Abstract
Background
Weight gain is commonly reported as a side effect of hormonal contraception and can lead to method discontinuation or reluctance to initiate the method. The purpose of this study was to investigate weight change in adolescent aged 15-19 years who were new users of depot-medroxyprogesterone acetate (DMPA), norethisterone enanthate (NET-EN), combined oral contraceptives (COCs), and non-users of hormonal contraception.
Study design
This longitudinal study recruited initiators of DMPA (n=115), NET-EN (n=115), COCs (n=116), and non-users of contraception (n=144). Participants were followed-up for 4-5 years and height, weight and contraceptive use was recorded at each visit.
Results
Women using DMPA or NET-EN throughout or switching between the two, had gained an average of 6.2 kg compared to average increases of 2.3 kg in the COC group, 2.8 kg in non-users and 2.8 kg among discontinued users of any method (p=0.02). There was no evidence of a difference in weight gain between women classified as non-obese or classified as overweight/obese in any of the four study groups at baseline.
Conclusion
There is fairly strong evidence that hormonal injectable users gain more weight than COC users, discontinuers and non-users of contraception.
Keywords: DMPA, NET-EN, COCs, weight, adolescents
1.Introduction
Weight gain is cited as a side effect of hormonal contraceptives [1-13] and. is given as a common reason for discontinuation of hormonal contraception [1-3]. In a survey of practitioners prescribing contraception in the United Kingdom (UK), over three-quarters perceived that oral contraceptives (COCs) and injectables caused weight gain (81.2% and 84.4% respectively) [14]. Conversely, a recent Cochrane Systematic Review found that there was insufficient evidence to determine the effect of COCs on weight [15] and other studies have found no significant weight gain in depot-medroxyprogesterone acetate (DMPA) users [16-18].
DMPA has however, been singled out as a method reported to cause weight gain in adolescents [2, 9-13]. Some studies comparing weight change in adolescent users of different hormonal contraceptives have found that adolescents using DMPA gained more weight than those using COCs [9, 12,13], and control nonusers [12]. There is some evidence that weight increases in DMPA users are associated with obesity status at commencement of use and that overweight and obese adolescent DMPA users are at greater risk of weight gain compared to non-obese women users [12,13]. However, one study found initial weight was not a risk factor [9]. Weight changes in adolescent users of norethisterone enanthante (NET-EN) users have been less investigated than DMPA and COC users and there is only data available for adult NET-EN users [1,4]. In one study [1] the NET-EN group gained slightly less weight at 12 months compared to DMPA but this difference was not significant.
The objective of this prospective study was to investigate long term weight change in a cohort of adolescent hormonal contraceptive users (DMPA, NET-EN and COCs with no past history of hormonal contraceptive use compared to nonusers and discontinuers of hormonal contraception).
2.Subjects and Methods
This was a longitudinal study of adolescents aged 15 to 19 years who had never used hormonal contraception prior to recruitment. Initiators of DMPA, NET-EN, COCs, and nonusers of contraception were enrolled from a family planning clinic in Durban, South Africa. The study cohort was recruited between July 2000 and July 2002 and follow-up continued until April 2006.
All DMPA users were started on a regimen of 150 mg every 12 weeks and NET-EN users on 200 mg every 8 weeks. Both DMPA and NET-EN were administered intramuscularly. The COCs used by women included a range of formulations, with almost all (93%) using low-dose formulations containing between 30-40 mcg of oestrogen. As the main study objective was to investigate bone mineral density (BMD) and hormonal contraceptive use, eligibility criteria required that women had not lactated or delivered in the past 6 months; were not currently or had never used medication known to effect calcium metabolism for more than 3 months; and did not have a chronic disease affecting calcium metabolism. At the baseline visit, participants’ height, weight and blood pressure were measured using a standard protocol and a questionnaire was administered to elicit information on demographic characteristics, regularity of the menstrual cycle, smoking, diet, exercise and caffeine and alcohol intake.
Study participants were followed-up at approximately six-monthly intervals. Height, weight and forearm BMD were measured at each follow-up visit. History of contraceptive use and dieting since last visit was recorded. Those recruited between July 2000 and April 2001 and who continued to participate until the end of the study completed 5 years of follow-up. Those recruited after April 2001 who continued until the end of the study, completed between 4 and 5 years of follow-up. Women continued the same follow-up schedule even if they stopped, changed or started another contraceptive method.
The study was powered to detect a half standard deviation difference in bone mass between users and non-users of hormonal contraceptives. Allowing for 10-15% loss to follow-up per year, this sample was increased to at least 110 women in each group to ensure adequate numbers in long-term follow-up. The non-user group was over recruited compared to other groups as, in addition to loss to follow-up, it was anticipated that some of these women would commence a method of contraception or become pregnant. Detailed methodology for this study is described elsewhere [19]
Differences in change of weight between contraceptive groups, and the associations between weight and selected characteristics of the study participants by contraceptive group were assessed using one-way analysis of variance. Weight was measured in non-users, contraceptive users and discontinuers of contraceptive methods. Women were classified into obesity status at each visit (normal: BMI 18.5-24.9, overweight: BMI 24.9-29.9 and obese: BMI =>30) to see if weight change differed between these groups. Women were also classified into three weight change groups: 1) stable weight = +/−2kg; 2) weight gain >2kg; and 3) weight loss >2kg at the end of the follow-up period. Data were analysed using STATA V.10 (College Station, TX, USA).
After four to five years of follow-up, many women presented with complicated contraceptive histories. Some had used more than one of the study contraceptive methods and others had discontinued hormonal contraception altogether. For the purpose of analysis women were classified into four categories according to their contraceptive histories as follows: 1) Injectable contraceptive users (IC users), including DMPA, NET-EN users and mixed DMPA/NET-EN users; 2) COC users; 3) Discontinuers of any of the three study methods, including women who had used more than one of the study methods (discontinuers were defined as women who had not used a method of contraception for at least 6 months, but had used one of the study methods for at least 18 months); and 4) Never-users/less than 6 months of ever use of hormonal contraception.
Ethical approval was granted by the University of the Witwatersrand, Human Subjects Research Committee (protocol number M981001), and by the Scientific and Ethical Review Group of the World Health Organization.
3.Results
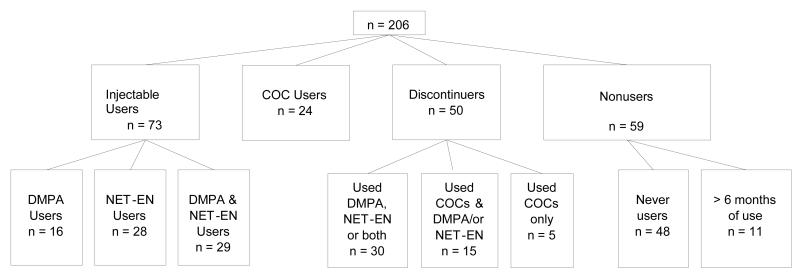
In total, 490 women aged 15-19 years were recruited. All women including those in the DMPA, NET-EN and COC groups had no past use of hormonal contraception before recruitment into the study. Overall, 64% of woman continued in the study for between 2-3 years and 42% completed between 4-5 years of follow-up, depending on time of recruitment. Main reasons for discontinuation from the study included: pregnancy, illness that could have affected BMD such as tuberculosis (TB), and moving out of the area. For this prospective study, all women who had completed between 4-5 years of follow-up in the study, were included in the analysis (n=206). The contraceptive history of these women is shown in Figure 1 and the following number of women were categorized into each analysis group:-
IC users :- 73 women had used either of the two injectables or both.
COC users:- 24 women had used COCs
Discontinuers:- 50 women were classified as discontinuers. Thirty of the discontinuers had used either one or both injectables, 15 had used both COCs and injectables, and 5 women had used only COCs before discontinuing.
Never users/less than 6 months of ever use of hormonal contraception:- 48 women had never used hormonal contraception and 11 had used a hormonal method for 6 months or less over the 4-5 year period. Eight of the 11 women had used the method in the first 6 months of follow-up and continued in the study as a non-user for the remainder of the follow-up period.
Figure 1.
Participant contraceptive history per study group
Although we were able to code all women into these primary categories, some women (n=14) in the IC group had used COCs for a short time and similarly some women in the COC group had used ICs (n=16). If use of one of the methods (either an injection or COC) was limited to six months or less, or if one method was only used in the first year of follow-up, they were classified into the group for the method they had used for the majority of the follow-up time.
Baseline information about the participants is shown in Table 1. Mean age was just under 18 years and most women were still in full-time education. The majority of women were African except in the COC group, which included 12.5% women of Indian origin and 6.3% Coloured women (mixed race). Mean weight of the 9 Indian women at baseline was 11 kgs lower than that of the African and Coloured women who were both just over 60 kg at baseline (p=0.019). There was no significant difference between the four groups in age, height or BMI at baseline. Although there was no significant difference in weight at baseline the discontinuers were between 3-4 kgs heavier than the other three groups (p=0.29).
Table 1. Baseline Characteristics of subjects by contraceptive user group.
| Characteristics |
nonusers (n=59) |
Injectable Users (n=72) |
COC users (n=24) |
discontinuers (n=50) |
P value |
|---|---|---|---|---|---|
|
| |||||
| Mean age, years (SD) | 17.1 (1.4) | 17.5 (1.3) | 17.7 (1.2) | 17.5 (1.2) | 0.17 |
| Mean highest education grade (SD) | 9.7 (1.4) | 10.4 (1.5) | 10.9 (1.3) | 10.7 (1.3) | <0.001 |
| Occupation | 0.08 | ||||
| Employed | 0 | 0 | 4.4 | 5.9 | |
| Unemployed | 5.4 | 7.8 | 13.1 | 7.8 | |
| Student/scholar | 94.6 | 92.2 | 82.6 | 86.3 | |
| Ethnicity % | 0.02 | ||||
| African | 97.5 | 96.2 | 81.3 | 93.8 | |
| Coloured | 0 | 2.8 | 6.3 | 3.1 | |
| Indian | 2.5 | 0.9 | 12.5 | 3.1 | |
| Ever pregnant % | 1.7 | 11.1 | 12.5 | 14.0 | 0.32 |
| Mean age at menarche, years(SD) | 13.4 (1.2) | 13.6 (1.3) | 13.4 (1.5) | 14.0 (1.4) | 0.09 |
| Exercise at least once a week (%) | 28.6 | 15.7 | 13.0 | 20.5 | 0.28 |
| Current smoker | 1.7 | 2.7 | 12.5 | 6.0 | 0.1 |
| Weight kg (SD) | 58.9 (10.9) | 59.4 (11.9) | 57.5 (12.3) | 62.2 (9.8) | 0.29 |
| Height cm (sd) | 155.6 (6.0) | 155.8 (5.5) | 156.6 (5.5) | 156.5 (5.9) | 0.8 |
| BMI kg/m2 (SD) | 24.4 (4.4) | 24.5 (4.3) | 23.5 (4.5) | 25.5 (3.8) | 0.24 |
| Overweight/obese% | 42.4 | 38.4 | 29.2 | 50.0 | 0.35 |
BMI = body mass index
Non-obese: BMI <25.0
Overweight: BMI 25-29.9
Obese: BMI ≥30.0
Across all contraceptive groups, women gained weight and height over time (Table 2). The injectable contraceptive group gained an average of 6.2 kgs over the follow-up period compared to smaller increases of 2.3 kgs in the COC users, 2.8 kgs in the nonusers and 2.8 kgs in the discontinuers (p=0.02). In total 68.5% of IC users gained more than 2kgs in weight compared with around half of women in the other 3 groups (50.8% nonusers, 54.2% COC users and 50% discontinuers). Numbers were too small in the IC sub groups (DMPA n=16, NET-EN n=28 or both n=29) to analyse separately. The exclusive DMPA users had gained the most weight in the IC user group (7.2kgs), mixed IC users (5.7 kgs) and the exclusive NET-EN users gained slightly less (5.9 kgs) but this was not significant p=0.83.
Table 2. Mean Weight Increase from baseline to end of 4/5 years follow-up according to contraceptive group.
| Variable | Nonusers | Injectable users |
COC users | Discontin uers |
P value |
|---|---|---|---|---|---|
|
| |||||
| Weight gain (kg) Mean SD | 2.8 (7.4) | 6.2 (8.4) | 2.3 (5.5) | 2.8 (7.6) | 0.02 |
| Height gain (cm) Mean SD | 2.0 (1.5) | 1.9 (1.6) | 1.9 (1.7) | 1.8 (1.6) | 0.92 |
We looked at the discontinuers to observe weight change at one year post discontinuation and found that just over half (56%) maintained their weight within 2 kgs of their last hormonal contraceptive use visit. Just under a quarter (22%) gained weight and the same percentage lost weight. Indian woman increased in weight by 6.4 over the follow-up period and this was higher than the other two ethnic groups but there was no evidence to suggest a difference (p=0.6) as numbers in this group were small (n=9) and two Indian women had put on considerable weight (12kgs and 17kgs).
As only a small proportion of women were classified as obese at baseline (10.7%), overweight and obese women were combined into one group, in order to see if they would be more likely to gain weight when compared with non-obese women (Table 3). There was no evidence of a difference in weight gain over time between women in the non-obese and overweight/obese categories (Table 3) in any of the four study groups.
Table 3. Percentage of non-obese and overweight/obese women gaining more than 2kg in weight during follow-up by contraceptive group at baseline.
| Contraceptive group |
Non-obese % |
Overweight/ obese % |
P value |
|---|---|---|---|
|
| |||
| Nonusers | 47.1 | 54.2 | 0.06 |
| Injectable users | 77.7 | 62.1 | 0.11 |
| COC users | 61.1 | 33.0 | 0.26 |
| Discontinuers | 42.3 | 42.3 | 0.65 |
Non-obese: BMI <25.0
Overweight: BMI 25-29.9
Obese: BMI ≥30.0
Women were asked at each follow-up visit if they had dieted since their last study visit. Twenty one women (10%) reported dieting at one or more of their follow-up visits. The average weight of women who dieted was 70 kgs, compared to 60kgs in those who did not diet during the study. The proportion of dieters in each group was similar, (11% from the injectable group, 10% of the non-users, 12.5% in the COC users and 8% in the discontinuers group). It is difficult to make any general comment on this group of women, as not all those reporting dieting actually lost weight and some who did lose weight regained the weight lost.
4.Discussion
Over half (57%) of women in this study gained weight over the follow-up period. This was expected as women were aged between 15 and 19 years at baseline and would still be considered to be growing. A small increase of approximately 2cm in height was seen in each study group. This study found that women who were using injectable contraception (DMPA, NET-EN or both) gained more than twice as much weight as those using COCs, never users of contraception/users with >6 months of use and discontinuers of contraception. Therefore, this study adds to the existing evidence that injectable contraceptive users gain more weight over time compared to COC users and nonusers [9,12,13]. The amount of weight gained over the 4-5 year follow-up in our study was proportionally lower than that shown in other studies including adolescents, where weight increases of approximately 4kgs were found at 1 year [13] and 18 months [12]. Our study reports on weight gain after long-term use of hormonal contraception and it maybe that weight gain seen in shorter studies would not have continued at the same rate over longer periods of time. The slightly higher weight gain in DMPA users compared to NET-EN and mixed DMPA/NET-EN users although not significant is in agreement with an earlier WHO multicentred trial [1]
Although there were no differences in weight gain between non-obese and overweight/obese women in our study, a difference between these groups has been reported in other studies [12,13]. However, our study had lower rates of obese adolescents at baseline (10%) compared to a similar study conducted in the United States (US) where 20.9% of adolescents were already obese at baseline [12] compared to 10.7% in our sample. The US study [12] combined normal and overweight women into a non-obese category and compared these with the obese group. Unfortunately the number of obese women in our study was too small a category to be analysed independently, and this may affect the comparability of results.
There have been limitations in some of the previous studies investigating weight change in contraceptive users. Only one study that has looked at adolescent weight change has included a non-hormonal contraceptive control group [12], and no other study on adolescents has included mixed contraceptive users or discontinuers. Dieting has also not been reported in studies investigating weight gain in hormonal contraceptive users. Our study found that the majority of adolescents either changed method or discontinued method during follow-up and so it is important to include these young women in future studies as they maybe far more prevalent than exclusive users of one method. Our study included a control group and a group of discontinuers, and found that long term weight gain was similar in both groups. The percentage of women gaining more than 2kg of weight after discontinuation of method was 22% and this was lower than the rates shown across the study in every group. This may indicate a stabilizing of weight post-discontinuation. It was interesting to note that although not significant the discontinuers had a mean weight approximately 3-4 kg higher than the other groups at baseline and had the highest proportion (50%) of women in the overweight and obese category. It may be that regardless of contraceptive method women who are already overweight when they start a contraceptive method may consider discontinuing contraception if they gain any further weight compared to a woman in the normal weight range who may be prepared to accept a small weight gain. However further research would need to be conducted to ascertain the exact reason for discontinuation.
Although the study was not designed to collect reasons for contraceptive change and discontinuation, anecdotal comments suggest that changes in relationship status and side effects of hormonal methods resulted in discontinuation. Side effects typically mentioned were bleeding problems and weight gain and this has been found in other studies documenting reasons for discontinuation of hormonal methods in adolescents [1-3]. As adolescents may not be in long-term relationships it can be expected that the end of a relationship may lead to contraceptive discontinuation until a new relationship starts and this has been reported previously [20].
Our study found no evidence of increase in weight in COC users compared to controls over the 4-5 year period. We found injectable users gained weight compared to COC users, discontinuers and non-user controls. Weight gain, whether real or perceived, is especially important in adolescents, as it may lead to contraceptive discontinuation and increase the risk of teenage pregnancy. Adolescents were found to be 15% more likely to discontinue contraception for method related reasons compared to women in other age groups in a national survey in the US [9]. Counseling new and continuing adolescent users of hormonal contraception should address weight gain as a possible side effect, in order to minimize discontinuation.
Limitations
In classifying women into the 4 study analysis groups we combined a number of sub-groups due to small numbers.
Acknowledgements
This study was supported by a grant from the World Health Organization and The William and Flora Hewlett Foundation.
References
- 1.World Health Organization Special Programme of Research, Development and Research Training in Human Reproductive Task Force on Long-acting Systemic Agents for the Regulation of Fertility Multinational comparative clinical evaluation of two long-acting injectable contraceptive steroids: Norethisterone enanthate given in two dosage regimens and depot medroxyprogesterone acetate: Final report. Contraception. 1983;23:1–20. [Google Scholar]
- 2.Harel Z, Biro FM, Kollar LM, Rauh JL. Adolescents reasons for and experience after discontinuation of the long-acting contraceptive Depo-Provera and Norplant. J Adolesc health. 1996;19(2):118–23. doi: 10.1016/1054-139X(95)00322-J. [DOI] [PubMed] [Google Scholar]
- 3.Gupta S. Weight gain on the combined pill--is it real? Human Reproduction Update. 2000;6(5):427–431. doi: 10.1093/humupd/6.5.427. [DOI] [PubMed] [Google Scholar]
- 4.Howard G, Blair M, Fotherby K, Elder MG, Bye P. Seven years clinical experience of the injectable contraceptive, norethisterone oenanthate. Br J Fam Plann. 1985;11:9–16. [Google Scholar]
- 5.Bahamondes L, Del Castillo S, Tabares G, Arce XE, Perrotti M, Petta C. Comparison of weight increase in users of depot medroxyprogesterone acetate and copper IUD up to 5 years. Contraception. 2001;64:223–5. doi: 10.1016/s0010-7824(01)00255-4. [DOI] [PubMed] [Google Scholar]
- 6.Espey E, Steinhart J, Ogburn T, Qualls C. Depo-Provera associated with weight gain in Navajo women. Contraception. 2000;62:55–8. doi: 10.1016/s0010-7824(00)00144-x. [DOI] [PubMed] [Google Scholar]
- 7.Clark MK, Dillon JS, Sowers M, Nichols S. Weight, fat mass, and central distribution of fat increase when woman use depot-medroxyprogesterone acetate for contraception. Int J Obes. 2005;29(10):1252–8. doi: 10.1038/sj.ijo.0803023. [DOI] [PubMed] [Google Scholar]
- 8.Amatayakul K, Sivasomboon B, Thanangkul O. A study of the mechanism of weight gain in medroxyprogesterone acetate users. Contraception. 1980;22:605–22. doi: 10.1016/0010-7824(80)90087-6. [DOI] [PubMed] [Google Scholar]
- 9.Risser WL, Gefter LR, Barratt MS, Risser JM. Weight change in adolescents who used hormonal contraception. J Adolesc Health. 1999;24(6):433–6. doi: 10.1016/s1054-139x(98)00151-7. [DOI] [PubMed] [Google Scholar]
- 10.Matson SC, Henderson KA, McGrath GJ. Physical findings and symptoms of depot medroxyprogesterone acetate use in adolescent females. J Pediatr Adolesc Gynecol. 1997;10(1):18–23. doi: 10.1016/s1083-3188(97)70039-1. [DOI] [PubMed] [Google Scholar]
- 11.Bonny A, Britto M, Huang B, Succop P, Slap GB. Weight gain, adiposity, and eating behavious among adolesent females on depot medroxyprogesterone acetate (DMPA) J Pediatr Adolesc Gynecol. 2004;17:109–115. doi: 10.1016/j.jpag.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Bonny AE, Ziegler J, Harvey R, Debanne S, Cromer BA. Weight gain in obese and nonobesse adolescent girls initiating depot medroxyprogesterone acetate, oral contraceptive pills, or no hormonal contraceptive method. Arch Pediatr Adolesc Med. 2006;160:40–45. doi: 10.1001/archpedi.160.1.40. [DOI] [PubMed] [Google Scholar]
- 13.Mangan SA, Larsen PG, Hudson S. Overweight teens at increased risk for weight gain while using depot medroxyprogesterone acetate. J Pediatr Adolesc Gynecol. 2002;15:79–82. doi: 10.1016/s1083-3188(01)00147-4. [DOI] [PubMed] [Google Scholar]
- 14.Wellings K, Zhihong Z, Krentel A, Barrett G, Glasier A. Attitudes towards long-acting reversible methods of contraception in general practice in the UK. Contraception. 2007;76:208–214. doi: 10.1016/j.contraception.2007.05.085. [DOI] [PubMed] [Google Scholar]
- 15.Gallo MF, Lopez LM, Grimes DA, Schulz KF, Helmerhorst FM. Combination contraceptives: effects on weight. Cochrane Database Syst Rev. 2008 Oct 8;(4):CD003987. doi: 10.1002/14651858.CD003987.pub2. [DOI] [PubMed] [Google Scholar]
- 16.Mainwaring R, Hales HA, Stevenson K, et al. Metabolic parameter, bleeding, and weight changes in US women using progestin only contraceptives. Contraception. 1995;51(3):149–53. doi: 10.1016/0010-7824(95)00011-x. [DOI] [PubMed] [Google Scholar]
- 17.Moore LL, Valuck R, McDougall C, Fink W. A comparative study of one-year weight gain among users of medroxyprogesterone acetate, levonorgestrel implants, and oral contraceptives. Contraception. 1995;52(4):215–9. doi: 10.1016/0010-7824(95)00189-h. [DOI] [PubMed] [Google Scholar]
- 18.Taneepanichskul S, Reinprayoon D, Khaosaad P. Comparative study of weight change between long-term DMPA and IUD acceptors. Contraception. 1998;58:149–51. [Google Scholar]
- 19.Beksinska ME, Kleinschmidt I, Smit J, Farley TMM. Bone mineral density in adolescents using norethisterone enanthate, depot-medroxyprogerterone acetate, or combined oral contraceptives for contraception. Contraception. 2007;75:438–43. doi: 10.1016/j.contraception.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Vaughan B, Trussell J, Kosy K, Singh S, Jones R. Discontinuation and resumption of contraceptive use: results from the 2002 National Survey of Family growth. Contraception. 2008;78:271–283. doi: 10.1016/j.contraception.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]