Abstract
A biphasic culture medium suitable for cultivation and sporulation of Clostridium perfringens, C. botulinum, and C. sporogenes was devised. The medium designed for use in a disposable, compartmented, plastic film container contained peptones, yeast extract, minerals, an anion exchange resin, and glucose in 4% agar as the solid phase and (NH4)2SO4 and 0.1% agar as the liquid phase. With the biphasic system, it was not necessary to use active cultures as inocula. Growth was at least equal to that obtained in conventional media, and spore production of 9 out of 12 strains of C. perfringens equalled or usually exceeded that of conventional media.
Full text
PDF
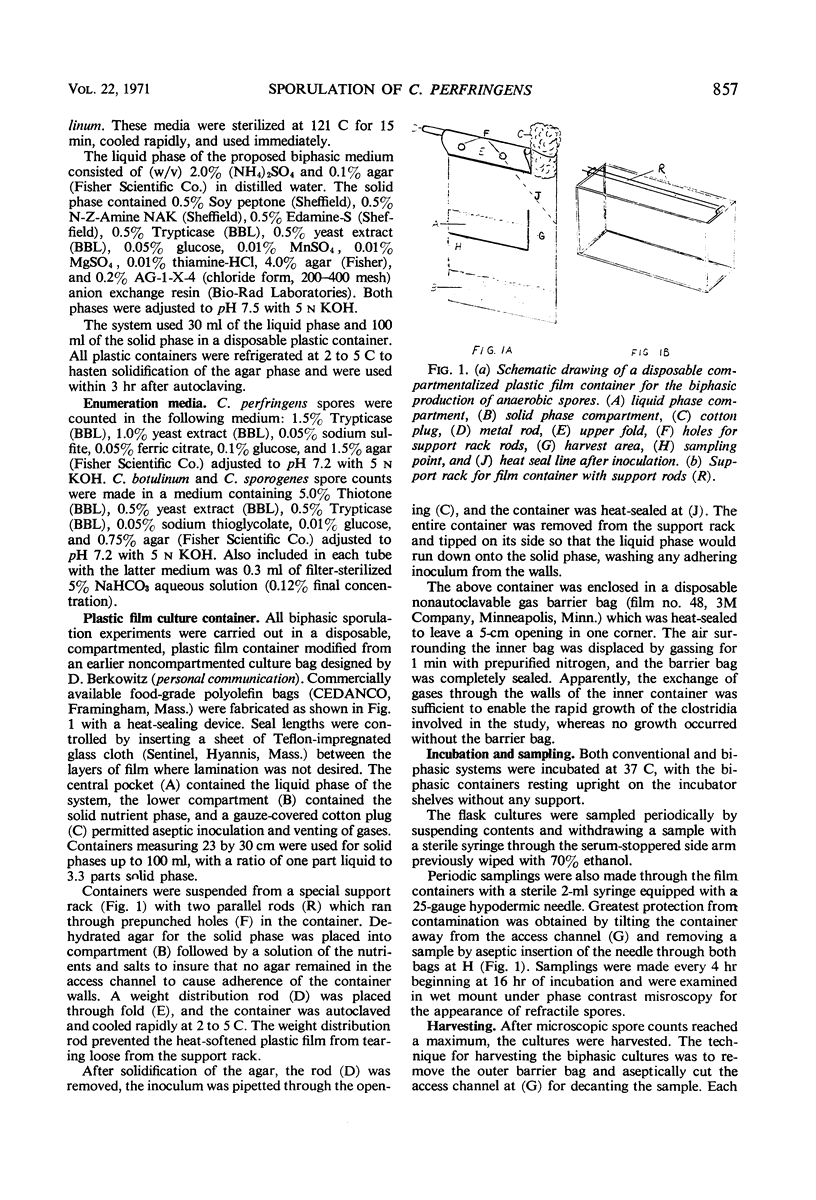

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANGELOTTI R., HALL H. E., FOTER M. J., LEWIS K. H. Quantitation of Clostridium perfringens in foods. Appl Microbiol. 1962 May;10:193–199. doi: 10.1128/am.10.3.193-199.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan C. L., Strong D. H. Improved medium for sporulation of Clostridium perfringens. Appl Microbiol. 1968 Jan;16(1):82–89. doi: 10.1128/am.16.1.82-89.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLNER P. D. A medium promoting rapid quantitative sporulation in Clostridium perfringens. J Bacteriol. 1956 Apr;71(4):495–496. doi: 10.1128/jb.71.4.495-496.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARDWICK W. A., GUIRARD B., FOSTER J. W. Antisporulation factors in complex organic media. II. Saturated fatty acids as antisporulation factors. J Bacteriol. 1951 Feb;61(2):145–151. doi: 10.1128/jb.61.2.145-151.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C. H., Cheney R., Woodburn M. Sporulation of Clostridium perfringens in a modified medium and selected foods. Appl Microbiol. 1967 Jul;15(4):871–876. doi: 10.1128/am.15.4.871-876.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALIN B., FINN R. K. The use of a synthetic resin in anaerobic media. J Bacteriol. 1951 Sep;62(3):349–350. doi: 10.1128/jb.62.3.349-350.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida S., Seo N., Nakagawa M. Sporulation, heat resistance, and biological properties of Clostridium perfringens. Appl Microbiol. 1969 Feb;17(2):303–309. doi: 10.1128/am.17.2.303-309.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHNEIDER M. D., GRECZ N., ANELLIS A. Sporulation of Clostridium botulinum types A, B, and E, Clostridium perfringens, and putrefactive anaerobe 3679 in dialysis sacs. J Bacteriol. 1963 Jan;85:126–133. doi: 10.1128/jb.85.1.126-133.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer P. Sporulation and the production of antibiotics, exoenzymes, and exotonins. Bacteriol Rev. 1969 Mar;33(1):48–71. doi: 10.1128/br.33.1.48-71.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz J. S., Gerhardt P. Dialysis culture of microorganisms: design, theory, and results. Bacteriol Rev. 1969 Mar;33(1):1–47. doi: 10.1128/br.33.1.1-47.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrey J. C., Kahn M. C., Salinger M. H. THE INFLUENCE OF H-ION CONCENTRATION ON THE SPORULATION OF B. WELCHII. J Bacteriol. 1930 Aug;20(2):85–98. doi: 10.1128/jb.20.2.85-98.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]



