). Indeed, the total calorie content of all wanted items (summed together) in the sleep-deprived condition was significantly greater compared to sleep rested state (T=2.07, p=0.05), representing an additional 600 ±289 s.d. Cal average increase. Additionally, the level of caloric content across food items significantly predicted the extent to which desirability ratings increased after sleep deprivation; such that the highest calorie foods accrued the largest increase in desirability ratings following sleep deprivation (Spearman’s r=0.23, p=0.04). Further implicating an association with insufficient sleep, increasing perceived severity of sleep deprivation across individuals, indexed by self-reported subjective sleepiness14, was positively and significantly correlated with the percentage of wanted high-calorie foods (Fig 3B), and this correlation remained significant when controlling for body mass index using linear regression (T=3.41, p=0.003). Confirming the specificity of this finding to the state of sleep deprivation, no such association between subjective sleepiness and percentage of wanted high-calorie foods was observed in the sleep rested state (r=0.19; p=0.39). Additionally, body mass index was not correlated with the percentage of high calorie choices in either the sleep rested or sleep deprived condition (r=−0.23, p=0.30, and r=−0.05, p=0.80, respectively), consistent with previous studies examining calories from snacks rather than meals15. Therefore, paralleling the observed change in the neural reactivity, sleep deprivation induced a concomitant behavioral profile of increased desire for weight-gain promoting (high-calorie) food choices, with inter-individual differences in the magnitude of such a change in food choice behavior being accounted for by the severity of perceived subjective sleepiness.
Fig. 3. Behavioral consequences of sleep deprivation on food desirability.
Behavioral responses (taken from in-scan ratings) are shown for the percentage of wanted high and low calorie items respectively (A) and the degree to which individual differences in sleepiness14 (after sleep deprivation) predict high-calorie choices (B). High/low calorie items are based on median split on Calories per serving; wanted items were collapsed across “somewhat” and “strongly” wanted ratings (* indicates p<0.05; paired t-test across 23 participants). Error bars are s.d.
Discussion
Taken together, these findings establish a disrupting impact of sleep deprivation that blunts activity in established appetitive evaluation regions5 within the human frontal and insula cortex during food desirability choices, yet a converse subcortical amplification of reactivity within the amygdala, known to code salience in the context of food decisions8. Furthermore, these neural changes were associated with a significant increase in appetitive desire for weight-gain promoting (high-calorie) food items following sleep loss, the magnitude of which was proportional to the subjective severity of sleep loss across participants. In addition, these changes occurred despite participants consuming more calories during the sleep deprivation session (provided in a controlled manner in order to offset any increased energy expenditure). Moreover, participants’ self-reported hunger levels were not different in the sleep rested and sleep deprivation session, suggesting that the condition of sleep loss, rather than metabolic need or hunger, as a primary factor influencing the observed changes.
The characterization of these neural and behavioral changes following sleep loss may provide several explanatory insights into a central nervous system (brain) mechanism by which insufficient sleep leads to the development/maintenance of obesity. First, these data describe a profile of bi-directional change in responsivity in appetitive-relevant brain regions following sleep deprivation. All three cortical regions of interest with recognized roles in appetitive stimulus evaluation demonstrated activity reductions following sleep loss in response to increasing food desire, while one of the two subcortical target regions of interest – the amygdala, associated with salience signaling of food items – expressed significant increases in response to food desirability. Interestingly, no significant differences in reactivity were observed in the classical reward region of the ventral striatum following sleep loss. It is important to note that while these brain areas do have specific and recognized functional roles in the context of appetitive food stimulus evaluation and choice, as we examined using the current task, theses regions are not limited to performing such functions. For example, the anterior cingulate has been associated with conflict monitoring16 as well as autonomic (especially cardiovascular) regulation17, the orbital frontal cortex has been associated with inhibitory control18, the anterior insula has been associated with interoception19 and the amygdala has been associated with fear and arousal processing20. While our interpretation of the impact of sleep loss on these regions is made within the context of appetitive food evaluation and choice, due to the nature of the task, they may nevertheless extend beyond appetitive processes, and include alterations in other functions such as those described above.
Second, this collection of brain changes may not only help account for recognized shifts in dietary intake and altered food choices following insufficient sleep3, but further reconcile potentially dissonant previous findings. Specifically, prior reports have demonstrated that sleep restriction leads to increased caloric intake following sleep loss under non-laboratory or “free-living” conditions (where food selection was not fixed)3, fitting with impoverished mechanisms of appetitive evaluation and choice regulated by the frontal lobe as well as heightened salience signaling within the amygdala. However, such altered food choices following sleep loss can also occur without any significant change in ratings of the hedonic qualities of food pleasantness or food desire when smelling foods directly3, consistent with our observations of unaltered responding in this reward-related region of ventral striatum. Furthermore, such a neural dissociation may additionally explain why some studies have failed to observe increases in caloric intake under sleep restriction when food choices are limited to small selection arrays and eating opportunities are fixed15,21, since increases in the motivated drive to eat in the absence of food choices has been primarily associated with activity in the ventral striatum (independent of the effects of sleep loss)9. Therefore, one plausible interpretation emerging from our data is that impoverished recruitment of cortical regions involved in appetitive choice selection following sleep loss, combined with enhanced responsivity from the amygdala, may result in improper valuation of food stimulus features, shifting behavioral choice-selection to high calorie desirable items driven more so by salience, when food is available. The current neural observations would therefore predict that if a range of freely attainable food choices and eating opportunities are offered (as is ecologically the case in the majority of real-world situations), then the effect of sleep deprivation would lead to a significant increase in food consumption choices considered non-optimal in the context of obesity (i.e. high calorie items).
Third, and congruent with these predictions, the changes in neural reactivity to food desirability under sleep deprivation were additionally accompanied by a significant shift in preferences for food items carrying the highest caloric content. While a shift in food desire ratings was observed following sleep deprivation, the controlled eating schedule of the study precluded the ability to measure actual changes in caloric intake under ad libitum (rather than the current controlled) food availability. Interestingly, the alteration in food desire observed here, coinciding with changes in brain activity, are consistent with previous behavioral findings describing increases in actual caloric intake following sleep loss when ad libitum food conditions are presented3,22 and increased cravings for higher caloric food categories (e.g. sweet, salty and starchy foods)23. Given the established increase in energy needs induced by sleep deprivation22,24,25, it is possible that this tendency toward increased caloric intake, and high calorie preferences reported here, supports an adaptive homeostatic function to recover such energy expended. However, a recent study which assessed ad libitum caloric intake as well as energy expenditure in sleep-restricted humans reported increased calorie consumption beyond that which could be explained by expended energy or altered metabolic rate22. Moreover, this increase in calorie intake resulted in significant gains in weight. This finding leads to the hypothesis that changes in central nervous system disruption due to sleep loss, such as the alterations in appetitive brain signaling described in the current study, may contribute to decisions that lead to increased calorie consumption in excess of energy expenditure changes, one consequence of which is weight gain. We additionally demonstrated that the magnitude of change (increase) in desire for high calorie foods was positively correlated with the perceived subjective severity of sleep deprivation across participants (indexed in the measure of sleepiness). Therefore, our data provide indirect support linking the state of sleep deprivation, and the subjective severity of this state, to altered internal homeostasis following extended time awake, and is consistent with already established alterations in metabolism and temperature regulation following sleep loss26,27. This may reflect a progressive deterioration in the brain and body systems that regulate and maintain optimal energy balance, potentially reflected in the current study by increases in energy consumption through heightened desire for high calorie foods.
Finally, and related to such whole organism considerations, elegant prior work has describe peripheral body changes in appetite and metabolic regulating hormones following sleep loss that can lead to weight-gain2,26,28. Our findings raise the presence of a central nervous system dysfunction that stands along side these increasingly well-described peripheral body changes following sleep deprivation that together, may converge on a common impact sleep loss on weight-gain potential. An important next step will be to examine if (and how) these peripheral and central nervous system pathways of sleep loss-dependent dysfunction may actively interact, thus providing the first whole-organism mechanistic account underlying a relationship between sleep loss and obesity.
Beyond the implications stated above, it is important to note that the current findings should be considered in the context of several limitations. First, this study used a carefully controlled feeding schedule that was standardized across participants which did not allow us to assess actual changes in calories consumed due to sleep deprivation (although see3,22 ) or to assess he relationship between the neural responses observed and behavioral shifts in actual calories consumed. Furthermore, due to this limitation it will be important for future studies to assess whether access to ad libitum high calorie food would normalize the observed brain responses under sleep deprivation due to potentially reduced motivational demands for high-calorie items after consumption. Second, all scan sessions for this study took place during the morning. Since both appetite and sleep patterns are significantly influenced by circadian phase29, future studies will be needed to examine the interactions of measurements at different circadian phases. Indeed, recent behavioral studies indicate that the largest impact of sleep loss on altered food choices occurs during the evening22,30 leading to the testable hypothesis that changes observed in the current study would be further exaggerated when repeated later in the day. Finally, it should be noted that the current findings were measured in a group of healthy young and lean participants (20.5±1.8 s.d. years of age; 23.0±1.8 s.d. BMI). An important future challenge will be to examine whether similar alterations caused by sleep deprivation are expressed across a broader age and body mass range; pertinent considering that hormones, metabolism as well as neural responses change over the life span31, and across a spectrum of lean to obese ranges32.
In summary, these findings contribute to a novel brain mechanism by which sleep loss may lead to the development and/or maintenance of obesity through the potentially maladaptive selection of foods carrying obesogenic (weight-gain) potential, thereby explaining the large-scale significant association between reduced sleep time and obesity reported in population level studies1. They further support the proposal of sufficient sleep as an important mechanistic factor promoting weight control, one pathway of which appears to be the regulation of central brain mechanisms governing appropriate food choices.
Methods
Experiment Overview
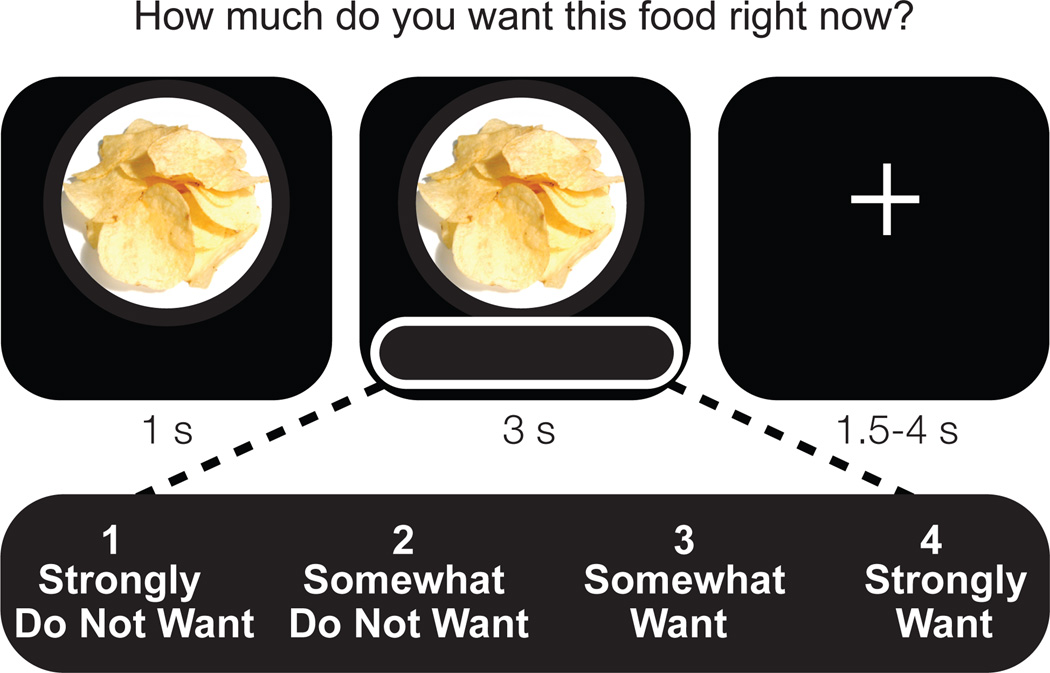
twenty-three healthy participants (13 female; age: 20.5± 1.8 s.d.; body mass index: 23.0±1.8 s.d.) underwent a repeated measures, counterbalanced cross-over design involving a night of normal rested sleep (average 8.2 hr asleep) and a night of monitored total sleep deprivation (average 24.6 hr awake), separated by at least 7 days. Participants ate a controlled snack (see Methods) at 2:30am in the sleep deprivation condition that contained a calorie amount sufficient to alleviate increased energy demands estimated to be expended due to staying awake. Together with a standardized breakfast in both conditions in the morning, this eating schedule resulted in hunger ratings (measured on 100mm visual analog scale23) that did not differ statistically (p=0.28) between sleep deprived (47.7±25.53 s.d. mm) and rested conditions (39.7±24.0 s.d. mm) preceding the MRI scan sessions. During each fMRI session (scan time 9:29AM±49min s.d.) participants rated 80 different food items that varied in calorie content on a 1–4 rating scale, according to how much they wanted that item “right now” (Fig 4). fMRI BOLD signal was correlated with these 1–4 ratings on a trial-to-trial basis, resulting in neural activation maps expressly sensitive to increasing food-choice desire. After the scan, participants actually received one food item based on their ratings, enhancing ecological incentive context and potentially reducing demand characteristics. Therefore, using this task we simultaneously monitored behavioral food desire as well as identified brain areas underlying these appetitive decisions, modeled and hence sensitive to increasing food desire.
Fig. 4. Food desire task trial structure.
Participants saw and rated 80 food items on a scale from 1–4 according to how much they wanted the food item at that moment under sleep rested and sleep deprived conditions.
Participants
The Institutional Review Board of the University of California, Berkeley approved the experimental protocol and we obtained written informed consent from participants. Participants were free of general medical, neurological, psychiatric or sleep disorder diagnoses and did not report any history of drug abuse or head trauma. Further, participants were free from MRI contraindications and no individuals had dietary restrictions or food allergies to any of the food stimuli. Participants abstained from drugs, alcohol and caffeine for 3-days before each session. Twenty-three participants were included in analysis (13 female; mean age: 20.5± 1.8 s.d.; mean body mass index: 23.0±1.8 s.d.; 1 participant was left handed). Three participants (from 26 original) were excluded due to, 1) failure to keep the sleep schedule (>2 hours deviation), 2) initiating a gluten free diet, 3) inability to stay awake during the deprivation scan session as evidence from online eye-tracking and omitting responses on 30% of trials. The remaining participants omitted 3.9± 4.3 s.d. trials under sleep deprivation and 0.2± 0.4 s.d trials when rested, all separately modeled as omitted trials in the fMRI analyses.
Sleep condition procedures
Participants completed two experimental sessions 1) a night of normal sleep in the lab monitored by PSG and 2) a night of total sleep deprivation monitored by lab personnel from 9pm and wrist actigraphy. Sessions were separated by a minimum of 7 days (average 10 days) and counter balanced in order across participants. Participants completed fMRI scanning sessions the morning after each experimental night, starting at 9:08 AM +- 45min (range: 8:05am to 10:39am) on the sleep deprivation day and 9:50 AM +- 45min (range: 8:17am to 11:00am) on the sleep rested day. To ensure that participants were well rested before each session, they kept a regular sleep schedule (7–9 hours time in bed between 10pm and 10am) for three days prior to each session, verified by daily sleep diaries and wrist actigraphy. Subjects attained 8.08±1.0 s.d. hours time in bed across the three nights proceeding the sleep deprivation and 8.07±1.0 s.d. hours preceding the sleep rested condition. Additionally, on these three days, participants used 5-point scales (1 indicating low/poor and 5 indicating high) to rate their subjective overall sleep quality (3.91±0.9 s.d. before sleep deprivation; 3.80±0.9 s.d. before sleep rested), how alert they felt (3.69±0.7 s.d. before sleep deprivation; 3.63±0.8 s.d. before sleep rested) and how well rested they felt (3.78±0.9 s.d. before sleep deprivation; 3.75±0.8 s.d. before sleep rested). Finally, participants rated their subjective sleepiness14 on the as 2.09±0.9 s.d. at their arrival in the rested session (~8:00pm) and as 2.35±1.1 s.d. at their arrival in the sleep deprivation session (~9:00pm). Taken together, these measures suggest that participants were well rested when entering both experimental sessions.
Sleep monitoring and recording procedures
On the sleep deprivation experimental night, subjects were monitored using wrist actigraphy throughout the day and then additionally monitored in the lab by lab personnel starting at 9 pm. On the experimental night in the sleep rested session, polysomnography (PSG) sleep monitoring was recorded in the laboratory using 19-channel electroencephalography (EEG) (locations according to international 10–20 system), together with electro-oculography (EOG) at right and left outer canthi and electromyography (EMG) via three chin electrodes33. Sleep-staging was performed in accordance with standardized techniques34 from the C3-A2 electrode derivation. During this PSG rested session, subjects obtained an average of 8.2± 0.84 s.d. hours time asleep (minutes and standard deviation): 48.68± 20.1 NREM Stage 1; 251.1± 39.6 NREM Stage 2; 83.58± 28.9 NREM slow wave sleep (Stages 3 & 4); 110.1± 28.7 REM sleep.
Eating schedule procedures
Participants ate according to their normal diet throughout enrollment. During the sleep deprivation night, participants were provided with a controlled snack from 2:30–3:00am. This consisted of calorie content sufficient to offset increased energy expenditure associated with one night of sleep loss over a 24 hr period (reported as 134±2.1 s.d. Cal)24. The snack contained the following four items: Fig Newtons (200 Cal), Gold Fish crackers (130 Cal), Ritz peanut butter crackers (150 Cal) and an apple (95 Cal), resulting in a total of 575 Cal available. On average, participants ate an estimated 485.2 Cal, with the least amount of calories consumed being approximately 160 Cal; and this was the only participant who consumed less than 300 Cal. Note that these estimates are based on calories per serving reported on the packaging and the proportion of the serving eaten by the participants (reported as none, half or all of the item). In addition, and in both sessions, all participants were given, and consumed, a small breakfast (one piece of toast with strawberry jam) approximately forty-five minutes before their scan session. Participants were monitored throughout both sessions to ensure that they did not eat anything in addition to this provided food (although water was not restricted). Self reported hunger levels (assessed on a 100 mm visual analog scale23) immediately preceding the scan session were no different between the rested and deprived conditions (p=0.28; also reported in Results). Both groups showed an increase in hunger levels compared to their study arrival baseline (Fig 2); important considering previous studies have shown that brain reactivity to food stimuli can be enhanced by subjective hunger35.
Food Desire Task
In the food desire task, participants saw 80 food items and rated them on a 1–4 scale according to how much they wanted that food right now (details provided in Fig 4). In order to control for lateralized motor effects, approximately half of the participants used their left hand to rate wanted items (1-strongly want; 2-somewhat want; 3-somewhat do not want; 4-strongly do not want) and the other half used their right hand (scale reversed). For all analyses, ratings were re-coded so that higher ratings indicated higher wanting. Participants were not informed of the hypotheses of the study nor were they told that they would be seeing foods that experimentally varied in terms of calorie content or food types that could otherwise establish preconceived biases.
In the task instructions, participants were informed that two food items would be revealed at the end of the scan, and a serving of whichever item they had rated as wanting more would be given to them to eat (which was carried out). They were further instructed that this meant it was in their best interest to rate each food item according to how much they actually wanted that item at the time of the session. This procedure was used to encourage participants to rate the food items according to their actual preferences (rather than according to experimental expectations or demand characteristics), and to ensure incentive compatibility in the task as in previous studies36.
Stimuli
The task used 80 pictures of food with no packaging collected from internet searches and cropped to standardized circles. The items were evenly distributed across five categories (salty, sweet, starchy, fruit or dairy) and varied in calorie content (Range: 7.2 – 523.4; Mean: 139.8± 94.8 s.d. Cal per serving based on USDA database listings (ndb.nal.usda.gov)). The same 80 food items but a different picture of each item was used in each experimental session.
fMRI scanning Acquisition
Blood oxygenation level-dependent contrast functional images were acquired with echo-planar T2*-weighted (EPI) imaging using a Siemens 3 Tesla MRI scanner with a 12-channel head coil. Each image volume consisted of 32 ascending 3.5mm slices (96 × 96 matrix; TR = 2000ms; TE = 28ms; voxel size 2.5 × 2.5 × 3.5 mm, FOV 224mm, flip angle = 90°). One high-resolution, T1 weighted structural scan was acquired at the end of the sleep rested session (256 × 256 matrix; TR=1900; TE = 2.52; flip angle = 9°; FOV 256mm; 1 × 1 × 1mm voxels). Concurrent eye tracking was utilized in order to further verify wakefulness. Each session was split into two 40-trial scanner acquisition runs.
fMRI scanning Preprocessing
Preprocessing and data analysis were performed using Statistical Parametric Mapping software implemented in Matlab (SPM8; Wellcome Department of Cognitive Neurology, London, UK). First, scan to scan variance was assessed for quality assurance using time-series difference analysis (http://imaging.mrc-cbu.cam.ac.uk/imaging/DataDiagnostics) and individual scans with supra-threshold shifts (indicating high subject movement) were removed and replaced with the average of surrounding scans, these time-points were modeled out with dummy regressors (this effected 6 subjects). Images were then slice time corrected, the time series was linearly detrended, then motion corrected, smoothed using a 6mm full-width-at-half-maximum (FWHM) Gaussian kernel and finally time-series were high pass filtered (width of 128s).
General Linear model
A separate general linear model was constructed for each subject which included 1) all trial onsets convolved with a canonical hemodynamic response function with a 3 second duration 2) A parametric regressor of the individual want ratings (1–4) for each food item convolved with a canonical hemodynamic response function with a 3 second duration (this was the regressor of interest) 3) The six movement-related covariates (three rigid-body translations and three rotations determined from the realignment preprocessing step). Separate regressors were used within the same model for each of the 2 scanner acquisition runs.
ROI Analysis
Guided by suggested ROI reporting policies37,38, regions of interests were taken as the average parameter estimates from 5 mm spheres centered around coordinates form previous literature examining food evaluation for the three cortical regions of interest as well as the amygdala, and reward responsively for the ventral striatum. MNI Coordinates [x, y, z] were: amygdala (18, −12, −22)8; ventral striatum (−12, 12, −10) 39; Anterior cingulated cortex (15, 6, 38)40; Lateral orbital frontal cortex (−36, 42, −10) 41; and bilateral anterior insula (−31, 22, 11 & 36, 17, 0) 42.
Behavioral comparisons
Behavioral comparisons between rested and deprived conditions were carried out using paired t-tests. Correlation analysis was used for results in Fig 3B. Spearman’s correlation analysis was used to investigate the relationship between calories across food items with the mean change in desire ratings across items.
Table 2.
Exploratory whole brain analysis
| Region | T | Cluster Size |
X | Y | Z |
|---|---|---|---|---|---|
| Sleep Rested > Sleep Deprived | |||||
| * L Putamen | 4.99 | 104 | −16 | 4 | 0 |
| * L Hippocampus | 4.00 | −14 | −10 | −8 | |
| * R Thalamus | 4.46 | 42 | 4 | −10 | −2 |
| * R Cingulate | 4.37 | 24 | 16 | 2 | 40 |
| * R Insula | 4.22 | 20 | 32 | 24 | −4 |
| * L Superior Parietal | 4.21 | 25 | −30 | −68 | 52 |
| L Parahippocampus | 4.18 | 4 | −20 | −32 | −8 |
| * L Middle Frontal | 3.91 | 17 | −34 | 30 | 54 |
| L Thalamus | 3.84 | 8 | −6 | −18 | −8 |
| L Middle Frontal | 3.72 | 2 | −36 | 38 | 46 |
| L Superior Frontal | 3.69 | 9 | −22 | 32 | 50 |
| L Orbital Frontal | 3.65 | 1 | −34 | 48 | −14 |
| R Postcentral gyrus | 3.58 | 7 | 32 | −28 | 42 |
| R Precuneus | 3.51 | 1 | 18 | −64 | 30 |
| Sleep Deprived > Sleep Rested | |||||
| * R Parahippocampu/Amygdala | 4.39 | 29 | 18 | −8 | −26 |
| * R Inferior temporal lobe | 4.11 | 30 | 48 | −48 | −18 |
| R Supperior temporal pole | 3.84 | 5 | 32 | 28 | −24 |
| L Fusiform | 3.69 | 3 | −30 | −22 | −28 |
| L Cerebellum | 3.69 | 7 | −14 | −72 | −38 |
| L Inferior temporal lobe | 3.68 | 6 | −56 | −64 | −22 |
| L Inferior temporal lobe | 3.60 | 3 | −50 | −56 | −24 |
| R Cerebellum | 3.55 | 1 | 58 | −50 | −32 |
| R Parahippocampal gryrus | 3.55 | 2 | 28 | −22 | −32 |
| R Superior temporal pole | 3.54 | 1 | 52 | 20 | −14 |
Exploratory whole brain analysis showing all peak activations (MNI coordinates) significant at p<0.001 (no cluster criteria) for paired comparison (Sleep Rested < > Sleep Deprived) of the parametric contrast of want ratings (i.e. regions correlated with increasing food desire and differing by condition). Bold indicates a priori regions of interest. Cluster size is in voxels; voxel size is 2 mm3.
Regions marked with * survive cluster correction criteria of ten voxels.
Acknowledgements
This work was supported by grants R01AG031164, R01MH093537, R21DA031939 (MPW) and F31DA035076 (SMG) from the National Institutes of Health. We would also like to thank the following research assistants: Alexis Casillas, Adam Krause, Jonathan Tam, Patrick Slattery, Jessica Hamel, Martine DeHuff, Amy Lai, Linda Nix, Anna Khazenzon, Connor Lemos and Jaimie Sallee.
Footnotes
Author contributions
S.M.G, A.N.G and M.P.W conceived and designed the study. S.M.G and A.N.G collected the data. S.M.G and M.P.W analyzed the data and wrote the paper.
The authors declare no competing financial interests.
References
- 1.Cappuccio FP, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31:619–626. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanlon EC, Van Cauter E. Quantification of sleep behavior and of its impact on the cross-talk between the brain and peripheral metabolism. Proc Natl Acad Sci U S A. 2011;108(Suppl 3):15609–15616. doi: 10.1073/pnas.1101338108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brondel L, Romer MA, Nougues PM, Touyarou P, Davenne D. Acute partial sleep deprivation increases food intake in healthy men. Am J Clin Nutr. 2010;91:1550–1559. doi: 10.3945/ajcn.2009.28523. [DOI] [PubMed] [Google Scholar]
- 4.Tang DW, Fellows LK, Small DM, Dagher A. Food and drug cues activate similar brain regions: a meta-analysis of functional MRI studies. Physiol Behav. 2012;106:317–324. doi: 10.1016/j.physbeh.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Small DM, Prescott J. Odor/taste integration and the perception of flavor. Exp Brain Res. 2005;166:345–357. doi: 10.1007/s00221-005-2376-9. [DOI] [PubMed] [Google Scholar]
- 6.Hollmann M, et al. Neural correlates of the volitional regulation of the desire for food. Int J Obes (Lond) 2012;36:648–655. doi: 10.1038/ijo.2011.125. [DOI] [PubMed] [Google Scholar]
- 7.Muzur A, Pace-Schott EF, Hobson JA. The prefrontal cortex in sleep. Trends Cogn Sci. 2002;6:475–481. doi: 10.1016/s1364-6613(02)01992-7. [DOI] [PubMed] [Google Scholar]
- 8.van der Laan LN, de Ridder DT, Viergever MA, Smeets PA. The first taste is always with the eyes: a meta-analysis on the neural correlates of processing visual food cues. Neuroimage. 2011;55:296–303. doi: 10.1016/j.neuroimage.2010.11.055. [DOI] [PubMed] [Google Scholar]
- 9.Lawrence NS, Hinton EC, Parkinson JA, Lawrence AD. Nucleus accumbens response to food cues predicts subsequent snack consumption in women and increased body mass index in those with reduced self-control. Neuroimage. 2012;63:415–422. doi: 10.1016/j.neuroimage.2012.06.070. [DOI] [PubMed] [Google Scholar]
- 10.Wang GJ, et al. Enhanced striatal dopamine release during food stimulation in binge eating disorder. Obesity (Silver Spring) 2011;19:1601–1608. doi: 10.1038/oby.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demos KE, Heatherton TF, Kelley WM. Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. J Neurosci. 2012;32:5549–5552. doi: 10.1523/JNEUROSCI.5958-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Venkatraman V, Chuah YM, Huettel SA, Chee MW. Sleep deprivation elevates expectation of gains and attenuates response to losses following risky decisions. Sleep. 2007;30:603–609. doi: 10.1093/sleep/30.5.603. [DOI] [PubMed] [Google Scholar]
- 13.Yoo SS, Gujar N, Hu P, Jolesz FA, Walker MP. The human emotional brain without sleep--a prefrontal amygdala disconnect. Curr Biol. 2007;17:R877–R878. doi: 10.1016/j.cub.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: a new approach. Psychophysiology. 1973;10:431–436. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- 15.Nedeltcheva AV, et al. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr. 2009;89:126–133. doi: 10.3945/ajcn.2008.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Botvinick MM. Conflict monitoring and decision making: reconciling two perspectives on anterior cingulate function. Cogn Affect Behav Neurosci. 2007;7:356–366. doi: 10.3758/cabn.7.4.356. [DOI] [PubMed] [Google Scholar]
- 17.Critchley HD, et al. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain. 2003;126:2139–2152. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- 18.Stuss DT. Functions of the frontal lobes: relation to executive functions. J Int Neuropsychol Soc. 2011;17:759–765. doi: 10.1017/S1355617711000695. [DOI] [PubMed] [Google Scholar]
- 19.Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 20.Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Res Brain Res Rev. 2003;41:88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]
- 21.Schmid SM, et al. Short-term sleep loss decreases physical activity under free-living conditions but does not increase food intake under time-deprived laboratory conditions in healthy men. Am J Clin Nutr. 2009;90:1476–1482. doi: 10.3945/ajcn.2009.27984. [DOI] [PubMed] [Google Scholar]
- 22.Markwald RR, et al. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc Natl Acad Sci U S A. 2013;110:5695–5700. doi: 10.1073/pnas.1216951110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 24.Jung CM, et al. Energy expenditure during sleep, sleep deprivation and sleep following sleep deprivation in adult humans. J Physiol. 2011;589:235–244. doi: 10.1113/jphysiol.2010.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Penev PD. Update on energy homeostasis and insufficient sleep. J Clin Endocrinol Metab. 2012;97:1792–1801. doi: 10.1210/jc.2012-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11:163–178. doi: 10.1016/j.smrv.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romeijn N, et al. Cold hands, warm feet: sleep deprivation disrupts thermoregulation and its association with vigilance. Sleep. 2012;35:1673–1683. doi: 10.5665/sleep.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Cauter E, et al. Impact of sleep and sleep loss on neuroendocrine and metabolic function. Horm Res. 2007;67(Suppl 1):2–9. doi: 10.1159/000097543. [DOI] [PubMed] [Google Scholar]
- 29.Saper CB. Staying awake for dinner: hypothalamic integration of sleep, feeding, and circadian rhythms. Prog Brain Res. 2006;153:243–252. doi: 10.1016/S0079-6123(06)53014-6. [DOI] [PubMed] [Google Scholar]
- 30.Baron KG, Reid KJ, Kern AS, Zee PC. Role of sleep timing in caloric intake and BMI. Obesity (Silver Spring) 2011;19:1374–1381. doi: 10.1038/oby.2011.100. [DOI] [PubMed] [Google Scholar]
- 31.Wilson MM, Morley JE. Invited review: Aging and energy balance. J Appl Physiol. 2003;95:1728–1736. doi: 10.1152/japplphysiol.00313.2003. [DOI] [PubMed] [Google Scholar]
- 32.Wang GJ, Volkow ND, Thanos PK, Fowler JS. Imaging of brain dopamine pathways: implications for understanding obesity. J Addict Med. 2009;3:8–18. doi: 10.1097/ADM.0b013e31819a86f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klem GH, Luders HO, Jasper HH, Elger C. The ten-twenty electrode system of the International Federation. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl. 1999;52:3–6. [PubMed] [Google Scholar]
- 34.Rechtschaffen A, Kales A. Los Angeles, CA: US Govenment Pringing Office, US Public Health Service; 1968. [Google Scholar]
- 35.Siep N, et al. Hunger is the best spice: an fMRI study of the effects of attention, hunger and calorie content on food reward processing in the amygdala and orbitofrontal cortex. Behav Brain Res. 2009;198:149–158. doi: 10.1016/j.bbr.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 36.Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- 37.Poldrack RA. Region of interest analysis for fMRI. Soc Cogn Affect Neurosci. 2007;2:67–70. doi: 10.1093/scan/nsm006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poldrack RA, Mumford JA. Independence in ROI analysis: where is the voodoo? Soc Cogn Affect Neurosci. 2009;4:208–213. doi: 10.1093/scan/nsp011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knutson B, Wimmer GE, Kuhnen CM, Winkielman P. Nucleus accumbens activation mediates the influence of reward cues on financial risk taking. Neuroreport. 2008;19:509–513. doi: 10.1097/WNR.0b013e3282f85c01. [DOI] [PubMed] [Google Scholar]
- 40.Small DM, et al. Dissociation of neural representation of intensity and affective valuation in human gustation. Neuron. 2003;39:701–711. doi: 10.1016/s0896-6273(03)00467-7. [DOI] [PubMed] [Google Scholar]
- 41.Beaver JD, et al. Individual differences in reward drive predict neural responses to images of food. J Neurosci. 2006;26:5160–5166. doi: 10.1523/JNEUROSCI.0350-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Small DM, et al. Human cortical gustatory areas: a review of functional neuroimaging data. Neuroreport. 1999;10:7–14. doi: 10.1097/00001756-199901180-00002. [DOI] [PubMed] [Google Scholar]