Abstract
Rationale: The stress-induced growth arrest and DNA damage–inducible α (GADD45a) gene is up-regulated by mechanical stress with GADD45a knockout (GADD45a−/−) mice demonstrating both increased susceptibility to ventilator-induced lung injury (VILI) and reduced levels of the cell survival and vascular permeability signaling effector (Akt). However, the functional role of GADD45a in the pathogenesis of VILI is unknown.
Objectives: We sought to define the role of GADD45a in the regulation of Akt activation induced by mechanical stress.
Methods: VILI-challenged GADD45a−/− mice were administered a constitutively active Akt1 vector and injury was assessed by bronchoalveolar lavage cell counts and protein levels. Human pulmonary artery endothelial cells (EC) were exposed to 18% cyclic stretch (CS) under conditions of GADD45a silencing and used for immunoprecipitation, Western blotting or immunofluoresence. EC were also transfected with mutant ubiquitin vectors to characterize site-specific Akt ubiquitination. DNA methylation was measured using methyl-specific polymerase chain reaction assay.
Measurements and Main Results: Studies exploring the linkage of GADD45a with mechanical stress and Akt regulation revealed VILI-challenged GADD45a−/− mice to have significantly reduced lung injury on overexpression of Akt1 transgene. Increased mechanical stress with 18% CS in EC induced Akt phosphorylation via E3 ligase tumor necrosis factor receptor–associated factor 6 (TRAF6)–mediated Akt K63 ubiquitination resulting in Akt trafficking and activation at the membrane. GADD45a is essential to this process because GADD45a-silenced endothelial cells and GADD45a−/− mice exhibited increased Akt K48 ubiquitination leading to proteasomal degradation. These events involve loss of ubiquitin carboxyl terminal hydrolase 1 (UCHL1), a deubiquitinating enzyme that normally removes K48 polyubiquitin chains bound to Akt thus promoting Akt K63 ubiquitination. Loss of GADD45a significantly reduces UCHL1 expression via UCHL1 promoter methylation resulting in increased Akt K48 ubiquitination and reduced Akt levels.
Conclusions: These studies highlight a novel role for GADD45a in the regulation of site-specific Akt ubiquitination and activation and implicate a significant functional role for GADD45a in the clinical predisposition to VILI.
Keywords: GADD45a, AKT, UCHL1, ubiquitin, mechanical stress
At a Glance Commentary
Scientific Knowledge on the Subject
Multiple lines of evidence support the contribution of genetic variants to acute lung injury (ALI) and ventilator-induced lung injury (VILI) susceptibility and outcomes. Several ALI-VILI candidate genes have now been identified including the growth arrest and DNA damage–inducible α (GADD45a) gene. The functional role of GADD45a in VILI, however, is poorly understood.
What This Study Adds to the Field
We identified a critical regulatory role for GADD45a, a novel ALI-VILI candidate gene, in the regulation of VILI responses via Akt ubiquitination and activation by mechanical stress. These data implicate a functional role for GADD45a in the clinical predisposition to VILI.
Acute lunfg injury (ALI) is a devastating disorder commonly encountered in the intensive care unit, with approximately 75,000 deaths annually in the United States (1). Although insults, such as infection and trauma, often precipitate in ALI, ALI morbidity and mortality are heavily influenced by excessive mechanical stress produced by mechanical ventilation. Mechanical ventilator support is required to provide adequate gas exchange but potentially generates excessive mechanical stress, a condition known as ventilator-induced lung injury (VILI) (2). Multiple lines of evidence, including marked ethnic and racial disparities in ALI mortality, support the contribution of genetic variants to ALI-VILI susceptibility and outcomes (3). Based on a systematic, multispecies gene expression profiling of preclinical ALI-VILI models we have identified several ALI-VILI candidate genes (4–7) including the growth arrest and DNA damage–inducible α gene GADD45a (8, 9).
The validation of GADD45a as a novel VILI candidate gene was subsequently confirmed by the enhanced susceptibility of GADD45a knockout (GADD45a−/−) mice to VILI with increased alveolar and vascular leak, increased leukocyte infiltration, and gene expression profiling evidence of marked inflammatory gene induction (9). Interestingly, bioinformatics analysis of GADD45a−/− mice dysregulated gene expression profiles identified PI3Kinase (PI3K) and the serine-threonine kinase, Akt, as a highly dysregulated canonical signaling pathway. Subsequent analyses confirmed markedly decreased Akt protein expression in GADD45a−/− mice and in GADD45a-silenced human lung endothelial cells (EC) (9). These observations were linked to increased Akt ubiquitination and proteasomal degradation (9). Of note, various reports have now identified augmented injury in animal models of both VILI and lipopolysaccharide-induced ALI associated with Akt inhibition (10, 11) and we previously reported increased agonist-induced barrier disruption in Akt1-silenced EC monolayers (9). These findings suggest that Akt depletion contributes directly to the elaboration of acute inflammatory lung injury via increased lung vascular permeability.
In this study, we explored the link between GADD45a and mechanical stress-induced Akt activation including the role of Akt in GADD45a−/− susceptibility to VILI. Our results demonstrate VILI-challenged GADD45a−/− mice were significantly protected by lung overexpression of a constitutively active (c/a) Akt1 transgene. Cyclic stretch (CS) (18%) –induced increases in mechanical stress resulted in increased Akt phosphorylation in human pulmonary artery EC, which was dependent on E3 ligase, tumor necrosis factor receptor–associated factor 6 (TRAF6) –mediated K63 ubiquitination resulting in Akt trafficking to the plasma membrane and kinase activation. In contrast, both GADD45a-silenced human endothelium and GADD45a−/− mice exhibit increased Akt K48 ubiquitination leading to proteasomal degradation caused by loss of ubiquitin carboxyl terminal hydrolase 1 (UCHL1), a deubiquitinating enzyme (DUB) that removes K48 poly-ubiquitin Akt chains in the presence of mechanical stress. Loss of GADD45a significantly reduces UCHL1 expression via UCHL1 promoter methylation resulting in increased Akt K48 ubiquitination and reduced Akt levels. These studies highlight a novel multifunctional role of GADD45a in site-specific Akt ubiquitination and activation and implicate active GADD45 participation in responses to excessive mechanical stress and clinical susceptibility to ventilator-mediated inflammatory injury. Some of the results of these studies have been previously reported in the form of an abstract (12, 13).
Methods
Antibodies, Silencing RNA, and Reagents
Antibodies against p-Akt ser/thr (Cell Signaling, Danvers, MA), total Akt (Santa Cruz Biotechnology, Santa Cruz, CA), GADD45a (Santa Cruz Biotechnology), myc-tag (Upstate, Billerica, MA), TRAF6 (Santa Cruz Biotechnology), UCHL1 (Cell Signaling), β-actin (Sigma, St. Louis, MO), proteasome 20S (Santa Cruz Biotechnology), PI3Kinase (Cell Signaling), horseradish peroxidase-conjugated anti-mouse and anti-rabbit secondary antibodies (Cell Signaling), and protein A/G horseradish peroxidase conjugated secondary antibody (Pierce, Rockford, IL) were purchased from the indicated sources. A smart pool of silencing RNA (siRNA) specific for GADD45a, TRAF6, UCHL1, or scrambled sequence was purchased from Dharmacon (Lafayette, CO). Other reagents used include the proteasome inhibitor MG132 (Boston Biochem, Cambridge, MA), DNA methylase inhibitor 5-aza-2’ deoxycytidine (5-aza-DC; Calbiochem, San Diego, CA), and PI3K inhibitors LY294002 and BAG 956 (EMD Biosciences, Rockland, MA).
Preclinical Model of VILI
Male 8- to 12-week-old wild-type (WT) C57BL/6 (Jackson Laboratory, Bar Harbor, ME) and GADD45a−/− mice (a gift of Dr. Michael O'Reilly, University of Rochester and Dr. Albert Fornace, Brigham and Women's Hospital) original 129/Ola background backcrossed onto a C57BL/6 background after eight generations as previously described (9) were administered inhaled isofluorane followed by intraperitoneal ketamine-acetylpromazine (150/15 mg/kg, respectively) before intubation with a 20-gauge angiocatheter and mechanical ventilation (Harvard Apparatus, Holliston, MA) with tidal volumes of 40 ml/kg, 65 breaths per minute, and positive end-expiratory pressure of 0 cm H2O for 4 hours. Ventilated mice were monitored for blood pressure and arterial blood gas to ensure adequate perfusion. Bronchoalveolar lavage (BAL) fluid was collected and indices of lung vascular leak were assessed as previously described (14). All animal experiments were approved by the Animal Care and Use Committees of the University of Illinois at Chicago, Chicago, IL.
Delivery of Akt1 Expression Vector In Vivo
c/a Akt1 mammalian expression vector (Millipore, Billercia, MA) in phosphate-buffered saline (50 μg) was given intratracheally to GADD45a−/− mice to induce lung overexpression of Akt1. The mice were subjected to mechanical ventilation (VT 40 ml/kg; 4 h) after 5 days of transfection. Control animals were administered a control vector.
Cell Culture and Model of Increased Mechanical Stress Via CS
Human pulmonary artery EC were cultured in growth medium (EGM-2) containing 10% fetal bovine serum (Clonetics, Walkersville, MD). Cells were grown in a 5% CO2 at 37°C and 95% humidity to achieve contact-inhibited monolayers. For CS experiments, EC were plated onto six-well silicone elastomer BioFlex plates coated with type I collagen (FlexCell International, Hillsborough, NC) and grown to confluence. Mechanical stretch was performed via the Flexcell Strain Unit (FX-3000; FlexCell International) placed in a 5% CO2 incubator at 37°C and 95% humidity. The device uses a controlled vacuum to produce 18% elongation at a frequency of 30 cycles per minute (0.5 Hz). Control Bioflex plates were kept in static condition in the same incubator. As we have previously reported, 18% CS corresponds to pathologically relevant levels of mechanical stress that result in phenotypic EC monolayer changes, increased susceptibility to barrier-disruptive agonists, but with preserved monolayer integrity even after prolonged exposure (48 h) (15).
Immunoblotting and Immunoprecipitation
Total proteins were extracted using NP-40 lysis buffer (50-mM Tris-HCl pH 7.4, 150-mM NaCl, 1% NP-40, and 5-mM ethylenediaminetetraacetic acid) supplemented with 0.4-mM sodium orthovanadate, 40-mM sodium fluoride, 0.2-mM phenylmethylsulfonyl fluoride, 10-mM N’ ethyl malamide, and cocktails of protease and phosphatase inhibitors (Calbiochem, San Diego, CA). Lung homogenates and cell lysates were briefly sonicated and were subjected to cycles of thawing and freezing on dry ice. The protein concentrations were measured using bicinchoninic acid protein assay kit (Pierce, Rockford, IL). Western blotting was performed using standard protocols and bands densities were determined using ImageJ (National Institutes of Health, http://imagej.nih.gov/ij/). Immunoprecipitation was performed by incubating protein extracts with primary antibodies (2 μg) overnight at 4°C. Immunocomplexes were collected by incubating with protein G or A Sepharose beads for 1 hour at 4°C and resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis followed by Western blotting.
Transfection of Human Lung EC
EC were transfected with pCMV myc-tagged mammalian expression vectors (Clontech, Mountain View, CA) containing WT or mutant ubiquitin with specific lysine residues 48 or 63 mutated to arginine (K48R or K63R, respectively) as we have previously described (16). Transfections were performed using Fugene 6 (Roche Diagnostic, Basel, Switzerland) in Opti-Mem medium (Invitrogen, Carlsbad, CA). The medium was changed to EGM-2 containing 2% fetal bovine serum after 4–5 hours of transfection and cells were used after 48 hours of transfection. Transfection of siRNA (100 nM) was performed using siPORT Amine transfection reagent (Ambion, Austin, TX) in serum-free conditions according to the manufacturer's protocol. Protein silencing was confirmed and cells were used after 72 hours of transfection.
Treatment of GADD45a−/− Mice with 5-aza-DC
The DNA methylase inhibitor 5-aza-DC was dissolved in 40% dimethyl sulfoxide and administered via intraperitoneal injection to GADD45a−/− mice. The mice were subjected to mechanical ventilation (VT 40 ml/kg; 4 h) 24 hours after drug administration. Control GADD45a−/− mice were administered 40% dimethyl sulfoxide alone.
RNA Isolation, Microarray Analysis, and Real-time Polymerase Chain Reaction
Total RNA was isolated from mouse lungs using Trizol (Invitrogen) and quantity was measured via NanoDrop spectrophotometer (NanoDrop Products, Wilmington, DE). Expression profiling was performed using Affymetrix Mouse 430 2.0 arrays and protocols (Affymetrix, Santa Clara, CA) as described previously (14). Chips were scanned using a GeneChip Scanner 3000 (Affymetrix). Chip quality and “present” calls were determined by Affymetrix GCOS software (Affymetrix). The chip data were normalized by “rank invariant set” method using dChip software (www.dchip.org). The differentially expressed genes between two experimental groups were identified using significance analysis of microarrays (17). The microarray data have been submitted to the NCBI's Gene Expression Omnibus database (GSE11662). cDNA was synthesized from total RNA and real-time polymerase chain reaction (RT PCR) was performed for Akt1, UCHL1, and GADD45a using ABI Prism 7700 Sequence Detector System (Applied Biosystems, Foster City, CA) according to the manufacturer's protocol. GAPDH was used as an endogenous control for normalization.
Immunofluorescent Imaging
EC were seeded on gelatin-coated coverslips in six-well plate or BioFlex stretch plates and then were silenced for UCHL1 (100 nM, 3 d) or subjected to 18% CS, respectively. Cells were fixed with 4% paraformaldehyde (pH 7.0) in phosphate-buffered saline for 20 minutes, permeabilized with 0.2% triton X-100, and blocked with 5% bovine serum albumin with goat serum for 1 hour. After overnight incubation at 4°C with primary antibodies cells were incubated with goat anti-rabbit Alexa Fluor 635 and goat anti-mouse Alexa Fluor 488 secondary antibodies. Nuclei were stained with Hoechst 33342 (Invitrogen) and coverslips were mounted on slides using mounting solution prolong gold (Invitrogen). To mount the cells on stretch plate, elastomer was cut and placed on the slide with cells facing up and were mounted with coverslip using prolong gold. Cells were then imaged using a Carl Zeiss LSM 510 laser scanning confocal microscope (Göttingen, Germany).
UCHL1 Methylation Assay
DNA was extracted from spontaneously breathing and VILI-challenged WT and GADD45a−/− mouse lungs using DNeasy Blood and Tissue kit (Qiagen, Valencia, CA). Bisulfite modification of DNA (500 ng) was performed using MethylCode Bisulfite conversion kit (Invitrogen) according to the manufacturer's protocol. Hypermethylated DNA (Zymo Research, Irvine, CA) was used as a control for bisulfite modification. Methyl-specific PCR was performed on bisulfite-modified DNA using methylated and nonmethylated primers. The primers were designed for the mouse UCHL1 locus 1,000 bp upstream of the translational start site using Methyl Primer Express v1.0 software (Applied Biosystems). Amplification reaction mixture contained 80 ng of template DNA, 1X PCR buffer (Invitrogen), 2 mM MgCl2, and 0.25 mM each dNTP and 40 pM of forward and reverse primer. The hot start PCR was performed for 35 cycles of denaturation at 93°C for 1 minute, annealing at 50°C for 45 seconds and extension at 72°C for 1 minute, followed by final extension at 72°C for 7 minutes. PCR products were resolved on 2% agarose gel and band intensity was observed in gel documentation system. The band densities were measured by software ImageJ.
Statistical Analysis
All results are expressed as mean ± SD. Statistical analysis was conducted using unpaired Student t test. Differences between experimental groups with P less than 0.05 were considered statistically significant.
Results
Role of Akt in the Increased Susceptibility of GADD45a−/− Mice to VILI
Previously, we reported increased susceptibility to VILI in GADD45a−/− mice and identified decreased lung expression of Akt associated with increased Akt ubiquitination and degradation (9). To determine the direct contribution of Akt loss to VILI susceptibility in GADD45a−/− mice, these animals were administered a c/a Akt1 mammalian expression vector delivered intratracheally before VILI challenge (high tidal volume mechanical ventilation, VT 40 ml/kg, 4 h). Immunoblots of lung homogenates confirmed overexpressed Akt in GADD45a−/− mice (Figure 1A). VILI-challenged GADD45a−/− mice with overexpressed Akt1 had significantly reduced BAL fluid total protein levels compared with VILI-challenged GADD45a−/− mice administered a control vector (Figure 1B), although BAL cell counts were not significantly different between these two groups. These data suggest a direct contributory role of Akt degradation in the exaggerated inflammatory phenotype in VILI-susceptible GADD45a−/− mice.
Figure 1.
Expression of a constitutively active Akt1 transgene in growth arrest and DNA damage–inducible α (GADD45a)−/− mice reverses ventilator-induced lung injury (VILI) susceptibility. (A) Western blots of lung homogenates from GADD45a−/− mice confirm increased Akt1 expression after intratracheal administration of constitutively active Akt1 mammalian expression vector (50 μg/mg × 5 d) in spontaneously breathing (SB) and VILI-challenged (VT 40 ml/kg, 4 h) GADD45a−/− mice. (B) Bronchoalveolar lavage (BAL) fluid total protein levels assessed after VILI challenge in GADD45a−/− mice with overexpressed Akt are comparable with the levels detected in uninjured, spontaneous breathing animals (n = 3 per group; *P < 0.05). WT = wild-type.
Differential Effects of Mechanical Stress and GADD45a Depletion on Akt Phosphorylation
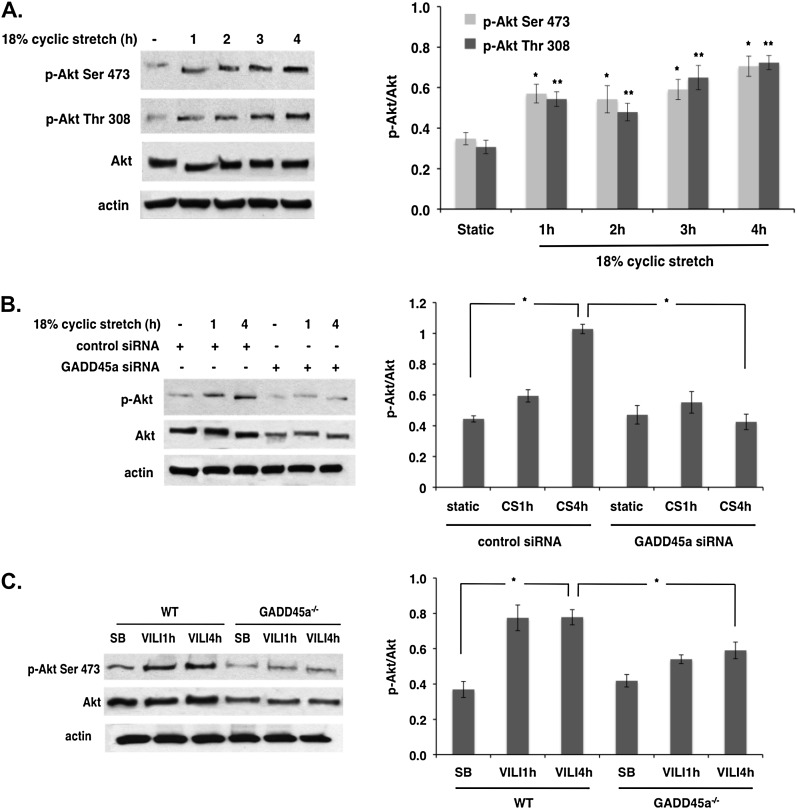
To explore the regulation of Akt by mechanical stress and GADD45a, EC cultured on BioFlex stretch plates were exposed to 18% CS as we have described (15). Compared with static cells, CS induced a significant increase in the levels of Akt phosphorylation at Ser 473 and Thr 308 in a time-dependent manner (1–4 h) (Figure 2A). In companion experiments, EC were transfected with siRNA specific for GADD45a (siGADD45a) and cultured on BioFlex plates before being subjected to 18% CS. Western blotting revealed significantly reduced phospho-Akt levels in GADD45a-silenced cells compared with unsilenced cells in response to 18% CS at 4 hours (Figure 2B) with total Akt levels also reduced in GADD45a-silenced cells. Consistent with our in vitro results, WT mice subjected to high tidal volume ventilation (VT 40 ml/kg) exhibited increased levels of phosphorylated Akt in whole-lung homogenates beginning at 1 hour and sustained at 4 hours (Figure 2C). This in vivo effect of mechanical stress on Akt phosphorylation was dose-dependent with the mechanical stress associated with VT 40 ml/kg producing greater Akt phosphorylation and activation than VT 30 ml/kg (9). These stress-induced alterations in Akt phosphorylation were markedly blunted in GADD45a−/− mice with concomitant reductions in the levels of total Akt expression.
Figure 2.
Differential effects of mechanical stress and growth arrest and DNA damage–inducible α (GADD45a) silencing on Akt phosphorylation. (A) Human pulmonary artery endothelial cell (EC) grown to confluence on Bioflex stretch plates were subjected to 18% cyclic stretch (CS) for 1–4 hours. Western blot shows significant time-dependent increases in phospho-Akt at Ser473 and Thr308 in response to CS compared with static controls. Densitometery confirms a relative increase in phospho-Akt levels compared with total Akt (n = 3 per condition; *P < 0.05 and **P < 0.05 compared with respective static controls). (B) EC transfected with GADD45a and control silencing RNA (100 nM, 3 d) were subjected to 18% CS (1 and 4 h). Immunoblots show significantly reduced Akt phosphorylation in response to CS in GADD45a-silenced EC compared with controls. (C) In separate experiments, wild-type (WT) and GADD45a−/− mice were subjected to ventilator-induced lung injury (VILI) (VT 40 ml/kg, 1 and 4 h). Immunoblots from whole-lung homogenates shows significantly increased phospho-Akt levels at 4 hours in WT animals compared with spontaneously breathing (SB) control animals, whereas both total and phospho-Akt levels are significantly reduced in VILI-challenged GADD45a−/− mice compared with VILI-challenged WT animals (n = 3 per group; *P < 0.05).
Role of Ubiquitination in Akt Regulation by GADD45a and Mechanical Stress
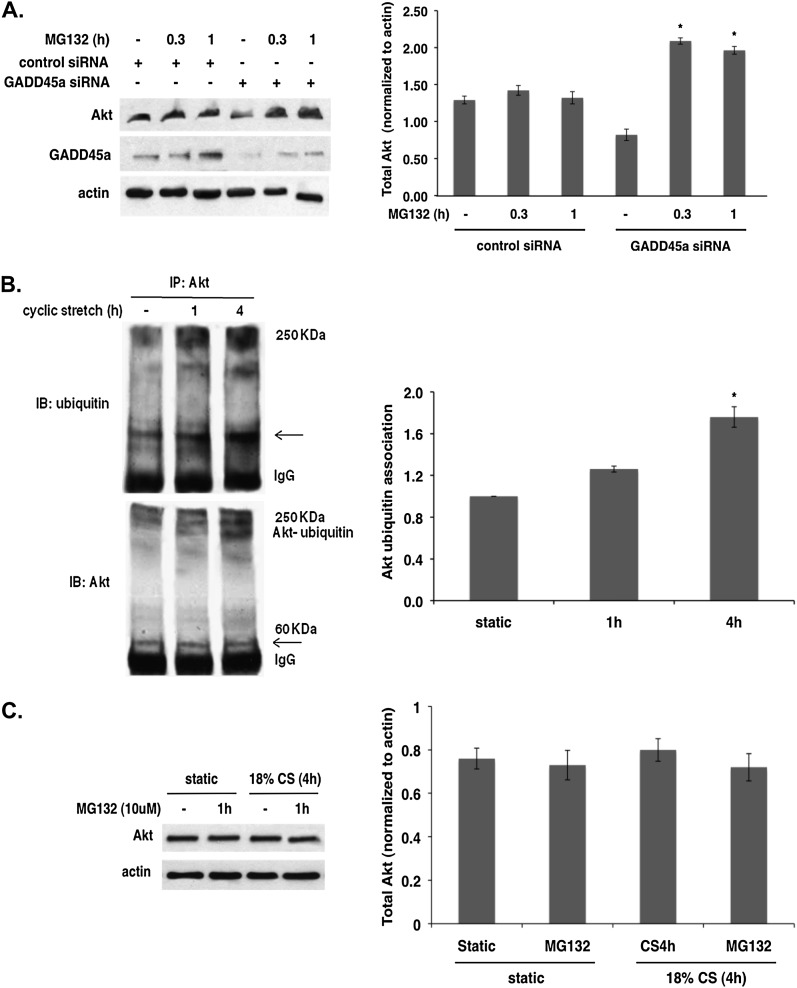
To determine if decreased Akt levels in GADD45a-silenced EC were caused by increased ubiquitination and subsequent proteasomal degradation of Akt, a pharmacologic inhibitor of proteasomal proteolytic activity, MG132, was used. Treatment with MG132 (10 μM) significantly restored the reduced Akt levels in GADD45a-silenced cells after 20 minutes (Figure 3A) confirming that increased Akt ubiquitination in GADD45a−/− mice leads to proteasomal degradation and represents a key mechanism of decreased Akt levels in these animals.
Figure 3.
Akt ubiquitination in growth arrest and DNA damage–inducible α (GADD45a) silenced and cyclic stretched endothelial cell (EC). (A) EC transfected with GADD45a and control silencing RNA (siRNA; 100 nM, 3 d) were treated with MG132 (10 μM, 20 min and 1 h), a potent proteasome inhibitor. Immunoblots show reduced Akt levels in GADD45a-silenced cells are significantly restored after MG132 treatment consistent with increased Akt proteasomal degradation in GADD45a-silenced cells (*P < 0.05 compared with GADD45a-silenced controls). (B) Immunoprecipitates of Akt from lysates of EC subjected to 18% cyclic stretch (CS) (4 h) immunoblotted for ubiquitin show increased binding of ubiquitin with Akt after 4-hour CS (n = 3 per condition; *P < 0.05). Ubiquitin levels were normalized to total Akt and densitometery results expressed as fold change relative to static cells. Similarly, immunoblotting for Akt reveals increased ubiquitin-bound Akt after 4-hour CS compared with static controls. (C) EC were treated with MG132 (10 μM, 1 h) followed by 18% CS (4 h). Mechanically stretched EC treated with MG132 show no changes in total or phospho-Akt levels suggesting that CS-dependent Akt ubiquitination does not lead to increased proteasomal degradation of Akt.
To assess the role of ubiquitination in Akt activation by mechanical stress, Akt was immunoprecipitated from EC lysates after exposure to 18% CS and immunoblotted for ubiquitin and Akt. These studies revealed significantly increased binding of ubiquitin to Akt in response to CS at 4 hours (Figure 3B). Furthermore, to determine if increased Akt ubiquitination on CS leads to proteasomal degradation of Akt, mechanically stretched EC were treated with MG132 (10 μM, 1 h). Treatment with MG132 did not alter the levels of total Akt or phospho-Akt compared with untreated control EC (Figure 3C) suggesting that Akt ubiquitination induced by mechanical stress directs a different fate of Akt.
Site-specific Ubiquitination of Akt in Response to Mechanical Stress
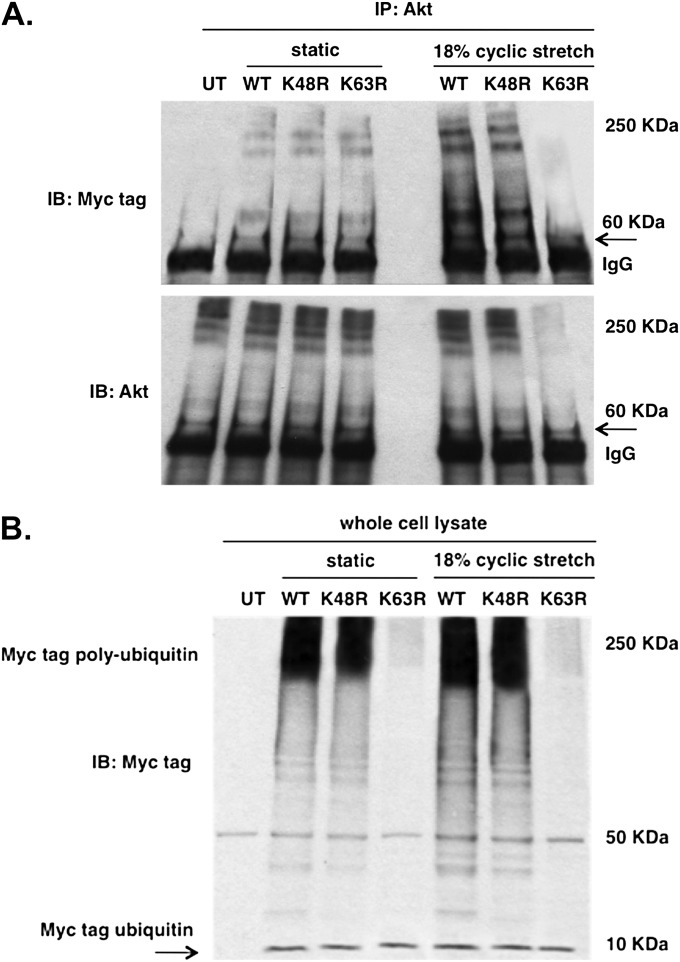
To characterize site-specific ubiquitination of Akt in CS-challenged cells, EC were transfected with myc-tagged WT or mutant ubiquitin vectors with specific lysine residues (K48 or K63) mutated to arginine. Immunoprecipitates of Akt from transfected EC subjected to mechanical stretch revealed abrogation of CS-induced Akt ubiquitination by the mutant K63R ubiquitin vector, although total Akt levels were unchanged (Figure 4A). Moreover, enhanced Akt-ubiquitin binding was observed after immunoblotting with myc tag in EC transfected with either WT or mutant K48R ubiquitin vector that were subjected to mechanical stretch compared with static controls. These results suggest that increased Akt ubiquitination in the presence of mechanical stress is characterized by K63-linked ubiquitin chains, findings consistent with the known role for K63 ubiquitination in the regulation of signal activation (18).
Figure 4.
Site-specific Akt ubiquitination in response to mechanical stress. (A) Endothelial cells (EC) were transfected with wild-type (WT), mutant K63R, or K48R ubiquitin myc-tagged expression vectors and subjected to static conditions or 18% cyclic stretch (CS; 4 h). Immunoprecipitates of Akt from cell lysate were immunoblotted with myc tag antibody reveal abrogated ubiquitin binding with Akt in cells expressing mutant K63R that were subjected to CS, whereas EC transfected with WT and mutant K48R ubiquitin vectors show enhanced ubiquitin binding with Akt in response to CS compared with static controls. Immunoblotting with Akt shows no binding of Akt with ubiquitin in K63R expressing cells as seen by the absence of Akt bands in the high-molecular-weight region. (B) Western blotting of whole-cell lysates for myc-tag (7 kD) confirms comparable transfection efficiency across conditions.
Role of TRAF6 in K63-linked Ubiquitination of Akt Induced by Mechanical Stress
To determine the functional role of K63 ubiquitination of Akt in response to CS we investigated a potential role for TRAF6, an E3 ligase that is known to catalyze K63-linked ubiquitination of Akt leading to membrane localization and phosphorylation of Akt (19). TRAF6 immunoprecipitated from EC lysates subjected to mechanical stress and then immunoblotted for total Akt and phosphorylated Akt revealed significantly increased binding of TRAF6 with both total Akt and phospho-Akt after exposure to 18% CS (4 h, densitometry shown in Figure 5A). Reciprocal immunprecipitates of Akt followed by immunoblotting for TRAF6 confirmed these results (Figure 5A). Consistent with TRAF6-mediated K63-linked Akt ubiquitination and phosphorylation, EC transfected with siRNA specific for TRAF6 demonstrated significantly attenuated Akt phosphorylation in response to mechanical stretch compared with unsilenced control cells (Figure 5B). Further, immunofluorescence images of mechanically stretched cells revealed increased Akt phosphorylation after 18% CS (1 h) and colocalization of phospho-Akt with PI3K (Figure 5C). In addition, immunoprecipitation of PI3K from EC subjected to 18% CS (1 h) confirmed an increased association with both phosphorylated and total Akt compared with static controls (Figure 5D). Finally, to study the role of PI3K and PI3K-dependent kinase1 (PDK1) in increased Akt phosphorylation induced by mechanical stretch, EC pretreated with LY294002 (10–25 μM), a PI3K inhibitor, or BAG 956 (10–25 μM), a dual PI3K and PDK1 inhibitor, were subjected to 18% CS (1 h). Both inhibitors significantly reduced CS-induced Akt phosphorylation consistent with PI3K/PDK1-dependent Akt activation in response to mechanical stress.
Figure 5.
Tumor necrosis factor receptor–associated factor 6 (TRAF6) mediates Akt K63 ubiquitination and phosphorylation in response to mechanical stress. (A) Akt and TRAF6 were immunoprecipated in separate experiments from endothelial cells (EC) subjected to 18% cyclic stretch (CS; 1 and 4 h) and immunoblotted with TRAF6 and Akt antibodies. Compared with static controls, TRAF6 was up-regulated at 4 hours and found to associate with Akt, whereas phospho-Akt levels were significantly increased at this same time point. Densitometery confirmed a nearly twofold increase in TRAF6 and Akt association in response to CS (results shown are from immunoprecipitation of Akt followed by immunoblottoing of TRAF6 and are normalized to total Akt; n = 3 per condition; *P < 0.05 compared with static controls). (B) In subsequent experiments, EC transfected with silencing RNA (siRNA) specific for TRAF6 (100 nM, 3 d) were subjected to 18% CS (1 and 4 h). Compared with unsilenced controls, Akt phosphorylation induced by CS was significantly attenuated in TRAF6-silenced EC under CS conditions. (C) Immunofluorescence of EC reveals CS (1 h) -induced increase in phospho-Akt levels and colocalization with PI3K (merge, bottom panel) consistent with increased Akt phosphorylation by CS. In contrast, no colocalization was observed in static cells (merge, top panel). (D) PI3K immunoprecipitates from lysates of EC subjected to 18% CS (1 h) demonstrate increased association of phosphorylated and total Akt compared with static controls. (E) EC pretreated with LY294002 or BAG956 (10–25 μM) and then subjected to 18% CS (1 h) exhibit reduced mechanical stress-induced Akt phosphorylation compared with untreated controls.
Differential Expression of Genes Involved in Ubiquitin Metabolism after Mechanical Stress and GADD45a Silencing
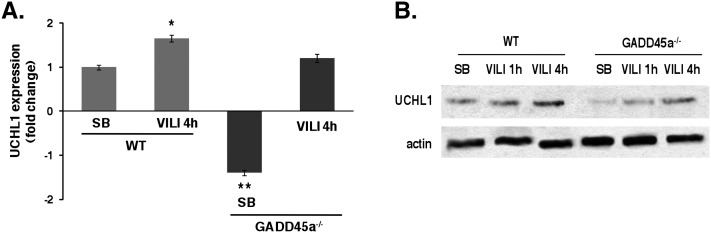
We next searched for genes involved in the differential, site-specific ubiquitination of Akt associated with mechanical stress and GADD45a depletion. Microarray gene expression profiles from lung homogenates of WT and GADD45a−/− mice identified 47 significantly up-regulated genes and 22 genes significantly down-regulated in response to VILI. Filtering of differentially expressed genes in VILI mice and GADD45a−/− mice led to the identification of UCHL1 as the most prominently differentially regulated DUB, significantly down-regulated in spontaneously breathing GADD45a−/− mice compared with WT animals and significantly up-regulated in response to VILI in both WT and GADD45a−/− mice compared with spontaneously breathing controls (see Figure E1 in the online supplement). These array findings were confirmed by real-time PCR (Figure 6A) and by measurement of UCHL1 protein levels in lungs of spontaneously breathing and VILI-challenged WT and GADD45a−/− mice (Figure 6B).
Figure 6.
Differential expression of ubiquitin carboxyl terminal hydrolase 1 (UCHL1) in ventilator-induced lung injury (VILI) challenged wild-type (WT) and growth arrest and DNA damage–inducible α (GADD45a)−/− mice. (A) Real-time polymerase chain reaction was performed on RNA isolated from lung tissue of spontaneously breathing (SB) and VILI-challenged (VT 40 ml/kg, 4 h) WT and GADD45a−/− mice. UCHL1 expression is significantly up-regulated in WT VILI-challenged mice and down-regulated in GADD45a−/− SB mice (* P < 0.05; n = 3 per group). UCHL1 is restored in VILI-challenged GADD45a−/− mice to an expression level comparable with basal UCHL1 levels in SB WT mice. These data are consistent with previous microarray data from lung tissue of SB and VILI-challenged (VT 40 ml/kg, 4h) WT and GADD45a−/− mice (see Figure E1 in the online supplement). (B) Western blotting of lung homogenates from SB and VILI-challenged WT and GADD45a−/− mice reveals markedly elevated protein levels of UCHL1 at 4 hours VILI in WT animal, whereas GADD45a−/− mice show relatively reduced UCHL1 protein levels, consistent with the real-time polymerase chain reaction data.
GADD45a Regulates Akt Activation via UCHL1
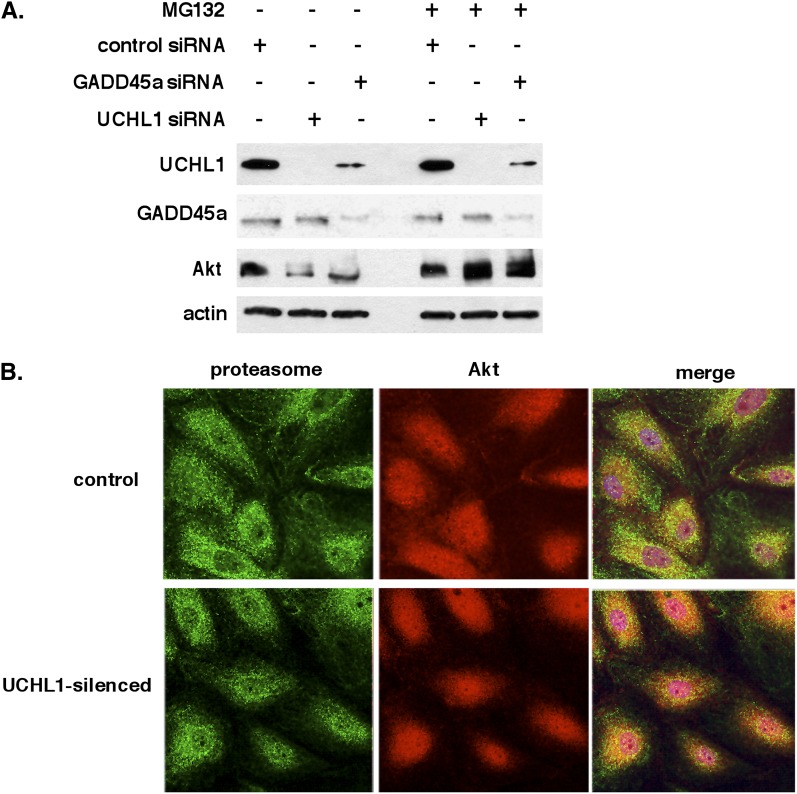
The potential role of UCHL1 in regulating Akt-bound ubiquitin chains was assessed by silencing of either GADD45a or UCHL1 in EC, which produced comparable decreases in total Akt levels. Reduced Akt levels were restored after treatment with MG132 in either GADD45a- or UCHL1-silenced cells (Figure 7A). Consistent with in vivo findings, silencing of GADD45a in EC produced significant reductions in UCHL1 protein levels (see Figure E2). Immunofluorescence images of UCHL1-silenced cells revealed increased colocalization of Akt and proteasomes (proteasome subunit 20S) in UCHL1-silenced cells compared with unsilenced controls (Figure 7B). These data indicate that UCHL1 maintains Akt levels by modulating K48 ubiquitination of Akt and thereby retarding proteasomal degradation.
Figure 7.
Role of ubiquitin carboxyl terminal hydrolase 1 (UCHL1) and proteasomal degradation in Akt regulation by growth arrest and DNA damage–inducible α (GADD45a). (A) Western blots of endothelial cell (EC) transfected with silencing RNA (siRNA) specific for GADD45a or UCHL1 (100 nM, 3 d) reveal comparable decreases in total Akt levels compared with controls that were similarly reversed by treatment with the proteasomal inhibitor MG132 (10 μM, 1 h). Notably, silencing of GADD45a was also associated with a significant decrease in UCHL1 protein levels. (B) Immunofluorescence of EC reveals a minimal association of Akt with proteasome (20S) under control conditions (merge, top panel). Conversely, in EC transfected with siRNA specific for UCHL1 (100 nM, 3 d) there is a marked colocalization of Akt with proteasomes (indicated by yellow in the merge image, lower panel) consistent with Akt proteasomal degradation in UCHL1-silenced cells.
GADD45a Regulation of UCHL1 Via Promoter Methylation
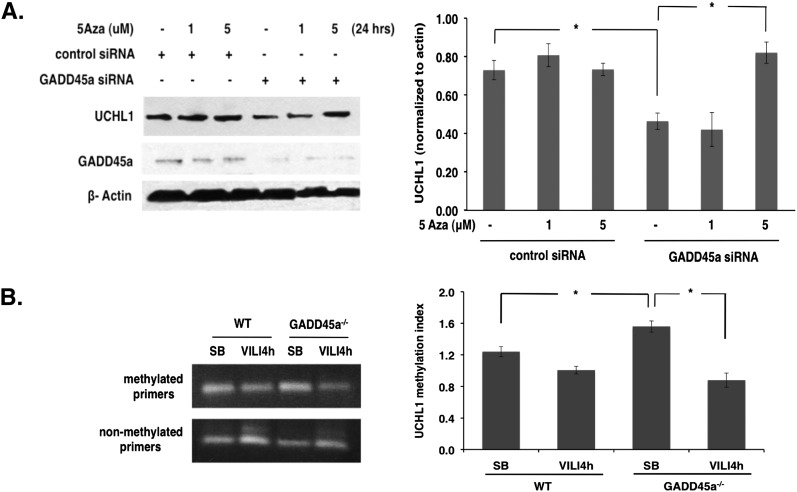
UCHL1 expression is potentially regulated by promoter methylation (20) and GADD45a is known to function as a global demethylase (21). Investigation of promoter methylation as a potential mechanism for GADD45a regulation of UCHL1 expression via treatment with 5-aza-DC (1–5 μM, 24 h), an inhibitor of DNA methylase activity, revealed significant restoration of UCHL1 levels in GADD45a-silenced EC compared with GADD45a-silenced control cells (Figure 8A). These results are consistent with increased DNA methylase activity in GADD45a-silenced cells resulting in increased UCHL1 promoter methylation and the observed decreased UCHL1 expression. In companion studies, increased UCHL1 promoter methylation was identified in the lungs of spontaneously breathing GADD45a−/− animals compared with WT animals (detected by methyl-specific UCHL1 promoter PCR using bisulfate-modified DNA), whereas comparable levels were detected in both groups after VILI-challenge (Figure 8B). These results further implicate hypermethylation as the mechanism responsible for reduced expression of UCHL1 under conditions of GADD45a depletion.
Figure 8.
Ubiquitin carboxyl terminal hydrolase 1 (UCHL1) regulation and demethylation by growth arrest and DNA damage–inducible α (GADD45a). (A) Compared with control cells, Western blotting of endothelial cell (EC) transfected with silenced GADD45a (100 nm, 3 d) demonstrates decreased UCHL1 expression, which is restored by treatment with 5-aza-2’ deoxycytidine (1–5 μM; 24 h; *P < 0.05; n = 3 per condition). (B) Methyl-specific polymerase chain reaction was performed on bisulfate-modified DNA from lung homogenates of wild-type (WT) and GADD45a−/− mice. GADD45a−/− mice demonstrate increased UCHL1 methylation under conditions of spontaneous breathing (SB) compared with WT animals (*P < 0.05; n = 3 per group). No difference is noted between groups in response to ventilator-induced lung injury challenge (VT 40 ml/kg; 4 h). siRNA = silenced RNA.
Treatment with 5-aza-DC Attenuates VILI Susceptibility of GADD45a−/− Mice
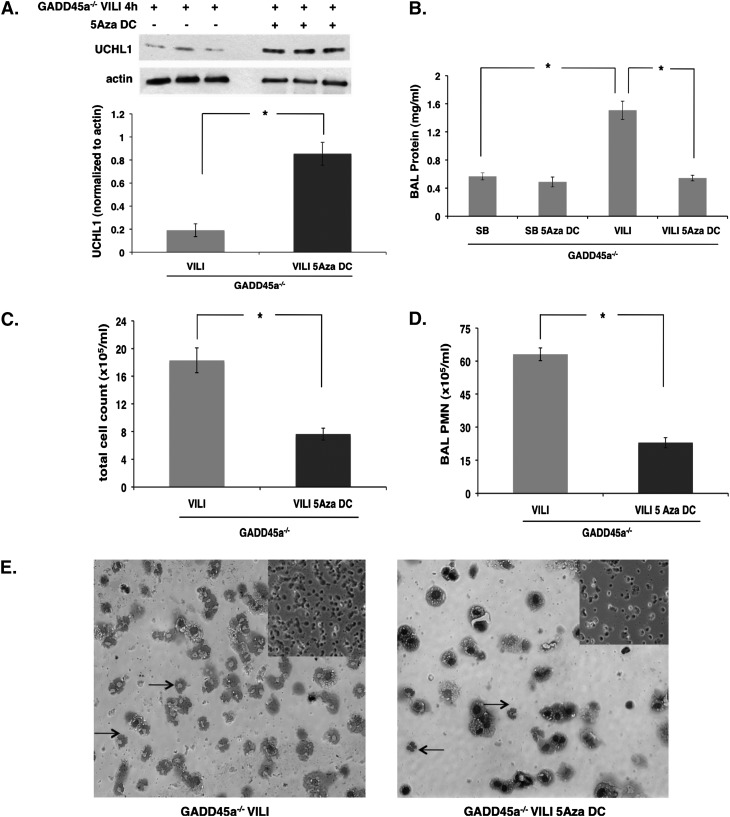
Finally, to firmly link increased DNA methylation to the increased VILI susceptibility of GADD45a−/− mice, these animals were treated with the DNA methylase inhibitor 5-aza-DC (5 mg/kg) or vehicle 24 hours before VILI-challenge (VT 40 ml/kg, 4 h). Consistent with our in vitro findings, Western blots of lung homogenates demonstrated increased UCHL1 expression in animals treated with 5-aza-DC compared with vehicle-treated GADD45a−/− controls (Figure 9A). In addition, compared with the GADD45a−/− mice treated with vehicle, animals treated with 5-aza-DC exhibited significantly reduced BAL fluid total protein levels, total cell counts, and polymorphonuclear cells (Figure 9B-D). The BAL fluid cell counts and polymorphonuclear cells were further supported by findings in the BAL fluid cytospin (Figure 9E). These data are consistent with our in vitro findings and support DNA hypermethylation as a key mechanism underlying increased VILI susceptibility of GADD45a−/− mice.
Figure 9.
Role of DNA methylation in ventilator-induced lung injury (VILI) susceptibility of growth arrest and DNA damage–inducible α (GADD45a)−/− mice. (A) Western blots of lung homogenates from VILI-challenged (VT 40 ml/kg, 4 h) GADD45a−/− mice demonstrate increased ubiquitin carboxyl terminal hydrolase 1 (UCHL1) expression in response to 5-aza-deoxycytidine treatment (5-aza-DC; 5 mg/Kg, intraperitoneal administration 24 h before mechanical ventilation). (B) 5-aza-DC treatment has no effect on bronchoalveolar lavage (BAL) fluid total protein levels in spontaneously breathing (SB) GADD45a−/− mice but does result in a significant attenuation in VILI-challenged GADD45a−/− mice. (C and D) Similarly, BAL fluid total cell counts and polymorphonuclear cells (PMNs) were significantly reduced in VILI-challenged GADD45a−/− mice treated with 5-aza-DC compared with vehicle-treated controls. (E) Consistent with these results, representative cytospin of BAL cells demonstrates significantly reduced total cells and PMNs (arrows) after 5-aza-DC treatment in VILI-challenged GADD45a−/− mice compared with vehicle-treated controls (original magnification ×20; inset original magnification ×10). (n = 3 per group; *P < 0.05).
Discussion
Using an approach relying on orthologous gene expression profiling, we previously identified GADD45a as a potential ALI candidate gene (22). In relevant in vitro and in vivo models, we now link mechanical stress-induced GADD45a expression to novel effects on ubiquitination components, including UCHL1 and TRAF6, and subsequent regulation of Akt phosphorylation (Figure 10). Our findings strongly implicate GADD45a as a critical determinant of lung responses to VILI via regulation of the serine-threonine protein kinase, Akt (also known as protein kinase B). Akt is involved in a number of cell processes including cell growth, proliferation, survival (23); angiogenesis; vascular maturation; regulation of EC permeability; and EC nitric oxide synthase activity (24, 25). We previously demonstrated the direct contribution of Akt to hepatocyte growth factor–mediated enhancement of lung vascular barrier properties during lung inflammation via GSK3-B activation (26). Evidence also supports an important role for Akt in the pathogenesis of VILI because pharmacologic inhibition of Akt results in augmented microvascular permeability in overdistended isolated mouse lungs (11). Having established that GADD45a depletion correlates with both decreased Akt expression and VILI severity, we have now confirmed a functional role for Akt in our murine model of VILI because VILI-challenged GADD45a−/− mice overexpressing a c/a Akt1 transgene showed a significant reduction in lung injury compared with VILI-challenged GADD45a−/− mice administered a control vector. These data suggest that altered Akt regulation is an essential mechanism in the linkage between GADD45a expression and pulmonary responses to excessive mechanical stress.
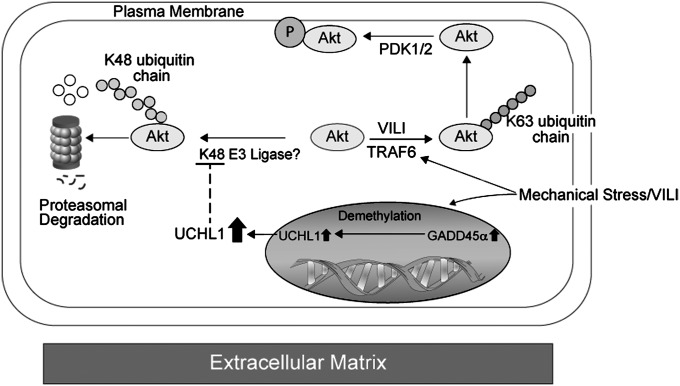
Figure 10.
Proposed regulation of Akt by mechanical stress and growth arrest and DNA damage–inducible α (GADD45a). Site-specific ubiqutination of Akt is differentially regulated by mechanical stress and GADD45a. Mechanical stress induces E3 ligase, tumor necrosis factor receptor–associated factor 6 (TRAF6), mediated K63-linked ubiquitination of Akt with subsequent trafficking to the cell membrane, and activation of Akt via PDK1/2. Mechanical stress also induces the up-regulation of GADD45a, which in turn drives the up-regulation of the deubiqutinating enzyme, ubiquitin carboxyl terminal hydrolase 1 (UCHL1), via increased demethylation of the UCHL1 promoter. UCHL1 inhibits K48-linked ubiquitination of Akt thereby attenuating trafficking of Akt to proteasomes and subsequent degradation. Our data suggest that via these pathways mechanical stress favors K63 ubiqutination of Akt and downstream Akt activation. Reductions in GADD45a availability have overriding effects on Akt via K48-linked ubiquitination and resulting in increased proteasomal degradation basally and results in significant attenuation of mechanical stress-induced Akt activation. VILI = ventilator-induced lung injury.
Protein ubiquitination, a covalent reaction that attaches ubiquitin, a 76–amino acid polypeptide, to one or more lysine residues is an important post-translational modification often associated with degradation of proteins and has emerged as a novel mechanism that regulates various cellular events ranging from apoptosis to intracellular protein trafficking and kinase activation (27, 28). Ubiquitination in turn is regulated by the sequential activity of ubiquitin-activating enzymes, ubiquitin-conjugating enzymes, and ubiquitin ligases, referred to as E1, E2, and E3, respectively. In addition, ubiquitination can be reversed by DUBs that remove ubiquitin chains from proteins. There are seven lysine residues within ubiquitin allowing for site-specific ubiquitination to occur at K6, K11, K27, K29, K33, K48, or K63 with variable consequences. For example, K48-linked ubiquitination targets proteins for proteasomal degradation, whereas K63-linked ubiquitination regulates protein trafficking and signaling (28).
Our data indicate that under homeostatic conditions Akt is phosphorylated in response to mechanical stress, an event that is associated with increased K63-linked ubiquitination, a key event in Akt activation and modulation of increased vascular permeability and VILI. We have also found that GADD45a depletion, either by siRNA approaches or in knockout mice, is associated with decreased levels of total and phosphorylated Akt in conjunction with increased K48-linked ubiquitination. Moreover, Akt levels and phosphorylation levels are restored by proteasomal inhibition (MG132) implicating Akt ubiquitination with subsequent proteasomal degradation. Thus, although ubiquitination of Akt is poorly understood, our data support the idea that Akt phosphorylation levels are dependent on differential, site-specific Akt ubiquitination.
Two important mediators of Akt ubiquitination identified in our studies include TRAF6 and UCHL1. Akt undergoes K63-linked ubiquitination by the E3 ligase, TRAF6, which leads to membrane translocation of Akt with binding to membrane-bound PIP3 through the pleckstrin homology domain and subsequent phosphorylation (19). Akt phosphorylation induced by mechanical stress is linked to augmented TRAF6 expression and association with Akt, whereas reduced TRAF6 expression decreased levels of Akt phosphorylation by mechanical stress. More surprising, we found evidence of K48-linked ubiquitination of Akt associated with GADD45a depletion with proteasomal inhibition (MG132) restoring Akt levels in GADD45a-silenced EC. These effects are mediated by UCHL1, a DUB known to be silenced in a variety of cancers (20, 29, 30) and implicated in the pathogenesis of Parkinson disease (31) but only recently linked to effects on Akt signaling (32). Our results now extend these previous findings and demonstrate for the first time evidence that total Akt expression levels are mediated directly by UCHL1. Moreover, we have identified demethylation of the UCHL1 promoter as the mechanism by which GADD45a regulates UCHL1 expression. Evidence that these findings are clinically relevant is suggested by decreased VILI susceptibility in GADD45a−/− mice treated with the DNA methylase inhibitor 5-aza-DC.
Our data are consistent with differential effects of GADD45a depletion and mechanical stress on Akt activation driven by site-specific ubiquitination of Akt. We speculate that basal levels of ubiquitinated Akt involve multiple sites including K48 and K63 but can be induced to favor K63-linked ubiquitination via the up-regulation of TRAF6 in response to mechanical stress. Conversely, GADD45a depletion is associated with increased K48-linked ubiquitination via the decreased expression of UCHL1 (29). The combined effects of GADD45a depletion and mechanical stress, however, reflect an overriding influence of decreased GADD45a in this context characterized by decreased Akt levels. Indeed, in support of this we have now linked decreased Akt levels directly to the increased susceptibility to VILI of GADD45a−/− mice. Evidence of differential regulation of other proteins via polyubiqutination involving both K48 and K63 has previously been described. For example, both RIP1, a mediator of tumor necrosis factor–induced nuclear factor-κB activation, and IRAK1, involved in IL-1β and Toll-like receptor signaling, are K63 ubiquitinated, but may also undergo K48-linked ubiquitination, which then targets them for proteasomal degradation (33). Our results now identify for the first time that Akt is similarly regulated via the effects of mechanical stress and GADD45a depletion.
Consistent with the clinical implications of this work, our preliminary findings suggest that patients with polymorphisms in GADD45a gene and aberrant expression of GADD45a are at increased risk for developing ALI-VILI (data not shown). GADD45a, Akt, TRAF6, and UCHL1 represent novel ALI-VILI candidate genes that warrant further investigations.
Acknowledgments
The authors are thankful to Ms. Lakshmi Natarajan, Ms. Carrie Evenoski, and Ms. Sara Camp for their excellent technical assistance.
Footnotes
Author Contributions: Conception and design, S.M. and J.G.N.G.; acquisition of data, S.M., S.S., D.L.B., N.J.M., S.M.D., and L.M.-V.; analysis and interpretation of data, S.M., J.G.N.G., and J.R.J.; drafting the article and revising it critically, S.M. and J.R.J.; final approval of the version to be published, J.G.N.G.
Originally Published in Press as DOI: 10.1164/rccm.201103-0447OC on August 18, 2011
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med 2005;353:1685–1693 [DOI] [PubMed] [Google Scholar]
- 2.Gajic O, Dara SI, Mendez JL, Adesanya AO, Festic E, Caples SM, Rana R, St Sauver JL, Lymp JF, Afessa B, et al. Ventilator-associated lung injury in patients without acute lung injury at the onset of mechanical ventilation. Crit Care Med 2004;32:1817–1824 [DOI] [PubMed] [Google Scholar]
- 3.Moss M, Mannino DM. Race and gender differences in acute respiratory distress syndrome deaths in the United States: an analysis of multiple-cause mortality data (1979–1996). Crit Care Med 2002;30:1679–1685 [DOI] [PubMed] [Google Scholar]
- 4.Flores C, Ma SF, Maresso K, Wade MS, Villar J, Garcia JG. IL6 gene-wide haplotype is associated with susceptibility to acute lung injury. Transl Res 2008;152:11–17 [DOI] [PubMed] [Google Scholar]
- 5.Gao L, Flores C, Fan-Ma S, Miller EJ, Moitra J, Moreno L, Wadgaonkar R, Simon B, Brower R, Sevransky J, et al. Macrophage migration inhibitory factor in acute lung injury: expression, biomarker, and associations. Transl Res 2007;150:18–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao L, Grant A, Halder I, Brower R, Sevransky J, Maloney JP, Moss M, Shanholtz C, Yates CR, Meduri GU, et al. Novel polymorphisms in the myosin light chain kinase gene confer risk for acute lung injury. Am J Respir Cell Mol Biol 2006;34:487–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye SQ, Simon BA, Maloney JP, Zambelli-Weiner A, Gao L, Grant A, Easley RB, McVerry BJ, Tuder RM, Standiford T, et al. Pre-B-cell colony-enhancing factor as a potential novel biomarker in acute lung injury. Am J Respir Crit Care Med 2005;171:361–370 [DOI] [PubMed] [Google Scholar]
- 8.Altemeier WA, Matute-Bello G, Gharib SA, Glenny RW, Martin TR, Liles WC. Modulation of lipopolysaccharide-induced gene transcription and promotion of lung injury by mechanical ventilation. J Immunol 2005;175:3369–3376 [DOI] [PubMed] [Google Scholar]
- 9.Meyer NJ, Huang Y, Singleton PA, Sammani S, Moitra J, Evenoski CL, Husain AN, Mitra S, Moreno-Vinasco L, Jacobson JR, et al. GADD45a is a novel candidate gene in inflammatory lung injury via influences on Akt signaling. FASEB J 2009;23:1325–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lionetti V, Lisi A, Patrucco E, De Giuli P, Milazzo MG, Ceci S, Wymann M, Lena A, Gremigni V, Fanelli V, et al. Lack of phosphoinositide 3-kinase-gamma attenuates ventilator-induced lung injury. Crit Care Med 2006;34:134–141 [DOI] [PubMed] [Google Scholar]
- 11.Miyahara T, Hamanaka K, Weber DS, Drake DA, Anghelescu M, Parker JC. Phosphoinositide 3-kinase, Src, and Akt modulate acute ventilation-induced vascular permeability increases in mouse lungs. Am J Physiol Lung Cell Mol Physiol 2007;293:L11–L21 [DOI] [PubMed] [Google Scholar]
- 12.Mitra S, Sammani S, Wang T, Meyer NJ, Ahn J, Mirzapoiazova T, Unzueta C, Evenoski C, Ma SF, Moreno-Vinasco L, et al. Role of ubiquitin proteasome pathway activation in Akt signaling in VILI-challenged GADD45a−/−mice [abstract]. Am J Respir Crit Care Med 2010;181:A3749 [Google Scholar]
- 13.Mitra S, Sammani S, Dudek SM, Moreno-Vinasco L, Garcia JG, Jacobson JR. GADD45A mediates Akt signaling in mechanical stress-induced lung injury via demethylation [abstract]. Am J Respir Crit Care Med 2011;183:A3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nonas SA, Moreno-Vinasco L, Ma SF, Jacobson JR, Desai AA, Dudek SM, Flores C, Hassoun PM, Sam L, Ye SQ, et al. Use of consomic rats for genomic insights into ventilator-associated lung injury. Am J Physiol Lung Cell Mol Physiol 2007;293:L292–L302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Birukov KG, Jacobson JR, Flores AA, Ye SQ, Birukova AA, Verin AD, Garcia JG. Magnitude-dependent regulation of pulmonary endothelial cell barrier function by cyclic stretch. Am J Physiol Lung Cell Mol Physiol 2003;285:L785–L797 [DOI] [PubMed] [Google Scholar]
- 16.Boone DL, Turer EE, Lee EG, Ahmad RC, Wheeler MT, Tsui C, Hurley P, Chien M, Chai S, Hitotsumatsu O, et al. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat Immunol 2004;5:1052–1060 [DOI] [PubMed] [Google Scholar]
- 17.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA 2001;98:5116–5121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanayama A, Seth RB, Sun L, Ea CK, Hong M, Shaito A, Chiu YH, Deng L, Chen ZJ. TAB2 and TAB3 activate the NF-kappaB pathway through binding to polyubiquitin chains. Mol Cell 2004;15:535–548 [DOI] [PubMed] [Google Scholar]
- 19.Yang WL, Wang J, Chan CH, Lee SW, Campos AD, Lamothe B, Hur L, Grabiner BC, Lin X, Darnay BG. The E3 ligase TRAF6 regulates Akt ubiquitination and activation. Science 2009;325:1134–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fellenberg J, Lehner B, Witte D. Silencing of the UCHL1 gene in giant cell tumors of bone. Int J Cancer 2010;127:1804–1812 [DOI] [PubMed] [Google Scholar]
- 21.Barreto G, Schafer A, Marhold J, Stach D, Swaminathan SK, Handa V, Doderlein G, Maltry N, Wu W, Lyko F, et al. Gadd45a promotes epigenetic gene activation by repair-mediated DNA demethylation. Nature 2007;445:671–675 [DOI] [PubMed] [Google Scholar]
- 22.Grigoryev DN, Ma SF, Irizarry RA, Ye SQ, Quackenbush J, Garcia JG. Orthologous gene-expression profiling in multi-species models: search for candidate genes. Genome Biol 2004;5:R34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell 2007;129:1261–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen J, Somanath PR, Razorenova O, Chen WS, Hay N, Bornstein P, Byzova TV. Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo. Nat Med 2005;11:1188–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 1999;399:601–605 [DOI] [PubMed] [Google Scholar]
- 26.Liu F, Schaphorst KL, Verin AD, Jacobs K, Birukova A, Day RM, Bogatcheva N, Bottaro DP, Garcia JG. Hepatocyte growth factor enhances endothelial cell barrier function and cortical cytoskeletal rearrangement: potential role of glycogen synthase kinase-3beta. FASEB J 2002;16:950–962 [DOI] [PubMed] [Google Scholar]
- 27.Mukhopadhyay D, Riezman H. Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science 2007;315:201–205 [DOI] [PubMed] [Google Scholar]
- 28.Pickart CM. Mechanisms underlying ubiquitination. Annu Rev Biochem 2001;70:503–533 [DOI] [PubMed] [Google Scholar]
- 29.Li L, Tao Q, Jin H, van Hasselt A, Poon FF, Wang X, Zeng MS, Jia WH, Zeng YX, Chan AT, et al. The tumor suppressor UCHL1 forms a complex with p53/MDM2/ARF to promote p53 signaling and is frequently silenced in nasopharyngeal carcinoma. Clin Cancer Res 2010;16:2949–2958 [DOI] [PubMed] [Google Scholar]
- 30.Okochi-Takada E, Nakazawa K, Wakabayashi M, Mori A, Ichimura S, Yasugi T, Ushijima T. Silencing of the UCHL1 gene in human colorectal and ovarian cancers. Int J Cancer 2006;119:1338–1344 [DOI] [PubMed] [Google Scholar]
- 31.Ragland M, Hutter C, Zabetian C, Edwards K. Association between the ubiquitin carboxyl-terminal esterase L1 gene (UCHL1) S18Y variant and Parkinson's disease: a HuGE review and meta-analysis. Am J Epidemiol 2009;170:1344–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hussain S, Foreman O, Perkins SL, Witzig TE, Miles RR, van Deursen J, Galardy PJ. The de-ubiquitinase UCH-L1 is an oncogene that drives the development of lymphoma in vivo by deregulating PHLPP1 and Akt signaling. Leukemia 2010;24:1641–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newton K, Matsumoto ML, Wertz IE, Kirkpatrick DS, Lill JR, Tan J, Dugger D, Gordon N, Sidhu SS, Fellouse FA, et al. Ubiquitin chain editing revealed by polyubiquitin linkage-specific antibodies. Cell 2008;134:668–678 [DOI] [PubMed] [Google Scholar]