Abstract
The pathogenesis of type 2 diabetes mellitus involves both peripheral insulin resistance and dysfunctional insulin secretion from the pancreatic β cell. Currently, there is intense research focus on delineating the etiologies of pancreatic β cell dysfunction in type 2 diabetes. However, there remains an unmet clinical need to establish therapeutic guidelines and strategies that emphasize the preservation of pancreatic β cell function in at-risk and affected individuals. Thiazolidinediones are orally active agents approved for use in type 2 diabetes and act as agonists of the nuclear hormone receptor PPAR-γ. These drugs improve insulin sensitivity, but there is also a growing appreciation of PPAR-γ actions within the β cell. PPAR-γ has been shown to regulate directly key β cell genes involved in glucose sensing, insulin secretion and insulin gene transcription. Further, pharmacologic PPAR-γ activation has been shown to protect against glucose-, lipid-, cytokine- and islet amyloid polypeptide (lAPP)-induced activation of numerous stress pathways. This article will review the mechanisms by which PPAR-γ activation acts to maintain β cell function and survival in type 2 diabetes mellitus and highlight some of the current controversies in this field.
Keywords: β cell, diabetes mellitus, PPAR-γ agonist
Introduction
Type 2 diabetes mellitus (T2DM) is a disease of disordered glucose homeostasis that affects an estimated 200–250 million individuals worldwide [1]. The pathogenesis of T2DM involves both peripheral insulin resistance and dysfunctional insulin secretion from the pancreatic β cell. Studies documenting the natural history of this disease suggest two roles for the pancreatic β cell [2,3]. The first is that ‘at-risk’ individuals possess an inherent tendency towards β cell failure. These individuals are therefore predisposed towards the development of diabetes in settings where insulin sensitivity is compromised [4–16]. States of obesity and decreased peripheral insulin sensitivity lead to elevated serum levels of glucose, adipocytokines and free fatty acids as well as other deleterious lipid intermediates [17]. These toxic mediators harm the β cell through several intersecting mechanisms including oxidative stress, mitochondrial dysfunction, epigenetic dysregulation and activation of endoplasmic reticulum (ER) stress pathways [18–21]. While there is a great overlap between many of these processes, the final common pathway that results is β cell dysfunction and death. Thus, the second component of this paradigm is that the altered metabolic milieu of T2DM leads to progressive β cell failure and loss of functional β cell mass over time.
Given this broader and evolving understanding of the role of the β cell in the pathogenesis of T2DM, there is a clinical need to identify treatment strategies and pharmacologic targets that prevent the development of diabetes in genetically or epigenetically ‘at-risk’ individuals. Further, there is a parallel need to maintain β cell function and mass in affected individuals. Thiazolidinediones (TZDs), which are peroxisome proliferator-activated receptor γ (PPAR-γ) agonists, were first approved for clinical use in the treatment of T2DM in 1997 [22]. Although these agents have been largely viewed as peripheral insulin-sensitizing agents, there is a growing appreciation for PPAR-γ regulated transcriptional and signalling events within the pancreatic β cell. This article will provide an overview of the mechanisms through which pharmacologic PPAR-γ activation may act to enhance pancreatic β cell homeostasis and highlight some of the current controversies associated with the use of these agents in T2DM.
Pharmacological PPAR-γ Activation and the Preservation of β Cell Function; Controversies in the Field
PPAR-γ (NR1C3) is a member of nuclear hormone receptor superfamily of ligand-activated transcription factors. TZDs are orally active agents that act as high-affinity activators of PPAR-γ [23]. There are currently two TZDs approved for use in type 2 diabetes in the United States: pioglitazone and rosiglitazone. Troglitazone was the first drug in the class approved by the Food and Drug Administration (FDA), but was subsequently withdrawn from the market after several patients developed fulminant hepatic failure [24]. TZD therapy in persons with diabetes has been shown to lower haemoglobin A1c levels, and initial trials showed that TZDs act to enhance peripheral insulin sensitivity in the liver, adipose and skeletal muscle [23,25]. However, the additional role of PPAR-γ-mediated events in the pancreatic β cells has been studied extensively since the confirmation of PPAR-γ expression in rodent and human pancreatic islets [26,27].
Results from several large clinical trials have supported the idea that PPAR-γ agonists, in addition to their known effects on insulin-sensitive tissues, may have complementary benefits in the β cell. A number of studies including the Diabetes Prevention Program (DPP) and Diabetes Reduction Assessment with Ramipril and Rosiglitazone Medication (DREAM) trial have shown that PPAR-γ agonists, when administered to individuals with impaired glucose tolerance (IGT), prevent or delay the onset of T2DM [28,29]. TZD therapy has also been shown to prevent the development of diabetes in individuals with a history of gestational diabetes mellitus (GDM). Those with GDM are considered to be an extremely high-risk population and have nearly a 50% chance of developing permanent diabetes within 5 years of the affected pregnancy [30–32]. Further, A Diabetes Outcome Progression Trial (ADOPT) showed that treatment with rosiglitazone in type 2 diabetes for a median of 4 years was associated with improved glycaemic durability as compared to sulphonylurea or metformin therapy [33].
These and other trials have also shown objective improvements in various measures of β cell function in T2DM. In persons with diabetes, TZDs have been shown to decrease the serum proinsulin to insulin ratio and improve glucose-entrained insulin secretion [34,35]. The proinsulin to insulin ratio is increased in diabetes and reflects dysfunctional processing of the insulin prohormone. An elevated ratio is also a marker of impaired β cell secretory response [36]. TZDs have further been shown to increase the insulin secretion/insulin resistance index as defined by oral glucose tolerance tests (OGTT) and euglycaemic insulin clamp studies. This index is defined as the change in incremental area under the curve (AUC) of plasma insulin during OGTT divided by the incremental AUC of plasma glucose during an OGTT. This ratio is then normalized for changes in insulin resistance, which is determined by euglycaemic clamp. Essentially, an improvement in the insulin secretion/insulin resistance index implies an improvement in the acute β cell insulin-secretory response to glucose. Because this assessment factors in changes in insulin sensitivity, the improvement in insulin secretion can be attributed directly to improvements in β cell function [37].
The homeostatic model of assessment-%B (HOMA-%B) is a method of mathematical modelling that allows for a surrogate assessment of β cell function during an intravenous glucose tolerance test (IVGTT). Using this method, Wallace et al. showed an improvement in HOMA-%B in subjects with diabetes who were treated with pioglitazone for 3 months [35]. The ADOPT trial further showed a slower rate of decline in HOMA-%B in rosiglitazone-treated subjects compared with those treated with either glyburide or metformin [33].
With regard to those at risk for the development of diabetes, including those with IGT, impaired fasting glucose (IFG) or a history of GDM, TZDs have been shown to produce similar results. Cavaghan and colleagues showed that 12 weeks of troglitazone treatment in individuals with IGT led to improved insulin secretion, when adjusted for insulin sensitivity, and improved β cell sensing during an oscillatory glucose infusion [38]. In a ‘prediabetic’ population, rosiglitazone treatment for 2 years, in the DREAM trial, led to a decreased fasting proinsulin to C-peptide ratio. Further, during OGTT, rosiglitazone-treated individuals manifested an improved insulinogenic index [39]. This measure is similar to the insulin secretion/insulin resistance index performed by Gastaldelli et al. except that insulin resistance was modelled from the homeostatic model of assessment (HOMA) calculation rather than directly measured from an insulin clamp. Finally, in patients with a history of GDM, the TRoglitazone In the Prevention of Diabetes (TRIPOD) and Pioglitazone in Prevention of Diabetes (PIPOD) studies have both shown that TZD use is associated with a stabilization of β cell function, as assessed by the disposition index. This is compared to placebo-treated controls, where worsening function was shown over the same time period [31,32].
The results of key trials, which have directly studied TZD effects on β cell function, are summarized in Table 1. In aggregate, these studies show that TZD use in persons with diabetes and those at risk for diabetes is associated with improvements in β cell secretory response and glucose sensing. Further, in persons with diabetes, TZD use led to improved glycaemic outcomes over time that can be attributed to changes in insulin sensitivity, but also importantly, to improvements in β cell function independent of any change in insulin sensitivity.
Table 1.
A summary of human trials which have included direct measurements of β cell function following PPAR-γ agonist treatment in persons with IGT, IFG, T2DM or a history of gestational diabetes.
| Study | Patient population | Drug | Length of treatment | Measurements performed | Outcome(s) of improved β cell Function |
|---|---|---|---|---|---|
| Cavaghan et al. [38] | IGT | Tro | 12 weeks | 75 g OGTT IVGTT |
Improved insulin secretion rates adjusted for insulin sensitivity |
| Graded glucose infusion Spectral analysis for oscillatory glucose infusion |
Improved β cell sensing during oscillatory glucose infusion | ||||
| Prigeon et al. [138] | T2DM | Tro | 12 weeks | Acute insulin response to arginine IVGTT | Reduced proinsulin to insulin ratio |
| Miyazaki et al. [139] | T2DM | Pio | 26 weeks | 75 g OGTT | Increased plasma insulin response during OGTT |
| Increased insulinogenic index (AUC insulin/AUC glucose) | |||||
| Buchanan et al. (TRIPOD) [31] | History of GDM | Tro | 120 weeks | 75 g OGTT IVGTT |
Troglitazone-treated controls had a stable β cell compensation (DI) for insulin resistance, compared to placebo-treated controls who showed decreasing DI |
| Juhl et al. [34] | T2DM | Rosi | 13 weeks | IVGTT Hyperglycaemic clamp with arginine stimulation |
Improved glucose-entrained insulin secretion |
| Assessment of baseline high-frequency insulin pulsatility by spectral analysis | |||||
| Hyperinsulinaemic euglycaemic clamp | |||||
| Wallace et al. [35] | T2DM | Pio | 12 weeks | HOMA IVGTT and hyperglycaemic clamp Euglycaemic hyperinsulinaemic clamp |
Increased HOMA-%B Reduced proinsulin to insulin ratio |
| Xiang et al. (PIPOD) [32] | History of GDM | Pio | 36 weeks after TRIPOD study | 75 g OGTT IVGTT |
Stabilization of AIRg and DI, compared to placebo-treated controls who had worsening of AIRg and DI over the same time period |
| Kahn et al. (ADOPT) [33] | T2DM | Rosi | 48 weeks | HOMA | Slower rate of decline in HOMA-%B in rosiglitazone-treated subjects compared to those treated with either glyburide or metformin |
| Gastaldelli et al. [37] | T2DM | Pio or Rosi | 16 weeks | 75 g OGTT Two-step euglycaemia insulin clamp |
Increased insulin secretion/ insulin resistance index (ISR AUC/glucose AUC + IR) |
| Improved insulin secretory response per incremental plasma glucose response expressed in relationship to the severity of insulin resistance (ISR/G0–120 + IR) | |||||
| Hanley et al. (DREAM) [39] | IGT and IFG | Rosi | At least 24 months | 75 g OGTT | Increased insulinogenic index which was corrected for HOMA-IR |
| Decreased fasting proinsulin-to-C-peptide ratio |
ADOPT, A Diabetes Outcome Progression Trial; AIRg, acute insulin secretion during IVGTT; AUC, area under the curve; DI, disposition index; DREAM, Diabetes Reduction Assessment with Ramipril and Rosiglitazone Medication; GDM, gestational diabetes mellitus; HOMA, homeostasis model of assessment; HOMA-IR, homeostasis model of assessment of insulin resistance; HOMA-%B, homeostasis model of assessment of β cell function; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; ISR, insulin secretory response; IVGTT, intravenous glucose tolerance test; OGTT, oral glucose tolerance test; pio, pioglitazone; rosi, rosiglitazone; T2DM, type 2 diabetes mellitus; TRIPOD, TRoglitazone In the Prevention Of Diabetes; tro, troglitazone.
Genetic Ablation of PPAR-γ in Pancreatic Islets
In spite of these aforementioned clinical observations, there has been continued controversy over whether PPAR-γ agonists have direct or indirect effects on the pancreatic islet. In part, this debate has been fuelled by conflicting data from rodent models of PPAR-γ ablation. Matsui et al. created a model of total body heterozygous PPAR-γ deletion. Interestingly, these mice were protected from high-fat diet-induced insulin resistance. Whereas peripheral tissues showed decreased triglyceride levels, there was an increase in intraislet triglyceride that led to impaired islet glucose oxidation. Administration of pioglitazone reversed these changes and restored islet function [40].
A pancreatic β cell-specific PPAR-γ knockout model (βγ KO mice) naturally followed and was generated by crossing mice with floxed PPAR-γ gene (PPARG) to mice expressing Cre driven by rat insulin II promoter (RIP-Cre). βγ KO mice displayed significant islet hyperplasia on normal chow diet, but had a blunted response towards β cell mass expansion when challenged with high-fat feeding. Isolated islets from βγ KO mice also exhibited a blunted TZD response towards glucose-stimulated insulin secretion (GSIS) yet normal physiological parameters after high-fat feeding. On the basis of these findings, the authors concluded that PPAR-γ had an inconsequential physiological role in the β cells, and these outcomes led support to the idea that the beneficial effects of PPAR-γ agonists are secondary solely to enhanced peripheral insulin sensitivity [41].
Recently, another pancreatic-specific KO model of PPAR-γ was generated by crossing mice with a floxed PPARG gene to mice expressing Cre driven by the pdx-1 promoter (PANC PPAR-γ−/−). Islets from PANC PPAR-γ−/− mice showed normal cytoarchitecture and no hyperplasia. Interestingly, the PANC PPAR-γ−/− mice exhibited glucose intolerance at baseline. Isolated islets show blunted glucose-stimulated insulin secretion as well as downregulation of pdx-1 and GLUT2 expression, with no effect on glucagon levels [42].
The apparent differences between the βγ KO and PANC PPAR-γ−/− mice are not fully understood, but several factors should be considered. First, it is plausible that pdx-1-driven Cre is expressed fairly early during development of the endocrine pancreas as compared to RIP-Cre. This difference may have developmental implications. Further, hypothalamic expression of the RIP-Cre has been described [43], and one could speculate that differences in the expression of PPAR-γ within the hypothalamus between the two models may have affected neuronal regulation of energy homeostasis and glucose metabolism. Although, hypothalamic expression of pdx-1 Cre has not yet been formally reported, pdx-1 is present in neural cells during brain development [44]. There are examples of models that have employed the pdx-1 Cre, which have specifically shown unaltered expression of the floxed gene within the hypothalamus [45,46]. Specifically, Gupta and colleagues show robust expression of PPAR-γ in the hypothalamus of their PANC PPAR-γ−/− model [42]. Notwithstanding this controversy, PPAR-γ immunoreactivity has been observed in a majority of neurons in the arcuate and ventromedial hypothalamic nuclei that control energy homeostasis and glucose metabolism [47]. The neuron-specific deletion of PPAR-γ, however, seems to have no significant effect on normal food intake or body weight on 4 weeks of normal chow. Finally, the RIP-Cre model has been reported to have glucose intolerance at baseline [48,49]; therefore, the findings in the βγ KO model are difficult to interpret. A long-term high-fat feeding or partial pancreatectomy study using the pdx-1 Cre-driven KO mouse model would potentially help to clarify the contribution of PPAR-γ-mediated signalling events in the β cells under stress conditions.
Mechanisms of PPAR-γ Action
Although there have been discrepant results from animal models of PPAR-γ deletion within the islet, a broader understanding of the role of PPAR-γ in the pancreas has been provided by a number of cell-based studies. To interpret these studies, it is useful to review the molecular mechanisms of PPAR-γ action. To function as a transcriptional regulator, PPAR-γ must be ligand activated, undergo heterodimerization with retinoid X receptors (RXRs: NR2B), recruit co-factors and recognize peroxisome proliferator response elements (PPREs) in the 5′ promoter region of a target gene. The consensus sequence for a PPRE consists of two direct repeats consisting of AGGTCA (direct repeat 1 and 2 or DR1 and DR2) separated by a nucleotide, although several variations of this consensus have been described [50]. Within a typical PPRE, the 5′-half is occupied by PPAR-γ, while the 3′-halfis occupied by RXR [51]. A recent comparison of known reported 73 DRl-like PPREs reflects the presence of heterogeneity, while the DR2 core sequence appears to be more highly conserved [52]. Further, stringent binding of RXR on the 3′-half of PPRE is more influential on the binding of PPAR-γ/RXR heterodimer than the ability of PPAR-γ to bind DNA In addition, PPAR-γ has been shown to bind as a homodimer to palindromic sequences separated by three nucleotides [53]. In the absence of ligand, PPAR-γ is bound by a co-repressor and the transcriptional effects are blocked [54].
PPAR-γ is expressed in a variety of tissues, although it is considered to be most abundantly expressed in adipose tissue [55]. However, protein levels of PPAR-γ in both rodent and human islets are comparable to levels observed in adipose tissue [26,56]. PPAR-γ expression in the islet may, however, change in response to environmental or stress conditions. Chronic exposure to high glucose has been shown to decrease PPAR-γ mRNA levels in mouse islets [57]. Further, Moibi et al. showed that PPAR-γ is upregulated 2 weeks after a 60% pancreatectomy procedure in rats. This coincided with a switch from a proliferative to a prodifferentiation state [58].
PPAR-γ Target Genes in the Pancreatic β Cell; Profunction Effects of PPAR-γ Activation
The β cell is the only cell in the body responsible for physiologic insulin secretion, thus it possesses a complement of highly specialized cellular machinery to accomplish the key tasks of glucose sensing and insulin release. Insulin secretion in the pancreatic β cell has been classically ascribed to an ATP-sensitive potassium (KATP) channel-dependent mechanism. In this model, glucose is taken up into the β cell through the glucose transporter (GLUT) [59]. In the rodent, GLUT2 is the predominant isoform; however, in human β cells, GLUT1 appears to play a more prominent role [60,61]. Upon entry into the β cell, glucose is converted to glucose-6-phosphate by glucokinase. Glucokinase, in contrast to other hexokinase isoforms in other tissue types, has a low affinity (high Km) for glucose, displays co-operativity with respect to glucose and serves as the rate-limiting step for glucose uptake [62]. Glucose-6-phosphate is subsequently converted to pyruvate in the glycolytic pathway, which is then converted to acetyl coenzyme A (CoA) and enters the tricarboxylic acid (TCA) cycle. The TCA cycle produces reducing equivalents and ATP resulting in an increase in the ATP/ADP ratio within the islet. This increase in the ATP/ADP ratio brings about closure of the KATP channels, leading to membrane depolarization and subsequent activation of voltage-gated Ca2+ channels as well as Ca2+ influx from the extracellular space and ER [63]. This increase in cytosolic Ca2+ ultimately serves to activate insulin granule docking and release from a readily releasable pool of secretory vesicles [64].
GSIS does not occur solely through this KATP-dependent mechanism. There are several alternative metabolic pathways that serve to amplify insulin secretion. These processes have been reviewed in detail elsewhere and include pyruvate carboxylase-mediated anapleurotic reactions, NADH shuttles and glutamate production from glutamate dehydrogenase [65,66]. In addition, the gut-derived incretin hormones, glucagon-like peptide-1 (GLP-1) and gastric inhibitory polypeptide (GIP) stimulate glucose-dependent insulin secretion through activation of their cognate receptors and subsequent downstream signalling, involving the production of cyclic adenosine monophosphate (cAMP) and activation of protein kinase A and guanine nucleotide exchange factors [67].
TZD administration has been shown to enhance glucose sensing and insulin secretion from diabetic rodent and human β cells [34,38,68,69]. With regard to PPAR-γ regulation of these KATP-independent processes, Jitrapakdee and colleagues showed that PPAR-γ is a direct transcriptional regulator of pyruvate carboxylase in white and brown adipose tissues [70]. This relationship, however, has not been studied directly in the β cell. Further, Gupta and colleagues recently showed a PPRE in the GIP receptor gene and showed, functionally, that this gene was a direct target of PPAR-γ [71].
PPAR-γ is also known to regulate components of the classical KATP-dependent GSIS pathway. The promoters of GLUT2 and glucokinase are reported to have functional PPREs that bind the PPAR-γ/RXRα heterodimer, and PPAR-γ activation leads to transcriptional upregulation of these genes in the β cell [72,73]. Further, the expression of these genes is impaired in rodent models of diabetes [68,74]. Little is known, however, about the regulation of GLUT1 by PPAR-γ in the human β cell. TZDs have been shown to increase expression levels and activity of the GLUT1 transporter in skeletal muscle and adipocytes [75–77].
Insulin secretion and transcription are closely linked in the pancreatic β cell, and insulin gene expression is carefully regulated through a network of interacting transcription factors [78–80]. Pdx-1 is often referred to as the master pancreatic transcription factor and plays a key role in normal pancreatic and β cell development as well as maintenance of the mature β cell phenotype (reviewed in [81]).Homozygous loss ofPdx-1 is associated with pancreatic agenesis, while Pdx-1 haploinsufficiency results in a subtype of the monogenic diabetes disorders: MODY4 or Maturity Onset Diabetes of the Young 4, a disease characterized by impaired insulin secretion and subsequent hyperglycaemia [82–84]. In the mature β cell, Pdx-1 binds the proximal insulin promoter and positively regulates glucose-stimulated insulin transcription [85]. Pdx-1 gene and protein expression are also known to be downregulated in rodent and in vitro models of diabetes [74,86,87]. Further, in db/db mice and a rat insulinoma cell line (INS-1) incubated with the ER stress-inducing compound, thapsigargin, the loss of Pdx-1 expression was restored with pioglitazone [68]. A recent report by Gupta and colleagues showed that Pdx-1 is, in fact, a direct target of PPAR-γ in the islet [42].
Pharmacologic PPAR-γ activation in normal and diabetic β cells has been shown to upregulate a number of other genes whose products mediate key aspects of normal function. Chronic administration of pioglitazone to db/db mice led to upregulation of the gene encoding Nkx6.1, a protein that regulates insulin transcription, secretion and replication [88]. More acute treatment of INS-1 cells with troglitazone showed the same results [58]. Other genes similarly affected included NeuroD1, Kcnj11, Irs1 and the insulin gene [68,89]. However, to date, these genes have not been investigated or validated as direct PPAR-γ targets.
Prosurvival Effects of PPAR-γ Activation in Diabetic β Cells
In addition to the profunction effects described earlier, PPAR-γ activation in the pancreatic β cell has been associated with a variety of prosurvival effects arising within the context of T2DM. There are a number of toxic mediators in T2DM that result in activation of stress pathways. These include elevated glucose, free fatty acids, proinflammatory cytokines and islet amyloid polypeptide (IAPP). These factors work to promote β cell dysfunction and death through several intersecting mechanisms, including oxidative stress, mitochondrial dysfunction, epigenetic dysregulation and activation of ER stress pathways [18–21,90–92]. In addition, although much less well studied, PPAR-γ activation may also enhance β cell function and survival through activation of non-genomic pathways.
Modulation of ER Stress Pathways
One mechanism through which chronic demand on the β cell to produce and secrete insulin leads to deleterious effects is through the activation of ER stress pathways. Accumulation of mis- or unfolded protein in the ER leads to a state of ER stress and subsequent activation of the unfolded protein response (UPR). The UPR is a multifaceted response aimed at limiting the delivery of new unfolded proteins to the ER. If left unchecked, though, perpetual stimulation of the UPR can lead to activation of apoptotic pathways and β cell death [93]. In recent years, ER stress pathways and their contribution towards progression of pancreatic β cell dysfunction have been the focus of intense investigation (reviewed in [94,95]). In genetic models prone to the development of ER stress, the administration of PPAR-γ agonists has been shown to reduce this process in the β cell. Mice deficient in Wolfram syndrome protein, WFS1, in combination with the agouti mutation develop severe insulin-deficient diabetes by 8 weeks of age. This process is characterized by robust activation of ER stress pathways and loss of β cell mass. Although activation of the UPR persisted in pioglitazone-treated mice, there was a marked protection against the development of diabetes, improvement in ER lumen dilation, with resulting positive effects on β cell mass. The exact mechanisms underlying these benefits, however, were not directly investigated [96].
In db/db mice, the reduction of ER stress with pioglitazone treatment was co-incident with the restoration of Pdx-1 expression, nuclear localization of the histone methyl-transferase Set7/9 and the maintenance of islet euchromatin architecture [68]. In support of these findings, Pdx-1 haploin-sufficient mice have recently been shown to develop diabetes when stressed with high-fat feeding. This phenotype is because of a failed compensatory gain in β cell mass and increased apoptosis, which was attributed to ER stress. Interestingly, in this same report, the WFS1 gene was also found to be a direct target of Pdx-1 [97]. In aggregate, these findings suggest that the modulation of ER stress by PPAR-γ activation may be closely linked with the maintenance and/or restoration of Pdx-1 levels.
Protection Against Islet Amyloid Polypeptide
Oligomerization of islet amyloid polypeptide into toxic amyloid deposits is also reported to play a prominent role in the progressive β cell dysfunction of type 2 diabetes [98]. Lin and colleagues showed that rosiglitazone was able to prevent IAPP-induced apoptosis in human islets through interference with IAPP toxic oligomer formation. In this report, these effects were mediated through activation of the PI3 kinase pathway and AKT phosphorylation [99]. Whereas supraphysiologic levels of IAPP result in the induction of ER stress pathways [100], a recent report suggests that at levels of IAPP seen in human type 2 diabetes, ER stress is not an obligatory pathway for IAPP-mediated β cell dysfunction [101]. Notwithstanding this controversy, PPAR-γ agonists may also act to modulate IAPP-induced ER stress, although this effect has not been directly characterized.
Reductions in Oxidative Stress
Hyperglycaemia, lipotoxicity and elevated cytokines have all been shown to induce oxidative stress and mitochondrial dysfunction in islets and β cell lines [102,103]. A simple definition of oxidative stress is any condition that results in the generation of reactive oxygen (ROS) or reactive nitrogen species (RNS) that cannot be dealt with appropriately through normal antioxidant mechanisms [104]. The β cell has limited natural antioxidant capacity and limited capacity for dealing with ROS and RNS, therefore making it a particularly fragile target for oxidative stress [105,106]. In the setting of elevated glucose, glycolytic pathways become saturated and ROS are generated from alternative metabolic pathways, while elevated lipid levels lead to increased lipid esterification and generation of ceramides, which likewise generate oxidative stress [107,108]. Cytokines have also been shown to increase the production of ROS and RNS and lead to impaired islet function. In this regard, Wang and colleagues recently showed that rosiglitazone and pioglitazone were able to block interleukin-1β (IL-1) and interferon-γ (IFN)-induced apoptosis and rescue GSIS in NIT-1 mouse insulinoma cells [109].
Treatment of db/db mice with pioglitazone for 6 weeks improved glucose homeostasis and serum free fatty acid levels as well as islet function and islet insulin content. Pioglitazone treatment reduced islet expression of both hydroxynonenal (HNE) and hemoxygenase 1, which are known to be toxic by-products of oxidative stress [110]. In a similar study, 2 weeks of treatment with pioglitazone was also found to decrease HNE expression in pioglitazone-treated db/db mouse islets. This correlated with an increased expression of the genes encoding several antioxidants including glutathione peroxidase, superoxide dismutase and catalase. Further, pioglitazone treatment decreased the expression of the stress-inducing gene NADPH oxidase [89].
Pioglitazone has been shown to have even shorter term effects to decrease oxidative stress in MIN6 cells treated with high glucose and palmitate. Pharmacological PPAR-γ activation in this system was able to restore partially GSIS and suppress intraislet triglyceride accumulation, while decreasing uncoupling protein-2 (UCP-2) mRNA levels and decreasing the production of reactive oxygen species [111]. UCP-2 is an inner mitochrondrial membrane protein that is induced by ROS and decreases the metabolic efficiency of the β cell by uncoupling oxidative processes from ATP generation. Induction of UCP-2 decreases the ability of the β cell to secrete insulin [112,113]. In aggregate, these results suggest that both short-term and long-term treatments with pioglitazone can decrease islet oxidative stress, which translates into preservation of β cell function and survival in type 2 diabetes.
Protection Against Lipotoxicity
Exposure to elevated serum free fatty acids, ceramides and other toxic lipid intermediates can lead to increased triglyceride accumulation within the islet, which can have profound effects on β cell health [114–117]. The islet-specific KO of the ATP-binding membrane cassette transporter protein A1 (ABCA1) as well as the heterozygous PPAR-γ KO model both show enhanced intraislet triglyceride accumulation and diminished glucose-stimulated insulin secretion [40,118]. ABCA1 is a membrane-associated protein, which plays a key role in intra-cellular cholesterol homeostasis. ABCA1 functions to efflux cellular cholesterol and phospholipids to apolipoproteins and mediates the formation of high-density lipoprotein particles. In the heterozygous PPAR-γ model, treatment with pioglitazone was able to restore GSIS. Rosiglitazone, however, had no effect on glucose intolerance in the ABCA1 KO model, suggesting that the effects of TZDs may be mediated, in part, through ABCA1 [118]. Rosiglitazone has also been found to restore GSIS and decrease apoptosis in isolated human lipotoxic islets. Interestingly, these effects were co-incident with a reduction in intraislet triglyceride accumulation and reduced islet inducible nitric oxide synthase (iNOS) expression [119,120].
Potential Non-genomic Effects of PPAR-γ Agonists
Ligands of PPAR-γ may also function in a receptor- or DNA-independent manner to affect cellular function. These include effects that are too rapid to be explained by changes in gene transcription as well as responses that persist in PPAR-γ null models or following treatment with a PPAR-γ antagonist. Whereas many of the PPAR-γ mediated non-genomic effects have been reviewed in detail elsewhere [121–123], there is limited data on potential non-genomic effects within the pancreatic β cell. Of relevance to the diabetic islet though, PPAR-γ agonists have been shown to suppress inflammatory gene transcription in PPAR-γ null macrophages, suggesting that, in part, the antiinflammatory effects of these agents may occur independent of the receptor [124]. In addition, PPAR-γ has been shown to physically interact with transcriptional co-activators, thereby preventing these factors from binding gene targets leading to repression of inflammatory gene expression [125].
PPAR-γ agonists may also modulate a number of other signalling pathways. For instance, troglitazone has been shown to activate rapidly extracellular signal-regulated kinase (ERK)1/2 in human colorectal cell lines [126]. ERK1/2 signalling has been shown to be important in β cell survival, proliferation and glucose-dependent insulin gene transcription [127–129]. Likewise, the activation of c-Jun N-terminal kinase (JNK) in the islet leads to apoptosis and β cell death. PPAR-γ agonists have been shown to inhibit cytokine-induced activation of JNK in an insulinoma cell line [130]. Further, a recent report examined the metabolic effects of macelignan, a natural compound isolated from Myristica fragrans, which functions as a dual PPAR-γ/α agonist. Treatment of db/db mice with this agent improved glucose homeostasis and reduced JNK activation and ER stress in liver and adipose tissues [131].
The effects of PPAR-γ agonists on insulin signalling in the β cell have also been characterized. Paracrine stimulation of the insulin-signalling pathway in the β cell through phosphoinositide 3 (PI3) kinase and AKT has important implications for glucose homeostasis and β cell survival. Mice with a β cell-specific KO of the insulin receptor show markedly impaired first-phase insulin secretion and progressive glucose intolerance [132]. PPAR-γ agonists have been shown to increase AKT phosphorylation in the setting of both IAPP-and lipid-inducted toxicity. These effects were blocked by PI3 kinase inhibitors and associated with increased levels of insulin receptor substrate 2 (IRS2) protein [133].
Conclusions
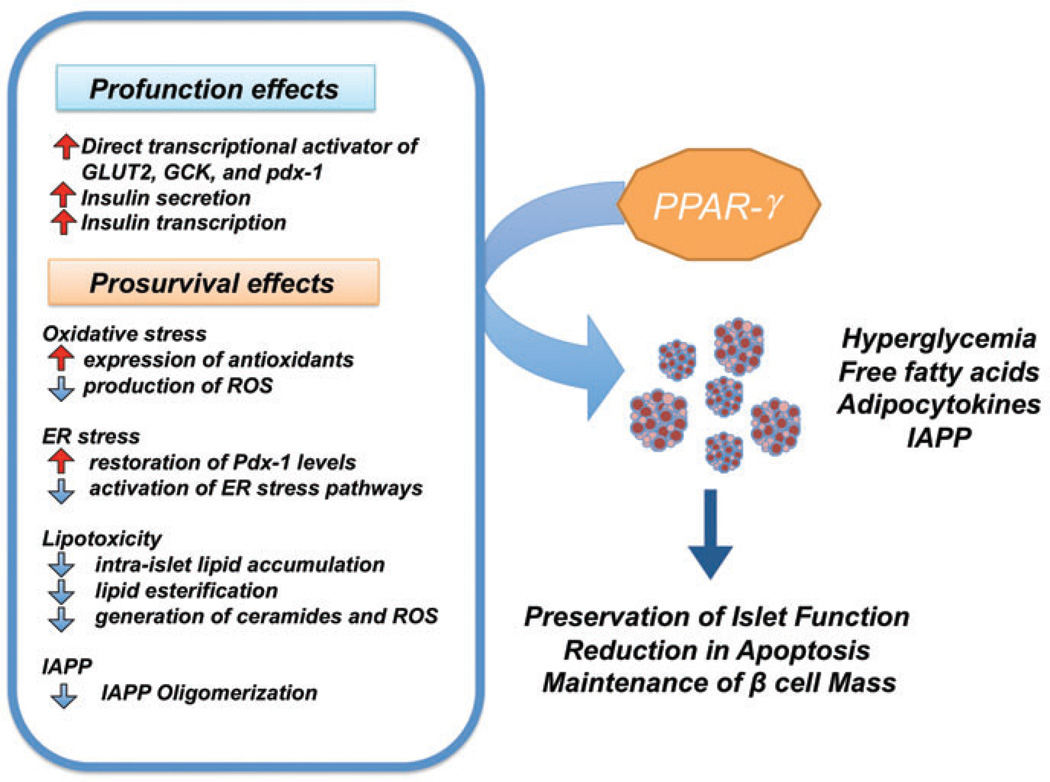
There is an abundance of preclinical and human data showing the β cell-specific profunction and prosurvival effects of pharmacological PPAR-γ activation in T2DM. These effects are summarized in figure 1, which illustrates an overall model of PPAR-γ mediated actions in the islet. Pharmacological activation of PPAR-γ has been shown to regulate transcription of several key β cell genes involved in glucose sensing, β cell development, GSIS and insulin gene transcription. Activation of these targets leads to enhanced insulin secretion and gene expression in the diabetic state. Further, treatment with PPAR-γ agonists has a number of prosurvival effects that limit death of the diabetic islet through reductions in cytokine-, lipid-, glucose- and IAPP-induced stress pathways.
Figure 1.
β cell-specific effects of peroxisome proliferator-activated receptor γ (PPAR-γ) agonists in type 2 diabetes mellitus. PPAR-γ directly regulates key β cell genes involved in glucose sensing, insulin secretion and insulin transcription including pdx-1, GLUT2 and glucokinase. Pharmacological PPAR-γ activation has also been shown to protect against glucose-, lipid-, cytokine- and IAPP-induced activation of stress pathways. These complementary profunction and prosurvival effects of PPAR-γ activation synergize to preserve β cell function and mass in type 2 diabetes mellitus.
Nevertheless, even in the context of this abundance of published data, there are several facets of PPAR-γ biology in the β cell that remain unexplained and require further investigation. First among those is a better understanding of the differences between the genetic models of islet-specific PPAR-γ deletion. More importantly, though, recent clinical data have questioned the safety of TZDs. In particular, use of these agents is associated with osteoporosis, fluid retention and congestive heart failure [134,135]. Even more serious, the use of rosiglitazone has been associated with an increased risk of adverse cardiac outcomes in diabetic patients [136]. In the clinical setting, these safety concerns should be weighed against the potential benefit of preserving β cell function in T2DM. As a research community, though, these remaining questions should serve as a ‘call to action’ to embark on studies aimed at explaining the tissue-specific effects of PPAR-γ agonism and understanding the differential molecular effects of specific TZDs. For instance, a recent report used microarray and chromatin immunoprecipitation to characterize regulation of gene expression and chromatin architecture by troglitazone, pioglitazone and rosiglitazone in 3T3-L1 adipocytes. Interestingly, the authors found that these three agents regulated similar but not completely overlapping sets of genes, supporting a model of selective PPAR modulation (SPPARM), analogous to the pharmacologically relevant selective estrogen receptor modulation (SERM) model [137]. These recently described safety concerns in combination with the SPPARM model speak to the importance of continued research into the biology of PPAR-γ . Importantly, these results suggest that ligands for PPAR-γ could and should be created to harness the profunction and prosurvival β cell-specific effects, while minimizing unintended consequences in the cardiovascular system and bone.
Acknowledgement
This work was supported by NIH grants T32 HL079995 (to T. K.) and K08 DK080225 and R03 DK 089147 (to C. E. M.).
Footnotes
Conflict of Interest
D. G., T. K. and C. E. M. worked together to create an outline for this review article. D. G. and T. K. worked on initial drafts, while C. E. M. was responsible for editing and compiling the final version.
All authors have no conflicts of interest.
References
- 1.World Health Organization. [Accessed 2 December 2010]; Available from URL: http://www.who.int.
- 2.Festa A, Williams K, D’Agostino RJ, Wagenknecht LE, Haffner SM. The natural course of beta-cell function in nondiabetic and diabetic individuals: the Insulin Resistance Atherosclerosis Study. Diabetes. 2006;55:1114–1120. doi: 10.2337/diabetes.55.04.06.db05-1100. [DOI] [PubMed] [Google Scholar]
- 3.Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA. 1999;281:2005–2012. doi: 10.1001/jama.281.21.2005. [DOI] [PubMed] [Google Scholar]
- 4.Sladek R, Rocheleau G, Rung J, et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445:881–885. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- 5.Florez JC. Newly identified loci highlight beta cell dysfunction as a key cause of type 2 diabetes: where are the insulin resistance genes? Diabetologia. 2007;51:1100–1110. doi: 10.1007/s00125-008-1025-9. [DOI] [PubMed] [Google Scholar]
- 6.Saxena R, Voight BF, Lyssenko V, et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316:1331–1336. doi: 10.1126/science.1142358. [DOI] [PubMed] [Google Scholar]
- 7.Zeggini E, Weedon MN, Lindgren CM, et al. Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science. 2007;316:1336–1341. doi: 10.1126/science.1142364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scott LJ, Mohlke KL, Bonnycastle LL, et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007;316:1341–1345. doi: 10.1126/science.1142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu Q, Song Y, Wang X, Won S, Cui Y, Elston RC. The effect of multiple genetic variants in predicting the risk of type 2 diabetes. BMC Proc. 2009;3(Suppl 7):S49. doi: 10.1186/1753-6561-3-s7-s49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haupt A, Staiger H, Schafer SA, et al. The risk allele load accelerates the age-dependent decline in beta cell function. Diabetologia. 2009;52:457–462. doi: 10.1007/s00125-008-1250-2. [DOI] [PubMed] [Google Scholar]
- 11.Villareal DT, Robertson H, Bell GI, et al. TCF7L2 variant rs7903 146 affects the risk of type 2 diabetes by modulating incretin action. Diabetes. 2010;59:479–485. doi: 10.2337/db09-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loos RJ, Franks PW, Francis RW, et al. TCF7L2 polymorphisms modulate proinsulin levels and beta-cell function in a British Europid population. Diabetes. 2007;56:1943–1947. doi: 10.2337/db07-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eftychi C, Howson JM, Barratt BJ, et al. Analysis of the type 2 diabetesassociated single nucleotide polymorphisms in the genes IRS 1, KCNJ1 1, and PPARG2 in type 1 diabetes. Diabetes. 2004;53:870–873. doi: 10.2337/diabetes.53.3.870. [DOI] [PubMed] [Google Scholar]
- 14.Knowler WC, Bennett PH, Hamman RF, Miller M. Diabetes incidence and prevalence in Pima Indians: a 19-fold greater incidence than in Rochester, Minnesota. Am J Epidemiol. 1978;108:497–505. doi: 10.1093/oxfordjournals.aje.a112648. [DOI] [PubMed] [Google Scholar]
- 15.Sam S, Sung YA, Legro RS, Dunaif A. Evidence for pancreatic beta-cell dysfunction in brothers of women with polycystic ovary syndrome. Metabolism. 2008;57:84–89. doi: 10.1016/j.metabol.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Staiger H, Machicao F, Fritsche A, Haring HU. Pathomechanisms of type 2 diabetes genes. Endocr Rev. 2009;30:557–585. doi: 10.1210/er.2009-0017. [DOI] [PubMed] [Google Scholar]
- 17.DeFronzo RA. Dysfunctional fat cells, lipotoxicity and type 2 diabetes. Int J Clin Pract Suppl. 2004;143:9–21. doi: 10.1111/j.1368-504x.2004.00389.x. [DOI] [PubMed] [Google Scholar]
- 18.Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. J Clin Invest. 2006;116:1802–1812. doi: 10.1172/JCI29103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaiser N, Leibowitz G. Failure of beta-cell adaptation in type 2 diabetes: lessons from animal models. Front Biosci. 2009;14:1099–1115. doi: 10.2741/3296. [DOI] [PubMed] [Google Scholar]
- 20.Robertson RP. Beta-cell deterioration during diabetes: what’s in the gun? Trends Endocrinol Metab. 2009;20:388–393. doi: 10.1016/j.tem.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boni-Schnetzler M, Ehses JA, Faulenbach M, Donath MY. Insulitis in type 2 diabetes. Diabetes Obes Metab. 2008;10(Suppl 4):201–204. doi: 10.1111/j.1463-1326.2008.00950.x. [DOI] [PubMed] [Google Scholar]
- 22.Gale EA. Lessons from the glitazones: a story of drug development. Lancet. 2001;357:1870–1875. doi: 10.1016/S0140-6736(00)04960-6. [DOI] [PubMed] [Google Scholar]
- 23.Yki-Jarvinen H. Thiazolidinediones. N Engl J Med. 2004;351:1106–1118. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- 24.Neuschwander-Tetri BA, Isley WL, Oki JC, et al. Troglitazone-induced hepatic failure leading to liver transplantation. A case report. Ann Intern Med. 1998;129:38–41. doi: 10.7326/0003-4819-129-1-199807010-00009. [DOI] [PubMed] [Google Scholar]
- 25.Nolan JJ, Ludvik B, Beerdsen P, Joyce M, Olefsky J. Improvement in glucose tolerance and insulin resistance in obese subjects treated with troglitazone. N Engl J Med. 1994;331:1188–1193. doi: 10.1056/NEJM199411033311803. [DOI] [PubMed] [Google Scholar]
- 26.Dubois M, Pattou F, Kerr-Conte J, et al. Expression of peroxisome proliferator-activated receptor gamma (PPARgamma) in normal human pancreatic islet cells. Diabetologia. 2000;43:1165–1169. doi: 10.1007/s001250051508. [DOI] [PubMed] [Google Scholar]
- 27.Braissant O, Foufelle F, Scotto C, Dauca M, Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology. 1996;137:354–366. doi: 10.1210/endo.137.1.8536636. [DOI] [PubMed] [Google Scholar]
- 28.Knowler WC, Hamman RF, Edelstein SL, et al. Prevention of type 2 diabetes with troglitazone in the Diabetes Prevention Program. Diabetes. 2005;54:1150–1156. doi: 10.2337/diabetes.54.4.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerstein HC, Yusuf S, Bosch J, et al. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet. 2006;368:1096–1105. doi: 10.1016/S0140-6736(06)69420-8. [DOI] [PubMed] [Google Scholar]
- 30.Azen SP, Peters RK, Berkowitz K, Kjos S, Xiang A, Buchanan TA. TRIPOD (TRoglitazone In the Prevention Of Diabetes): a randomized, placebocontrolled trial of troglitazone in women with prior gestational diabetes mellitus. Control Clin Trials. 1998;19:217–231. doi: 10.1016/s0197-2456(97)00151-7. [DOI] [PubMed] [Google Scholar]
- 31.Buchanan TA, Xiang AH, Peters RK, et al. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk hispanic women. Diabetes. 2002;51:2796–2803. doi: 10.2337/diabetes.51.9.2796. [DOI] [PubMed] [Google Scholar]
- 32.Xiang AH, Peters RK, Kjos SL, et al. Effect of pioglitazone on pancreatic beta-cell function and diabetes risk in Hispanic women with prior gestational diabetes. Diabetes. 2006;55:517–522. doi: 10.2337/diabetes.55.02.06.db05-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–2443. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 34.Juhl CB, Hollingdal M, Porksen N, Prange A, Lonnqvist F, Schmitz O. Influence of rosiglitazone treatment on beta-cell function in type 2 diabetes: evidence of an increased ability of glucose to entrain high-frequency insulin pulsatility. J Clin Endocrinol Metab. 2003;88:3794–3800. doi: 10.1210/jc.2002-021181. [DOI] [PubMed] [Google Scholar]
- 35.Wallace TM, Levy JC, Matthews DR. An increase in insulin sensitivity and basal beta-cell function in diabetic subjects treated with pioglitazone in a placebo-controlled randomized study. Diabet Med. 2004;21:568–576. doi: 10.1111/j.1464-5491.2004.01218.x. [DOI] [PubMed] [Google Scholar]
- 36.Roder ME, Porte DJ, Schwartz RS, Kahn SE. Disproportionately elevated proinsulin levels reflect the degree of impaired B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1998;83:604–608. doi: 10.1210/jcem.83.2.4544. [DOI] [PubMed] [Google Scholar]
- 37.Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. Thiazolidinediones improve beta-cell function in type 2 diabetic patients. Am J Physiol Endocrinol Metab. 2007;292:E871–E883. doi: 10.1152/ajpendo.00551.2006. [DOI] [PubMed] [Google Scholar]
- 38.Cavaghan MK, Ehrmann DA, Byrne MM, Polonsky KS. Treatment with the oral antidiabetic agent troglitazone improves beta cell responses to glucose in subjects with impaired glucose tolerance. J Clin Invest. 1997;100:530–537. doi: 10.1172/JCI119562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanley AJ, Zinman B, Sheridan P, Yusuf S, Gerstein HC. Effect of rosiglitazone and ramipril on {beta}-cell function in people with impaired glucose tolerance or impaired fasting glucose: the DREAM trial. Diabetes Care. 2010;33:608–613. doi: 10.2337/dc09-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsui J, Terauchi Y, Kubota N, et al. Pioglitazone reduces islet triglyceride content and restores impaired glucose-stimulated insulin secretion in heterozygous peroxisome proliferator-activated receptor-gammadeficient mice on a high-fat diet. Diabetes. 2004;53:2844–2854. doi: 10.2337/diabetes.53.11.2844. [DOI] [PubMed] [Google Scholar]
- 41.Rosen ED, Kulkarni RN, Sarraf P, et al. Targeted elimination of peroxisome proliferator-activated receptor gamma in beta cells leads to abnormalities in islet mass without compromising glucose homeostasis. Mol Cell Biol. 2003;23:7222–7229. doi: 10.1128/MCB.23.20.7222-7229.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gupta D, Jetton TL, Mortensen RM, Duan SZ, Peshavaria M, Leahy JL. In vivo and in vitro studies of a functional peroxisome proliferator-activated receptor gamma response element in the mouse pdx-1 promoter. J Biol Chem. 2008;283:32462–32470. doi: 10.1074/jbc.M801813200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gannon M, Shiota C, Postic C, Wright CV, Magnuson M. Analysis of the Cre-mediated recombination driven by rat insulin promoter in embryonic and adult mouse pancreas. Genesis. 2000;26:139–142. doi: 10.1002/(sici)1526-968x(200002)26:2<139::aid-gene12>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 44.Perez-Villamil B, Schwartz PT, Vallejo M. The pancreatic homeodomain transcription factor IDX1/IPF 1 is expressed in neural cells during brain development. Endocrinology. 1999;140:3857–3860. doi: 10.1210/endo.140.8.7048. [DOI] [PubMed] [Google Scholar]
- 45.Morioka T, Asilmaz E, Hu J, et al. Disruption of leptin receptor expression in the pancreas directly affects beta cell growth and function in mice. J Clin Invest. 2007;117:2860–2868. doi: 10.1172/JCI30910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Niswender KD, Magnuson MA. Obesity and the beta cell: lessons from leptin. J Clin Invest. 2007;117:2753–2756. doi: 10.1172/JCI33528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sarruf DA, Yu F, Nguyen HT, et al. Expression of peroxisome proliferator-activated receptor-gamma in key neuronal subsets regulating glucose metabolism and energy homeostasis. Endocrinology. 2009;150:707–712. doi: 10.1210/en.2008-0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee JY, Gavrilova O, Davani B, Na R, Robinson GW, Hennighausen L. The transcription factors Stat5a/b are not required for islet development but modulate pancreatic beta-cell physiology upon aging. Biochim Biophys Acta. 2007;1773:1455–1461. doi: 10.1016/j.bbamcr.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee JY, Ristow M, Lin X, White MF, Magnuson MA, Hennighausen L. RIP-Cre revisited, evidence for impairments of pancreatic beta-cell function. J Biol Chem. 2006;281:2649–2653. doi: 10.1074/jbc.M512373200. [DOI] [PubMed] [Google Scholar]
- 50.Venkatachalam G, Kumar AP, Yue LS, Pervaiz S, Clement MV, Sakharkar MK. Computational identification and experimental validation of PPRE motifs in NHE1 and MnSOD genes of human. BMC Genomics. 2009;10(Suppl 3):S5. doi: 10.1186/1471-2164-10-S3-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.IJpenberg A, Jeannin E, Wahli W, Desvergne B. Polarity specific sequence requirements of peroxisome proliferator-activated receptor (PPAR)/retinoid X receptor heterodimer binding to DNA. A functional analysis of the malic enzyme gene PPAR response element. J Biol Chem. 1997;272:20108–20117. doi: 10.1074/jbc.272.32.20108. [DOI] [PubMed] [Google Scholar]
- 52.Lemay DG, Hwang DH. Genome-wide identification of peroxisome proliferator response elements using integrated computational genomics. J Lipid Res. 2006;47:1583–1587. doi: 10.1194/jlr.M500504-JLR200. [DOI] [PubMed] [Google Scholar]
- 53.Okuno M, Arimoto E, Ikenobu Y, Nishihara T, Imagawa M. Dual DNA-binding specificity of peroxisome-proliferator-activated receptor gamma controlled by heterodimer formation with retinoid X receptor alpha. Biochem J. 2001;353:193–198. doi: 10.1042/0264-6021:3530193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guan HP, Ishizuka T, Chui PC, Lehrke M, Lazar MA. Corepressors selectively control the transcriptional activity of PPARgamma in adipocytes. Genes Dev. 2005;19:453–461. doi: 10.1101/gad.1263305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM. mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994;8:1224–1234. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- 56.Welters HJ, McBain SC, Tadayyon M, Scarpello JH, Smith SA, Morgan NG. Expression and functional activity of PPARgamma in pancreatic beta cells. Br J Pharmacol. 2004;142:1162–1170. doi: 10.1038/sj.bjp.0705844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chuang JC, Cha JY, Garmey JC, Mirmira RG, Repa JJ. Research resource: nuclear hormone receptor expression in the endocrine pancreas. Mol Endocrinol. 2008;22:2353–2363. doi: 10.1210/me.2007-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moibi JA, Gupta D, Jetton TL, Peshavaria M, Desai R, Leahy JL. Peroxisome proliferator activated receptor-{gamma} regulates expression of PDX-1 and NKX6. 1 in INS-1 cells. Diabetes. 2007;56:88–95. doi: 10.2337/db06-0948. [DOI] [PubMed] [Google Scholar]
- 59.Straub SG, James RF, Dunne MJ, Sharp GW. Glucose activates both K(ATP) channel-dependent and K(ATP) channel-independent signaling pathways in human islets. Diabetes. 1998;47:758–763. doi: 10.2337/diabetes.47.5.758. [DOI] [PubMed] [Google Scholar]
- 60.De Vos A, Heimberg H, Quartier E, et al. Human and rat beta cells differ in glucose transporter but not in glucokinase gene expression. J Clin Invest. 1995;96:2489–2495. doi: 10.1172/JCI118308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Elsner M, Tiedge M, Lenzen S. Mechanism underlying resistance of human pancreatic beta cells against toxicity of streptozotocin and alloxan. Diabetologia. 2003;46:1713–1714. doi: 10.1007/s00125-003-1241-2. [DOI] [PubMed] [Google Scholar]
- 62.Iynedjian PB. Molecular physiology of mammalian glucokinase. Cell Mol Life Sci. 2009;66:27–42. doi: 10.1007/s00018-008-8322-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tarasov A, Dusonchet J, Ashcroft F. Metabolic regulation of the pancreatic beta-cell ATP-sensitive K+ channel: apas de deux. Diabetes. 2004;53(Suppl 3):S113–S122. doi: 10.2337/diabetes.53.suppl_3.s113. [DOI] [PubMed] [Google Scholar]
- 64.Hou JC, Min L, Pessin JE. Insulin granule biogenesis, trafficking and exocytosis. Vitam Horm. 2009;80:473–506. doi: 10.1016/S0083-6729(08)00616-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jensen MV, Joseph JW, Ronnebaum SM, Burgess SC, Sherry AD, Newgard CB. Metabolic cycling in control of glucose-stimulated insulin secretion. Am J Physiol Endocrinol Metab. 2008;295:E1287–E1297. doi: 10.1152/ajpendo.90604.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jitrapakdee S, Wutthisathapornchai A, Wallace JC, MacDonald MJ. Regulation of insulin secretion: role of mitochondrial signalling. Diabetologia. 2010;53:1019–1032. doi: 10.1007/s00125-010-1685-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.De Leon DD, Crutchlow MF, Ham JY, Stoffers DA. Role of glucagon-like peptide-1 in the pathogenesis and treatment of diabetes mellitus. Int J Biochem Cell Biol. 2006;38:845–859. doi: 10.1016/j.biocel.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 68.Evans-Molina C, Robbins RD, Kono T, et al. PPAR-{gamma} activation restores islet function in diabetic mice through reduction of ER stress and maintenance of euchromatin structure. Mol Cell Biol. 2009;29:2053–2067. doi: 10.1128/MCB.01179-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim HI, Ahn YH. Role of peroxisome proliferator-activated receptorgamma in the glucose-sensing apparatus of liver and beta-cells. Diabetes. 2004;53(Suppl 1):S60–S65. doi: 10.2337/diabetes.53.2007.s60. [DOI] [PubMed] [Google Scholar]
- 70.Jitrapakdee S, Slawik M, Medina-Gomez G, et al. The peroxisome proliferator-activated receptor-gamma regulates murine pyruvate car-boxylase gene expression in vivo and in vitro. J Biol Chem. 2005;280:27466–27476. doi: 10.1074/jbc.M503836200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gupta D, Peshavaria M, Monga N, Jetton TL, Leahy JL. Physiologic and pharmacologic modulation of glucose-dependent insulinotropic polypeptide (GIP) receptor expression in beta-cells by peroxisome proliferator-activated receptor (PPAR)-gamma signaling: possible mechanism for the GIP resistance in type 2 diabetes. Diabetes. 2010;59:1445–1450. doi: 10.2337/db09-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Im SS, Kim JW, Kim TH, et al. Identification and characterization of peroxisome proliferator response element in the mouse GLUT2 promoter. Exp Mol Med. 2005;37:101–110. doi: 10.1038/emm.2005.14. [DOI] [PubMed] [Google Scholar]
- 73.Kim HI, Kim JW, Kim SH, Cha JY, Kim KS, Ahn YH. Identification and functional characterization of the peroxisomal proliferator response element in rat GLUT2 promoter. Diabetes. 2000;49:1517–1524. doi: 10.2337/diabetes.49.9.1517. [DOI] [PubMed] [Google Scholar]
- 74.Laybutt DR, Hawkins YC, Lock J, et al. Influence of diabetes on the loss of beta cell differentiation after islet transplantation in rats. Diabetologia. 2007;50:2117–2125. doi: 10.1007/s00125-007-0749-2. [DOI] [PubMed] [Google Scholar]
- 75.Liao W, Nguyen MT, Yoshizaki T, et al. Suppression of PPAR-gamma attenuates insulin-stimulated glucose uptake by affecting both GLUT 1 and GLUT4 in 3T3-L1 adipocytes. Am J Physiol Endocrinol Metab. 2007;293:E219–E227. doi: 10.1152/ajpendo.00695.2006. [DOI] [PubMed] [Google Scholar]
- 76.Tafuri SR. Troglitazone enhances differentiation, basal glucose uptake, and Glut1 protein levels in 3T3-L 1 adipocytes. Endocrinology. 1996;137:4706–4712. doi: 10.1210/endo.137.11.8895337. [DOI] [PubMed] [Google Scholar]
- 77.Park KS, Ciaraldi TP, Abrams-Carter L, Mudaliar S, Nikoulina SE, Henry RR. Troglitazone regulation of glucose metabolism in human skeletal muscle cultures from obese type II diabetic subjects. J Clin Endocrinol Metab. 1998;83:1636–1643. doi: 10.1210/jcem.83.5.4764. [DOI] [PubMed] [Google Scholar]
- 78.Leibiger B, Wahlander K, Berggren PO, Leibiger IB. Glucose-stimulated insulin biosynthesis depends on insulin-stimulated insulin gene transcription. J Biol Chem. 2000;275:30153–30156. doi: 10.1074/jbc.M005216200. [DOI] [PubMed] [Google Scholar]
- 79.Goodison S, Ashcroft SJ. Trans-acting factor(s) confer glucose-responsive transcriptional regulation in the insulin gene. Adv Exp Med Biol. 1997;426:97–100. doi: 10.1007/978-1-4899-1819-2_13. [DOI] [PubMed] [Google Scholar]
- 80.Melloul D, Marshak S, Cerasi E. Regulation of insulin gene transcription. Diabetologia. 2002;45:309–326. doi: 10.1007/s00125-001-0728-y. [DOI] [PubMed] [Google Scholar]
- 81.Babu DA, Deering TG, Mirmira RG. A feat of metabolic proportions: Pdx1 orchestrates islet development and function in the maintenance of glucose homeostasis. Mol Genet Metab. 2007;92:43–55. doi: 10.1016/j.ymgme.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hani EH, Stoffers DA, Chevre JC, et al. Defective mutations in the insulin promoter factor-1 (IPF1) gene in late-onset type 2 diabetes mellitus. J Clin Invest. 1999;104:R41–R48. doi: 10.1172/JCI7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stoffers DA, Zinkin NT, Stanojevic V, Clarke WL, Habener JF. Pancreatic agenesis attributable to a single nucleotide deletion in the human IPF 1 gene coding sequence. Nat Genet. 1997;15:106–110. doi: 10.1038/ng0197-106. [DOI] [PubMed] [Google Scholar]
- 84.Fajans SS, Bell GI, Polonsky KS. Molecular mechanisms and clinical pathophysiology of maturity-onset diabetes of the young. N Engl J Med. 2001;345:971–980. doi: 10.1056/NEJMra002168. [DOI] [PubMed] [Google Scholar]
- 85.Le Lay J, Stein R. Involvement of PDX-1 in activation of human insulin gene transcription. J Endocrinol. 2006;188:287–294. doi: 10.1677/joe.1.06510. [DOI] [PubMed] [Google Scholar]
- 86.Suzuki R, Tobe K, Terauchi Y, et al. Pdx1 expression in Irs2-deficient mouse beta-cells is regulated in a strain-dependent manner. J Biol Chem. 2003;278:43691–43698. doi: 10.1074/jbc.M307004200. [DOI] [PubMed] [Google Scholar]
- 87.Moritoh Y, Takeuchi K, Asakawa T, Kataoka O, Odaka H. Combining a dipeptidyl peptidase-4 inhibitor, alogliptin, with pioglitazone improves glycaemic control, lipid profiles and beta-cell function in db/db mice. Br J Pharmacol. 2009;157:415–426. doi: 10.1111/j.1476-5381.2009.00145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schisler JC, Fueger PT, Babu DA, et al. Stimulation of human and rat islet beta-cell proliferation with retention of function by the homeodomain transcription factor Nkx6. 1. Mol Cell Biol. 2008;28:3465–3476. doi: 10.1128/MCB.01791-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kanda Y, Shimoda M, Hamamoto S, et al. Molecular mechanism by which pioglitazone preserves pancreatic {beta} cells in obese diabetic mice: evidence for acute and chronic actions as a PPAR{gamma} agonist. Am J Physiol Endocrinol Metab. 2010;298:278–286. doi: 10.1152/ajpendo.00388.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cunha DA, Hekerman P, Ladriere L, et al. Initiation and execution of lipotoxic ER stress in pancreatic beta-cells. J Cell Sci. 2008;121:2308–2318. doi: 10.1242/jcs.026062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Leahy JL. Mary, Mary, quite contrary, how do your beta-cells fail? Diabetes. 2008;57:2563–2564. doi: 10.2337/db08-0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ritzel RA, Meier JJ, Lin CY, Veldhuis JD, Butler PC. Human islet amyloid polypeptide oligomers disrupt cell coupling, induce apoptosis, and impair insulin secretion in isolated human islets. Diabetes. 2007;56:65–71. doi: 10.2337/db06-0734. [DOI] [PubMed] [Google Scholar]
- 93.Schroder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res. 2005;569:29–63. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 94.Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev. 2008;29:42–61. doi: 10.1210/er.2007-0015. [DOI] [PubMed] [Google Scholar]
- 95.Fonseca SG, Burcin M, Gromada J, Urano F. Endoplasmic reticulum stress in beta-cells and development of diabetes. Curr Opin Pharmacol. 2009;9:763–770. doi: 10.1016/j.coph.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Akiyama M, Hatanaka M, Ohta Y, et al. Increased insulin demand promotes while pioglitazone prevents pancreatic beta cell apoptosis in Wfs1 knockout mice. Diabetologia. 2009;52:653–663. doi: 10.1007/s00125-009-1270-6. [DOI] [PubMed] [Google Scholar]
- 97.Sachdeva MM, Claiborn KC, Khoo C, et al. Pdx1 (MODY4) regulates pancreatic beta cell susceptibility to ER stress. Proc Natl Acad Sci U S A. 2009;106:19090–19095. doi: 10.1073/pnas.0904849106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Haataja L, Gurlo T, Huang CJ, Butler PC. Islet amyloid in type 2 diabetes, and the toxic oligomer hypothesis. Endocr Rev. 2008;29:303–316. doi: 10.1210/er.2007-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lin CY, Gurlo T, Haataja L, Hsueh WA, Butler PC. Activation of peroxisome proliferator-activated receptor-gamma by rosiglitazone protects human islet cells against human islet amyloid polypeptide toxicity by a phosphatidylinositol 3’-kinase-dependent pathway. J Clin Endocrinol Metab. 2005;90:6678–6686. doi: 10.1210/jc.2005-0079. [DOI] [PubMed] [Google Scholar]
- 100.Matveyenko AV, Gurlo T, Daval M, Butler AE, Butler PC. Successful versus failed adaptation to high-fat diet-induced insulin resistance: the role of IAPP-induced beta-cell endoplasmic reticulum stress. Diabetes. 2009;58:906–916. doi: 10.2337/db08-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hull RL, Zraika S, Udayasankar J, Aston-Mourney K, Subramanian SL, Kahn SE. Amyloid formation in human IAPP transgenic mouse islets and pancreas, and human pancreas, is not associated with endoplasmic reticulum stress. Diabetologia. 2009;52:1102–1111. doi: 10.1007/s00125-009-1329-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Oprescu AI, Bikopoulos G, Naassan A, et al. Free fatty acid-induced reduction in glucose-stimulated insulin secretion: evidence for a role of oxidative stress in vitro and in vivo. Diabetes. 2007;56:2927–2937. doi: 10.2337/db07-0075. [DOI] [PubMed] [Google Scholar]
- 103.Tanaka Y, Tran PO, Harmon J, Robertson RP. A role for glutathione peroxidase in protecting pancreatic beta cells against oxidative stress in a model of glucose toxicity. Proc Natl Acad Sci U S A. 2002;99:12363–12368. doi: 10.1073/pnas.192445199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li N, Frigerio F, Maechler P. The sensitivity of pancreatic beta-cells to mitochondrial injuries triggered by lipotoxicity and oxidative stress. Biochem Soc Trans. 2008;36:930–934. doi: 10.1042/BST0360930. [DOI] [PubMed] [Google Scholar]
- 105.Tiedge M, Lortz S, Drinkgern J, Lenzen S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes. 1997;46:1733–1742. doi: 10.2337/diab.46.11.1733. [DOI] [PubMed] [Google Scholar]
- 106.Grankvist K, Marklund SL, Taljedal IB. CuZn-superoxide dismutase, Mnsuperoxide dismutase, catalase and glutathione peroxidase in pancreatic islets and other tissues in the mouse. Biochem J. 1981;199:393–398. doi: 10.1042/bj1990393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shimabukuro M, Zhou YT, Levi M, Unger RH. Fatty acid-induced beta cell apoptosis: a link between obesity and diabetes. Proc Natl Acad Sci U S A. 1998;95:2498–2502. doi: 10.1073/pnas.95.5.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Robertson RP. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. J Biol Chem. 2004;279:42351–42354. doi: 10.1074/jbc.R400019200. [DOI] [PubMed] [Google Scholar]
- 109.Wang AP, Li X, Liu BL, et al. Inhibitory effects of thiazolidinedione upon cytokine-induced apoptosis in pancreatic beta-cell line. Zhonghua Yi Xue Za Zhi. 2009;89:1989–1993. [PubMed] [Google Scholar]
- 110.Ishida H, Takizawa M, Ozawa S, et al. Pioglitazone improves insulin secretory capacity and prevents the loss of beta-cell mass in obese diabetic db/db mice: possible protection of beta cells from oxidative stress. Metabolism. 2004;53:488–494. doi: 10.1016/j.metabol.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 111.Saitoh Y, Chun-Ping C, Noma K, Ueno H, Mizuta M, Nakazato M. Pioglitazone attenuates fatty acid-induced oxidative stress and apoptosis in pancreatic beta-cells. Diabetes Obes Metab. 2008;10:564–573. doi: 10.1111/j.1463-1326.2007.00749.x. [DOI] [PubMed] [Google Scholar]
- 112.Chan CB, Kashemsant N. Regulation of insulin secretion by uncoupling protein. Biochem Soc Trans. 2006;34:802–805. doi: 10.1042/BST0340802. [DOI] [PubMed] [Google Scholar]
- 113.Chan CB, Saleh MC, Koshkin V, Wheeler MB. Uncoupling protein 2 and islet function. Diabetes. 2004;53(Suppl 1):S136–S142. doi: 10.2337/diabetes.53.2007.s136. [DOI] [PubMed] [Google Scholar]
- 114.Hagman DK, Hays LB, Parazzoli SD, Poitout V. Palmitate inhibits insulin gene expression by altering PDX-1 nuclear localization and reducing MafA expression in isolated rat islets of Langerhans. J Biol Chem. 2005;280:32413–32418. doi: 10.1074/jbc.M506000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kelpe CL, Moore PC, Parazzoli SD, Wicksteed B, Rhodes CJ, Poitout V. Palmitate inhibition of insulin gene expression is mediated at the transcriptional level via ceramide synthesis. J Biol Chem. 2003;278:30015–30021. doi: 10.1074/jbc.M302548200. [DOI] [PubMed] [Google Scholar]
- 116.Poitout V, Hagman D, Stein R, Artner I, Robertson RP, Harmon JS. Regulation of the insulin gene by glucose and fatty acids. J Nutr. 2006;136:873–876. doi: 10.1093/jn/136.4.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kawai T, Hirose H, Seto Y, et al. Troglitazone ameliorates lipotoxicity in the beta cell line INS-1 expressing PPAR gamma. Diabetes Res Clin Pract. 2002;56:83–92. doi: 10.1016/s0168-8227(01)00367-9. [DOI] [PubMed] [Google Scholar]
- 118.Brunham LR, Kruit JK, Pape TD, et al. Beta-cell ABCA1 influences insulin secretion, glucose homeostasis and response to thiazolidinedione treatment. Nat Med. 2007;13:340–347. doi: 10.1038/nm1546. [DOI] [PubMed] [Google Scholar]
- 119.Vandewalle B, Moerman E, Lefebvre B, et al. PPARgamma-dependent and -independent effects of rosiglitazone on lipotoxic human pancreatic islets. Biochem Biophys Res Commun. 2008;366:1096–1101. doi: 10.1016/j.bbrc.2007.12.088. [DOI] [PubMed] [Google Scholar]
- 120.Lupi R, Del Guerra S, Marselli L, et al. Rosiglitazone prevents the impairment of human islet function induced by fatty acids: evidence for a role of PPARgamma2 in the modulation of insulin secretion. Am J Physiol Endocrinol Metab. 2004;286:E560–E567. doi: 10.1152/ajpendo.00561.2002. [DOI] [PubMed] [Google Scholar]
- 121.Feinstein DL, Spagnolo A, Akar C, et al. Receptor-independent actions of PPAR thiazolidinedione agonists: is mitochondrial function the key? Biochem Pharmacol. 2005;70:177–188. doi: 10.1016/j.bcp.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 122.Papageorgiou E, Pitulis N, Msaouel P, Lembessis P, Koutsilieris M. The non-genomic crosstalk between PPAR-gamma ligands and ERK1/2 in cancer cell lines. Expert Opin Ther Targets. 2007;11:1071–1085. doi: 10.1517/14728222.11.8.1071. [DOI] [PubMed] [Google Scholar]
- 123.Luconi M, Cantini G, Serio M. Peroxisome proliferator-activated receptor gamma (PPARgamma): is the genomic activity the only answer? Steroids. 2010;75:585–594. doi: 10.1016/j.steroids.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 124.Chawla A, Barak Y, Nagy L, Liao D, Tontonoz P, Evans RM. PPAR-gamma dependent and independent effects on macrophage-gene expression in lipid metabolism and inflammation. Nat Med. 2001;7:48–52. doi: 10.1038/83336. [DOI] [PubMed] [Google Scholar]
- 125.Chung SW, Kang BY, Kim SH, et al. Oxidized low density lipoprotein inhibits interleukin-12 production in lipopolysaccharide-activated mouse macrophages via direct interactions between peroxisome proliferator-activated receptor-gamma and nuclear factor-kappa B. J Biol Chem. 2000;275:32681–32687. doi: 10.1074/jbc.M002577200. [DOI] [PubMed] [Google Scholar]
- 126.Baek SJ, Wilson LC, Hsi LC, Eling TE. Troglitazone, a peroxisome proliferator-activated receptor gamma (PPAR gamma) ligand, selectively induces the early growth response-1 gene independently of PPAR gamma. A novel mechanism for its anti-tumorigenic activity. J Biol Chem. 2003;278:5845–5853. doi: 10.1074/jbc.M208394200. [DOI] [PubMed] [Google Scholar]
- 127.Arnette D, Gibson TB, Lawrence MC, et al. Regulation of ERK1 and ERK2 by glucose and peptide hormones in pancreatic beta cells. J Biol Chem. 2003;278:32517–32525. doi: 10.1074/jbc.M301174200. [DOI] [PubMed] [Google Scholar]
- 128.Khoo S, Gibson TB, Arnette D, et al. MAP kinases and their roles in pancreatic beta-cells. Cell Biochem Biophys. 2004;40:191–200. doi: 10.1385/cbb:40:3:191. [DOI] [PubMed] [Google Scholar]
- 129.Lawrence MC, McGlynn K, Park BH, Cobb MH. ERK1/2-dependent activation of transcription factors required for acute and chronic effects of glucose on the insulin gene promoter. J Biol Chem. 2005;280:26751–26759. doi: 10.1074/jbc.M503158200. [DOI] [PubMed] [Google Scholar]
- 130.Maggi LBJ, Sadeghi H, Weigand C, Scarim AL, Heitmeier MR, Corbett JA. Anti-inflammatory actions of 1 5-deoxy-delta 12, 14-prostaglandin J2 and troglitazone: evidence for heat shock-dependent and -independent inhibition of cytokine-induced inducible nitric oxide synthase expression. Diabetes. 2000;49:346–355. doi: 10.2337/diabetes.49.3.346. [DOI] [PubMed] [Google Scholar]
- 131.Han KL, Choi JS, Lee JY, et al. Therapeutic potential of peroxisome proliferator-activated receptor-alpha/gamma dual agonist with alleviation of endoplasmic reticulum stress for the treatment of diabetes. Diabetes. 2008;57:737–745. doi: 10.2337/db07-0972. [DOI] [PubMed] [Google Scholar]
- 132.Kulkarni RN, Bruning JC, Winnay JN, Postic C, Magnuson MA, Kahn CR. Tissue-specific knockout of the insulin receptor in pancreatic beta cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell. 1999;96:329–339. doi: 10.1016/s0092-8674(00)80546-2. [DOI] [PubMed] [Google Scholar]
- 133.Yang L, An HX, Deng XL, Chen LL, Li ZY. Rosiglitazone reverses insulin secretion altered by chronic exposure to free fatty acid via IRS-2-associated phosphatidylinositol 3-kinase pathway. Acta Pharmacol Sin. 2003;24:429–434. [PubMed] [Google Scholar]
- 134.Schwartz AV, Sellmeyer DE, Vittinghoff E, et al. Thiazolidinedione use and bone loss in older diabetic adults. J Clin Endocrinol Metab. 2006;91:3349–3354. doi: 10.1210/jc.2005-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lipscombe LL, Gomes T, Levesque LE, Hux JE, Juurlink DN, Alter DA. Thiazolidinediones and cardiovascular outcomes in older patients with diabetes. JAMA. 2007;298:2634–2643. doi: 10.1001/jama.298.22.2634. [DOI] [PubMed] [Google Scholar]
- 136.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 137.Sears DD, Hsiao A, Ofrecio JM, Chapman J, He W, Olefsky JM. Selective modulation of promoter recruitment and transcriptional activity of PPARgamma. Biochem Biophys Res Commun. 2007;364:515–521. doi: 10.1016/j.bbrc.2007.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Prigeon RL, Kahn SE, Porte DJ. Effect of troglitazone on B cell function, insulin sensitivity, and glycemic control in subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab. 1998;83:819–823. doi: 10.1210/jcem.83.3.4641. [DOI] [PubMed] [Google Scholar]
- 139.Miyazaki Y, Matsuda M, DeFronzo RA. Dose-response effect of pioglitazone on insulin sensitivity and insulin secretion in type 2 diabetes. Diabetes Care. 2002;25:517–523. doi: 10.2337/diacare.25.3.517. [DOI] [PubMed] [Google Scholar]