Abstract
Transcranial direct current stimulation (tDCS) affects neurons at both cortical and subcortical levels. The subcortical effects involve several descending motor systems but appeared to be relatively weak, as only small increases in the amplitude of subcortically initiated descending volleys and a minute shortening of latencies of these volleys were found. The aim of the present study was therefore to evaluate the consequences of facilitation of these volleys on the ensuing muscle activation. The experiments were carried out on deeply anaesthetized rats without neuromuscular blockade. Effects of tDCS were tested on EMG potentials recorded from neck muscles evoked by weak (20–60 μA) single, double or triple stimuli applied in the medial longitudinal fascicle (MLF) or in the red nucleus (RN). Short latencies of these potentials were compatible with monosynaptic or disynaptic actions of reticulospinal and disynaptic or trisynaptic actions of rubrospinal neurons on neck motoneurons. Despite only weak effects on indirect descending volleys, the EMG responses from both the MLF and the RN were potently facilitated by cathodal tDCS and depressed by anodal tDCS. Both the facilitation and the depression developed relatively rapidly (within the first minute) but both outlasted tDCS and were present for up to 1 h after tDCS. The study thus demonstrates long-lasting effects of tDCS on subcortical neurons in the rat, albeit evoked by an opposite polarity of tDCS to that found to be effective on subcortical neurons in the cat investigated in the preceding study, or for cortical neurons in the humans.
Key points
Previously demonstrated facilitation of activation of subcortical neurons by transcranial direct current stimulation (tDCS) in acute experiments on deeply anaesthetized animals was fairly weak. It resulted in only small increases in the amplitude and in a slight shortening of latencies of subcortically initiated descending volleys.
Here we show that despite weak effects on descending volleys, EMG responses evoked in neck muscles by reticulospinal and rubrospinal neurons in deeply anaesthetized non-paralysed rats are potently facilitated by tDCS and that the facilitation outlasts tDCS.
We further show that the facilitatory subcortical effects of tDCS in the rat are evoked by cathodal rather than anodal polarization, i.e. by a polarity that is the reverse of that most often found to be effective in humans and in the cat. Anodal polarization depressed activation of the same rat subcortical neurons.
These findings should assist further studies of mechanisms of tDCS in vivo in rodents.
Introduction
Transcranial direct current stimulation (tDCS) was recently found to evoke prolonged facilitation of activation of subcortical neurons in the cat (Bolzoni et al. 2013), replicating tDCS effects on cortical neurons in both humans and animals (for recent reviews see e.g. Boggio et al. 2008; Di Lazzaro et al. 2008; Brunoni et al. 2011, 2012; Stagg & Nitsche, 2011). The facilitation involved both direct and transsynaptic activation of rubrospinal, reticulospinal and vestibulospinal neurons, as judged from the descending volleys induced in their axons. However, since Bolzoni et al. (2013) investigated the effects of tDCS on deeply anaesthetized animals in which neuromuscular transmission was blocked, they were unable to evaluate the functional consequences of relatively small increases in the amplitude of these descending volleys and of only marginal shortening of their latencies. In order to address this problem the present study was carried out on similarly deeply anaesthetized rats without neuromuscular blockade in which responses to subcortically applied MLF and RN stimuli could be monitored by EMG potentials.
Monitoring the effects of tDCS on EMG potentials also had further advantages. One of these was that by using weak (20–60 μA) MLF stimuli we could evoke EMG potentials in only very few motor units, in extreme cases in only a single unit. As activation of these units at threshold was very labile, EMG potentials were a much more sensitive marker of the outcome of tDCS than the descending volleys. Another advantage was that, unlike the descending volleys, any changes in EMG responses were easily detectable during the experiment, helping to optimize the stimulus parameters at the beginning of each experiment.
It will be shown that tDCS facilitated EMG responses of neck muscles evoked by near-threshold MLF or RN stimulation to a much greater extent than descending volleys evoked by the same stimuli and that the facilitation manifested itself both during tDCS and during the subsequent periods of up to 1 h. Moreover our results show that subcortical effects of tDCS in the rat differ from those in the cat in that cathodal rather than anodal tDCS is facilitatory while effects of anodal tDCS are depressive.
Methods
Ethical approval
All experiments were approved by the Regional Ethics Committee for Animal Research (Göteborgs Djurförsöksetiska Nämnd) and complied with NIH and EU guidelines for animal care and the ethical policies and regulations of The Journal of Physiology (Drummond, 2009). The animals were bred and housed under veterinary supervision at the Laboratory of Experimental Biomedicine at Sahlgrenska Academy where the experiments were carried out.
Preparation
The experiments were performed on 23 deeply anaesthetized adult rats of both sexes weighing 200–300 g (15 Sprague–Dawley, 8 Wistar). The anaesthesia was induced with isoflurane (Baxter Medical AB, Sweden) and was followed by either medetomidine hydrochloride (Dormitor, Orion Pharma, Finland; 1 mg kg−1 i.p.) and fentanyl (Leptanal, Janssen, 0.2 mg kg−1 i.p.) or α-chloralose (Rhône-Poulenc Santé, France; 60– 70 mg kg−1, i.p. supplemented by pentobarbital sodium (APL, Sweden; 10–20 mg kg−1 i.p.). Under Dormitor and Leptanal anaesthesia the heart rate was low (about 150–200 beats min−1) during the first 1–2 h and returned to normal rates of about 250–450 beats min−1 after only 2–3 h. In contrast, the level of chloralose/pentobarbital anaesthesia sufficient to abolish withdrawal reflexes was stable during 6–8 h, with heart rate in the normal range (350–400 beats min−1) from the very beginning to the end of the experiments. The majority of experiments were therefore carried out under pentobarbital/chloralose anaesthesia. During the preliminary dissection the rats were intubated and the respiration was assisted by connecting the tracheal tube to a high frequency (60–70 Hz) and low volume respiratory pump to maintain the CO2 level in the expired air at about 3.5–4%. The same parameters of artificial respiration were used in two rats when the neuromuscular transmission was blocked by pancuronium bromide (Pavulon, Organon, Sweden; 0.3 mg kg−1 i.v.). The core body temperature was kept at about 38°C by servo-controlled heating lamps. In order to compensate for fluid loss 10 ml of acetate buffer was injected subcutaneously at the beginning of the experiments. The experiments were terminated by a near-lethal dose of pentobarbital i.p., formalin perfusion and removal of the brain for histological control.
Following the anaesthesia and tracheal canulation, the head of the rat was placed in a stereotactic frame, keeping the upper incisor level either at the inter-aural line or 3.3 mm below this line. Thereafter, the caudal part of the cerebellum was exposed to the foramen magnum and the area between the C1 and C2 spinal segments by laminectomy. Electrodes to be placed in the left MLF were inserted through the cerebellum at an angle of 20 deg (with the tip directed rostral). They were introduced about 0.1–0.2 mm lateral to the midline, aiming at a location about 2 mm rostral of the obex at a depth about 1 mm below the surface of 4th ventricle, as subsequently verified histologically (see Fig. 1A). Electrodes to be placed in the right RN were introduced at an angle of 90 deg with respect to the horizontal plane aiming at coordinates 5.3 mm caudal to the bregma, 1 mm from the midline and 7.5 mm from the surface according to the stereotaxic atlas of Paxinos and Watson (Ginanneschi et al. 2005) using a Neurostar motorized stereotactic system (Neurostar GmbH, Germany). The final depths of both the MLF and RN electrodes were adjusted taking into account descending volleys evoked while the electrodes were lowered down. They were left at the locations from which the lowest threshold (10–20 μA) direct descending volleys were evoked by single stimuli and indirect volleys and EMG responses followed a train of 2–4 stimuli at 330 or 400 Hz.
Figure 1. Stimulation sites in and around the MLF and the RN.
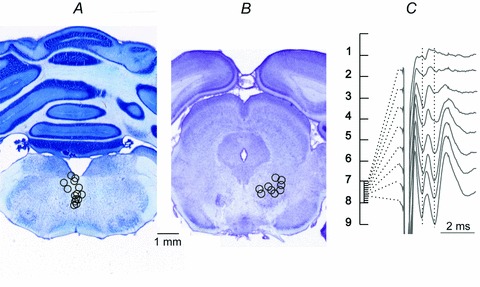
A and B, reconstructions of the locations of the stimulating electrodes in and around the MLF and the RN, indicated on representative sections of the medulla and the mesencephalon, respectively. The locations were defined by electrolytic lesions made at the end of the experiments (0.2 mA constant current for 15 s), verified on 100 μm thick sections, cut in the plane of the electrode insertions using a vibratome, mounted on slides, counterstained with cresyl violet and scanned. Stimulation sites are indicated by circles corresponding to the centres of the lesions. The diameters of these circles reflect the areas of approximate spread of current from these sites, within 0.2–0.5 mm for stimuli of 20–50 μA (see Fig. 11 in Gustafsson & Jankowska, 1976). C, indirect volleys (with the onset indicated by the first vertical dotted line) and EMG potentials (indicated by the second dotted line) evoked by 60 μA single stimuli applied along one of the most medial electrode tracks in the RN. Averages of 20 potentials recorded every 100 μm at depths 7.0–7.7 mm from the surface of the skull. Note that the maximal EMG potentials and indirect volleys were evoked from the same electrode sites within RN.
Stimulation and recording
MLF and RN were stimulated monopolarly via a tungsten electrode (impedance 30–150 kΩ; manufactured from 0.2 mm wire, electrolytically sharpened and insulated except for the very tip) with the silver reference electrode in contact with one of the neck muscles on the right side. Single 0.2 ms rectangular stimuli and trains of two to four stimuli at 330–400 Hz were used at intensities up to 60 μA. The intensity and the number of stimuli were selected to ensure that they induced submaximal indirectly evoked volleys and just detectable muscle twitches.
EMG responses were recorded using a silver ball electrode (∼1 mm) in contact with a twitching neck muscle against a larger electrode touching other muscles. When choosing the recording site the preference was given to negative–positive EMG potentials with the onset of the negative phase not exceeding 2 ms from the effective stimulus and thus compatible with monosynaptic or disynaptic activation of neck motoneurons by reticulospinal or rubrospinal neurons (Wilson & Yoshida, 1969, Peterson et al. 1978); see first section of Results. However, EMG potentials were also picked up by electrodes aimed to record descending volleys from the surface of the spinal cord. They were usually triphasic and reflected compound rather than unitary muscle activity. Those that were more synchronous and more stable than potentials recorded from the surface of the muscle were therefore sometimes easier to quantify.
The descending volleys were recorded with one electrode in contact with the intact dura mater over the C1–2 segments and the reference electrode in contact with neck or back muscles. Collection of any fluid that would decrease the stability of the recording was prevented by a cotton wool wig.
Both single records and averages of 20–50 successive records were stored on-line (with the time resolution of 30 μs per address) and were analysed off-line using software for sampling and analysis developed by E. Eide, T. Holmström and N. Pihlgren (University of Gothenburg).
Transcranial polarization was applied via a sponge (about 6 mm × 8 mm), soaked with saline attached with agar-agar to the skull (1–2 mm from the midline at the level of bregma on the right side), against a larger sponge attached to the left ear lobe, the lower jaw, or the chest via a crocodile clip. The 0.2 mA current intensities used corresponded to about 4–5 μA mm−2. They exceeded the stimulus intensity of 0.3 μA mm−2 used in humans (1 mA over 35 cm2) but were within the range of intensities that were used in the original acute experiments on anaesthetized rats (0.25 or 10 μA mm−2; Bindman et al. 1964) or cats (1 or 2.5 μA mm−2; Bolzoni et al. 2013), awake rabbits or cats (about 10 μA mm−2; Morrell, 1961), awake rats (about 30 μA mm−2; Liebetanz et al. 2006; Laste et al. 2012) or unanaesthetized decerebrate cats (30–80 μA mm−2; Purpura & McMurtry, 1965). As in the previous study in the cat (Bolzoni et al. 2013), the reason for using higher current intensities than in studies in humans was that the density of current within the target area at depth drops significantly when the size of the electrode is decreased (Miranda et al. 2009; Paulus, 2011). Using a smaller polarization area but aiming at subcortical regions several millimetres below cortex would thus require the use of higher current intensities than in humans.
Polarization was applied during 5 min periods alternating with 5 min periods which will be referred to as ‘post-polarization periods’, repeated 5–7 times, taking into account that effects of tDCS on descending volleys in the cat were sometimes detected only after 4–5 such periods (Bolzoni et al. 2013) and that intermittent stimuli are more effective than stimuli applied for longer periods (see Paulus, 2011).
Analysis
Effects on compound EMG potentials and descending volleys were estimated by comparing averaged (n= 20) potentials evoked by MLF stimuli prior to, during, or after tDCS. The areas of early components of these potentials were quantified in arbitrary units, within time windows between the onset and the peak of the potentials, as indicated in Figs 2, 3 and 6, and were expressed as a percentage of control areas. Whenever EMG responses were evoked in single motor units (in an all-or-none fashion) the effects of tDCS on these unitary responses were estimated from changes in their latency and firing index. Differences between data sets were assessed for statistical significance by Student's t test or ANOVA (using Statistica 5.1 StatSoft or SigmaPlot 12.5 Systat software Inc. and following the recommendations of Drummond & Vowler, 2011).
Figure 2. Examples of EMG responses evoked from the MLF.
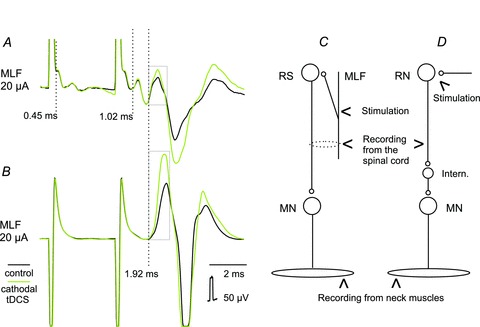
A, records from the surface of the spinal cord at C1–C2 level illustrating potentials evoked by double 20 μA stimuli. Averages of 20 traces. B, simultaneously obtained records from neck muscles.The three dotted vertical lines indicate the most likely onset of the direct volleys after the first stimulus, the indirect volleys evoked by the 2nd stimulus and EMG potentials evoked by these stimuli, respectively. Boxes indicate the time windows within which the areas of the early components of the EMG responses were measured. C, diagram of the most direct connections between fibres stimulated in the MLF and motoneurons (MN) innervating neck muscles. It takes into account <0.5 ms latencies of direct volleys in axons of reticulospinal (RS) neurons, <1 ms additional delays of indirect volleys and <1 ms latencies of EMG responses with respect to the indirect volleys evoked by the second stimulus (none appearing after the first stimulus) which would not leave time for additional relay neurons. D, similar diagram of the most direct connections between neurons stimulated in the red nucleus (RN) and motoneurons innervating neck muscles. The ∼1.5 ms longer latencies of EMG responses would require one or two additional synaptic delays, related to transsynaptic and not direct activation of rubrospinal neurons and their trans-interneuronal, and not direct, actions on neck motoneurons.
Figure 3. Effects of tDCS on descending volleys evoked from the MLF and the RN.
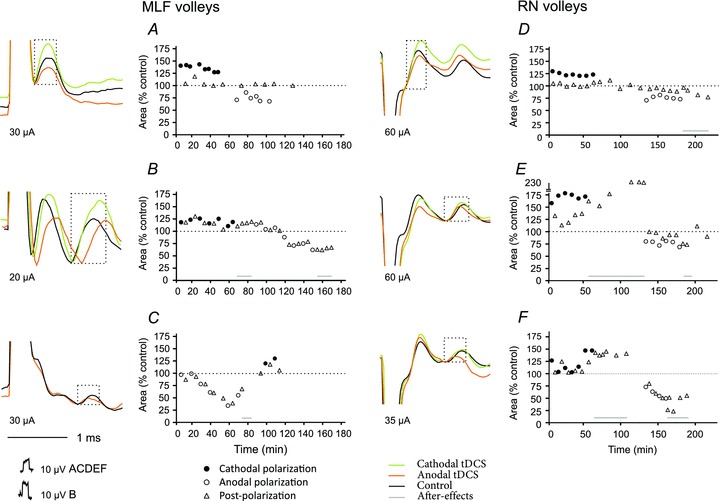
Examples of direct (A and D) and indirect (B, C, E and F) volleys recorded at C1–C2 spinal levels following MLF or RN stimulation, as indicated. Averages of 20 records during maximal effects of tDCS. Plots show changes in the areas of the volleys in multiples or fractions of areas of control volleys (ordinate indicated by horizontal dotted lines) in successive tests (abscissa) in six experiments. The areas were measured within the indicated time windows (boxes), during tDCS and during periods following tDCS as indicated. Note facilitation occurring during cathodal polarization and depression during anodal polarization. Note also that both facilitation and depression of indirect volleys often outlasted periods of tDCS application. Statistically significant differences were found between descending volleys evoked during cathodal or anodal polarization and control data in all tests illustrated in A–F, except for effects of anodal tDCS on direct volleys from RN and cathodal tDCS on direct volleys from MLF (see Table 1).
Results
Tests used to evaluate effects of tDCS
The effects of tDCS were evaluated on four kinds of potentials evoked by electrical stimuli applied in the MLF or RN. These included: (i) compound EMG potentials recorded from deep neck muscles, (ii) large potentials recorded from the surface of the spinal cord corresponding to those recorded from the muscles, (iii) single motor unit EMG responses, and (iv) direct and indirect descending volleys recorded from the C1–C2 segments. EMG potentials evoked from the muscles (illustrated in Fig. 2B) provided the most direct measure of any changes in the excitability of motoneurons. However, records from the surface of the spinal cord (illustrated in Fig. 2A) often included large potentials evoked at a very similar latency (see the third dotted vertical line in Fig. 2). These potentials were present only in preparations in which neuromuscular transmission was intact and disappeared when it was blocked by pancuronium bromide. They thus reflected activity in either the same muscle from which the EMG was recorded or in neighbouring muscles. A particular advantage with these potentials was that they allowed the onset of the EMG potentials to be related to the descending volleys that preceded them and hence assess the synaptic coupling in the pathways activated by MLF and RN stimuli. They also provided the means to select the earliest and most synchronous potentials evoked in a given experiment for the analysis.
Figure 2 illustrates the most commonly encountered effects of stimuli applied within the MLF, EMG potentials being evoked following the second but not the first stimulus. The earliest components of EMG potentials associated with muscle contractions were evoked at latencies of 1.5–2.0 ms from the second stimulus and were delayed by <1 ms from the indirect volleys (2nd vertical dotted line in Fig. 2A). The synaptic delay between reticulospinal fibres and neck motoneurons located in C1–C3 segments would be about 0.5 ms with respect to the arrival of nerve impulses in these fibres, the conduction time from motoneurons to neck muscles would be no more than 0.2 ms and the delay of the initiation of propagated action potentials in neck muscle fibres about 0.3 ms. In total these delays would add about 1 ms to the latencies of the indirect descending volleys and would leave no time for synaptic actions relayed by additional neurons. They would accordingly define the earliest EMG potentials as being most likely the result of monosynaptic actions of reticulospinal tract fibres on motoneurons, or to disynaptic actions evoked via indirectly activated reticulospinal neurons, as indicated in Fig 2C. The effects of tDCS on EMG potentials, or on EMG-related spinal cord potentials, should therefore be exerted either on MLF fibres or on reticulospinal neurons, current spread to motoneurons being unlikely. The earliest EMG responses evoked by RN stimuli appeared at latencies about 1.5 ms longer (3.4–3.8 ms from the effective stimuli; Fig. 3) than those evoked from the MLF and were less synchronous. They are therefore likely to be relayed by one or two additional neurons as indicated in Fig. 2D. Indirect activation of rubrospinal neurons by stimuli applied in RN would require one supplementary synaptic delay but the additional delay might also be related to the interneuronally, and not directly, mediated synaptic actions of rubrospinal neurons on neck motoneurons.
Comparison of effects of cathodal and anodal tDCS on descending volleys
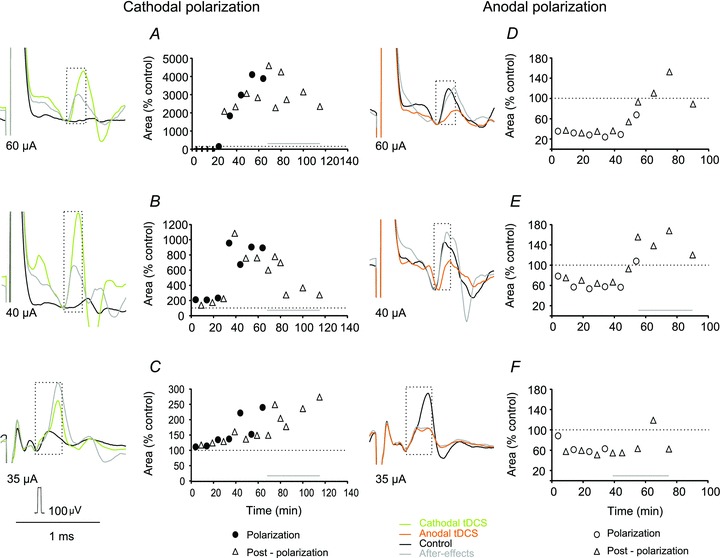
Figure 2A shows that descending volleys of a critical amplitude (like those after the 2nd stimulus) were needed for EMG potentials to be evoked and it was expected that tDCS would affect EMG potentials in parallel with the volleys. However, effects of tDCS on the descending volleys were detectable in only some experiments. Measurements of direct volleys from the MLF were usually precluded by the overlap between these volley and stimulus artefacts, while indirect volleys were often distorted by EMG responses which either preceded or followed them. Hence the effects of tDCS could only be examined on a small sample of descending volleys: five direct and nine indirect volleys. The effects of tDCS were nevertheless consistent in showing that facilitation of all of these volleys was evoked by cathodal polarization and depression by anodal polarization, in contrast to facilitation evoked under similar conditions by anodal polarization in the cat (Bolzoni et al. 2013).
Direct volleys were facilitated or depressed primarily during on-going tDCS (Fig. 3A and D). The facilitation amounted up to 129–175% of control areas during cathodal tDCS and the depression to 64–68% of control areas during anodal tDCS. Changes in indirect volleys were similar, or more marked, but longer lasting (Fig. 3B, C, E and F). The facilitation amounted to 180–214% during cathodal tDCS and the depression to 23–36% during anodal tDCS (for details see Table 1). Whenever tested, they outlasted the last period of polarization by at least 20 min to 1 h. Facilitation of four indirect volleys was associated with shortening of their latencies, and depression of two volleys with latency lengthening (Fig. 3B), even though both changes were of only about 0.1–0.2 ms. Facilitation of indirect descending volleys of subcortical origin evoked in the rat by cathodal tDCS thus showed the same features as the facilitation evoked by anodal tDCS found in the cat (Bolzoni et al. 2013) while the depression was evoked by anodal tDCS.
Table 1.
Degree of facilitation and depression of descending volleys and EMG responses evoked from the MLF and RN by cathodal and anodal tDCS
| Direct volleys | ||||
|---|---|---|---|---|
| MLF | P | RN | P | |
| Cathodal | 129 ± 21%, n= 52 | ** | 103 ± 12%, n= 68 | 0.253 |
| Anodal | 87 ± 15%, n= 12 | 0.106 | 87 ± 11%, n= 39 | ** |
| Indirect volleys | ||||
|---|---|---|---|---|
| MLF | P | RN | P | |
| Cathodal | 124 ± 22%, n= 63 | ** | 131 ± 26%, n= 170 | ** |
| Anodal | 76 ± 22%, n= 46 | ** | 81 ± 22%, n= 73 | ** |
| EMG | ||||
|---|---|---|---|---|
| MLF | P | RN | P | |
| Cathodal | 165 ± 82%, n= 167 | ** | 796 ± 1114%, n= 154 | ** |
| Anodal | 59 ± 38%, n= 149 | ** | 70 ± 34%, n= 118 | ** |
Average areas of direct and indirect volleys and of EMG potentials as a percentage of control areas ± standard deviation, and number of analysed records n. **P < 0.01; Mann–Whitney rank sum test for data with non-normal distribution.
Comparison of effects of anodal and cathodal tDCS on EMG responses
EMG responses evoked by near-threshold stimuli were affected in all of the rats in a similar way to the descending volleys, being facilitated by cathodal tDCS and depressed by anodal tDCS. In Fig. 4 these effects are illustrated on responses of single motor units, in Fig. 5 on compound EMG responses evoked from MLF and in Fig. 6 on compound EMG responses evoked from RN.
Figure 4. Opposite effects of anodal and cathodal transcranial polarization on MLF-evoked unitary EMG responses.
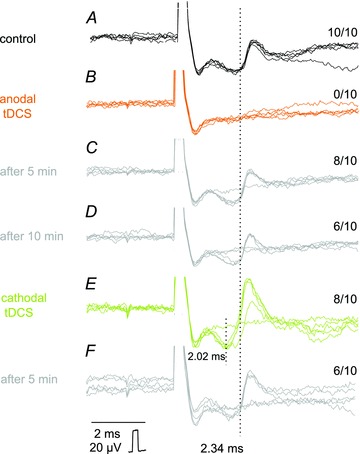
A–F, individual records from a neck muscle evoked by single 30 μA stimuli (five superimposed records). These were gathered before tDCS (control), during the positive or the negative tDCS and at the end of the following 5 min periods, as indicated to the left. All-or-none appearance of these potentials indicates that they most likely represented EMG responses of single motor units (in A–D) or two motor units (in E and F) and the rate of their appearance could therefore be counted (figures to the right are for 10 single records of the unit in A). It can be seen that positive tDCS prevented activation of EMG responses evoked at latencies of about 2.34 ms and the negative tDCS ensured a regular appearance of these responses and in addition activation of two other motor units, one at the same and another at a shorter latency. Dotted lines indicate the onset of the shorter and longer EMG potentials. Note that after the end of tDCS the responses tended to return to the approximately pre-tDCS level. In this and the following figures the negativity is upward and the largest shock artefacts are truncated.
Figure 5. Opposite effects of transcranial cathodal and anodal polarization on compound EMG responses evoked from MLF.
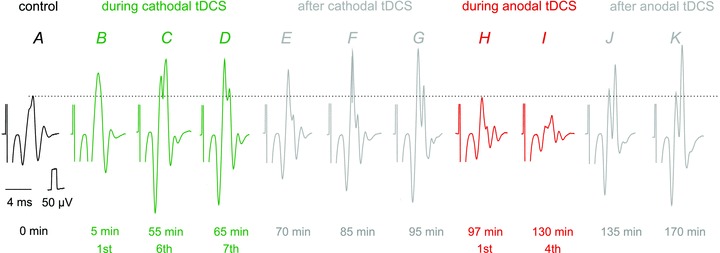
Series of EMG potentials evoked in neck muscles by stimuli applied in the MLF (30 μA). The potentials were evoked by the 3rd stimulus in a train, the earlier parts of the records being cropped off-line. They include EMG potentials evoked before tDCS (A), during three 5 min periods of cathodal tDCS (B–D) alternating with after-polarization periods, during the following 30 min (E–G), during two 5 min periods of anodal tDCS (H and I), commencing immediately after records in G and during the following post-stimulation period of 40 min (J and K). The timing of the records is indicated with respect to the beginning of the first period of cathodal polarization. The horizontal dotted line indicates the amplitude of the control responses for comparison between effects of cathodal and anodal tDCS. It will be noted that the moderate facilitation by the first cathodal tDCS was enhanced during and after successive tDCS periods and that it was counteracted by anodal tDCS but with the return to the facilitated responses following termination of tDCS.
Figure 6. Opposite effects of cathodal and anodal tDCS on compound EMG potentials evoked from RN and the timing of these effects.
A–F, examples of RN-evoked muscle EMG responses recorded from neck muscles before, during and after tDCS, and changes in the areas of these potentials. Changes in areas within the indicated time windows (boxed; ordinate) are plotted for 5 min periods during the total of about 2 h (abscissa). They are expressed as a percentage of the areas of control responses taken as 100%, indicated by the horizontal dotted lines, except for A where the area of EMG potentials appearing during the third polarization period was used for control, as no measurable EMG responses were seen before this period. Horizontal lines above abscissa indicate periods during which facilitatory effects of tDCS outlasted periods of tDCS application. Statistically significant differences were found between EMG responses evoked during cathodal or anodal polarization and control data in all tests illustrated in A–F (see Table 1).
EMG responses which appeared in an all-or-none fashion following minute changes in the stimulus intensity were classified as evoked in single motor units (Fig. 4C and D). As seen in Fig. 4B MLF-evoked responses present in panel A disappeared during anodal tDCS and reappeared, during the successive 10 min, albeit at a lower firing index. Cathodal tDCS following the anodal tDCS resulted in a further increase in the firing index (with less failures in panel E) but also in the appearance of responses of two motor units which were not activated under control conditions: one at the same latency and one at a shorter latency. The earliest responses disappeared during the subsequent 5 min (panel F) but the later ones remained. Similar effects of tDCS on responses of single motor units were seen in four experiments.
Changes in compound EMG potentials (Figs 5–7) were less dramatic than in unitary potentials, but always marked. Records in Fig. 5 illustrate these effects on EMG potentials evoked by the third stimulus of a train of three stimuli applied in the MLF. Records B, C and D show that the facilitation increased during successive 5 min periods of cathodal polarization, with the increase in the earliest components and the appearance of the second components. After the last cathodal tDCS (panel E) the facilitation was somewhat weaker but it was enhanced rather than reduced after 20 and 30 min (panels F and G), the final effect exceeding even that during the final cathodal stimulation (D). H and J show opposite and similarly accruing effects following anodal polarization, with the decrease in the EMG response as compared to responses evoked before (A) and during (B–D) cathodal polarization. Finally an unexpected increase occurred during 25 min following the last anodal polarization. As seen in J and K, the EMG responses which were evoked then exceeded not only the control responses but also those evoked during cathodal polarization; this might suggest that the previously evoked facilitation was only temporarily counteracted by anodal polarization.
Figure 7. Earliest effects of anodal and cathodal tDCS on MLF-evoked compound EMG potentials.
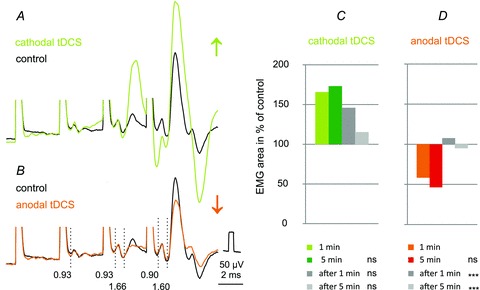
A and B, averaged records (n= 20) from the surface of the spinal cord in the C1 segment of responses evoked by 25 μA stimuli applied in the MLF. The facilitation was evoked during the first minute of cathodal tDCS and depression during the first minute of anodal tDCS. Vertical dotted lines indicate latencies of the indirect volleys (0.93 ms) and of the most likely disynaptically evoked responses from neck motoneurons (1.6 ms). It will be noted that cathodal tDCS not only enhanced EMG responses following the 4th stimulus but also advanced activation of the muscle (by the third rather than the fourth stimulus). C and D, mean areas of the early parts of EMG potentials (n= 14) recorded from neck muscles or from the surface of the spinal cord during the first and fifth minutes of application of tDCS and during the first and fifth minutes after the end of tDCS. The areas are expressed as a percentage of areas of control EMG potentials. Note in C that substantial increases had occurred already during the first minute of polarization and were not much stronger after 5 min. Note also that the facilitation outlasted the first 5 min period of cathodal tDCS by 1 min but to a much smaller extent by 5 min. However, no statistically significant differences were found between the mean areas of potentials recorded during the first minute of tDCS and later on (Kruskal–Wallis one-way ANOVA on ranks, n= 20, H= 7.1, d.f. = 3, P= 0.070). In D both after-effects of anodal tDCS were statistically significantly smaller than those during the first minute (one-way ANOVA, n= 14, F(3,44)= 10.626, P < 0.001). ***Significant differences with respect to records obtained during the first minute (P < 0.05, post hoc test, Holm–Sidak method); ns, not significant.
Stimuli applied in RN evoked EMG potentials in only 5 of 8 experiments. Nevertheless, all of these EMG potentials were consistently facilitated by cathodal polarization (Fig. 6A–C) and depressed by anodal polarization (Fig. 6D–F). The degree of facilitation by tDCS greatly depended on the test responses. Increases of the two largest compound EMG potentials were within the same range as that of MLF-evoked EMG potentials, with a maximal effect up to about 200% of control (Fig. 6C). In contrast, increases of two smaller EMG potentials and of the potential that appeared primarily only after tDCS were 10- to 40-fold (Fig. 6A and B). Plots of tDCS effects in individual experiments (Fig. 6A–F) may thus be more informative than pooled data (Fig. 8C and D).
Figure 8. Time course of effects of tDCS on EMG responses.
A and B, areas of EMG potentials (ordinate) evoked from MLF during or after 5 min periods of cathodal (n= 12) and anodal (n= 11) tDCS, respectively, for the total of about 2 h (abscissa). In most experiments effects of cathodal and anodal tDCS were examined subsequently but the order of polarization was reversed in every second experiment. The areas are expressed as multiples or fractions of the areas of control responses. C and D as in A and B except for the EMG potentials (C: n= 5; D: n= 5) evoked from RN for all of which cathodal tDCS was applied before anodal tDCS. Horizontal lines above abscissa indicate periods of outlasting effects of tDCS. *Statistically significant differences between areas of EMG responses evoked during, or after tDCS and of control responses (Holm–Sidak or Dunn's method post hoc analysis, P < 0.05). For further details see Table 2.
Timing of effects of transcranial polarization on EMG responses evoked from the MLF
Records illustrated in Figs 4, 5 and 6 show that tDCS not only altered the effectiveness of muscle activation evoked by reticulospinal and rubrospinal neurons but also that at least some effects of tDCS outlasted the duration of the polarization. This indicates that the effects of tDCS are not restricted to transient changes in the excitability of the tested neurons but reflect longer-lasting modulation of activation of these neurons.
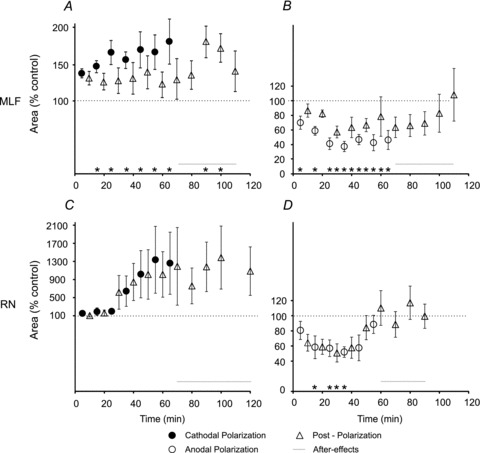
The increases or decreases of test responses evoked from the MLF had most often commenced already during the first minute of cathodal or anodal polarization but pooled data for the whole sample show that the effects were only slightly stronger after 5 min than after 1 min (Fig. 7C and D), the difference not being statistically significant. Furthermore, the facilitation evoked by the first period of polarization outlasted the tDCS for hardly more than 1 min and the early depression appeared to be only transient.
Repeated tDCS gradually enhanced the effects on EMG responses evoked from the MLF. Maximal effects most often developed during the 3rd to 5th periods of polarization, i.e. after 15–25 min of intermittent polarization, or within about 30–50 min from the beginning of the first sequence. Examples for these accruing effects are shown in Figs 5 and 6, and the data for the whole sample in Fig. 8. Facilitation of responses evoked by cathodal tDCS immediately following post-polarization periods was generally weaker but was found in 11/12 experiments. The degree of facilitation was at about the same level as after the first period of polarization but increased after the last tDCS. Mean responses exceeded control responses during the whole post-polarization period of testing in all experiments (up to 30–40 min) even though only some of the differences were found to be statistically significant. Significant differences were found between responses evoked before and during cathodal polarization and during post-polarization periods. Depressive effects of anodal tDCS likewise outlasted the duration of the polarization in all experiments but lasted more than 15–20 min in only 7/11 experiments. Significant differences were found between responses evoked before and during but not after anodal polarization (for details see Tables 1 and 2). The depression appeared also to decline faster than the facilitation. The decline of the facilitation during the period after the last tDCS was seen both when the anodal tDCS was applied after and before the cathodal tDCS so that the residual effects of cathodal facilitatory actions could not be the only explanation of this decline.
Table 2.
Analysis of pooled data for effects of tDCS on EMG responses summarized in Fig. 8
| Polarization | Post-polarization | After-polarization | |
|---|---|---|---|
| MLF | |||
| Cathodal (n= 12) | H= 29.69, d.f. = 8, P < 0.001 | H= 8.36, d.f. = 8, P= 0.399 | H= 21.61, d.f. = 4, P < 0.001 |
| Anodal (n= 10) | F(7,71)= 9.768, P < 0.001 | H= 23.89, d.f. = 7, P= 0.001 | H= 8.08, d.f. = 4, P= 0.089 |
| RN | |||
|---|---|---|---|
| Cathodal (n= 5) | H= 11.36, d.f. = 7, P= 0.123 | H= 12.03, d.f. = 7, P= 0.099 | H= 13.78, d.f. = 5, P= 0.017 |
| Anodal (n= 5) | F(6,27)= 2.898, P= 0.029 | F(6,24)= 3.289, P= 0.017 | F(3,16)= 0.537, P= 0.663 |
Data for changes in the areas of EMG responses evoked during 5 min periods of application of cathodal and anodal tDCS, during 5 min post-polarization periods and during post-polarization periods following the last polarization. ANOVA one-way analysis of variance was used for data with normal distribution and equal variance (F, value; P, value) while Kruskal–Wallis one-way analysis of variance on ranks was used for data with non-normal distribution (H, value; d.f., degrees of freedom; P, value). n, sample size.
The timing of tDCS effects from RN was similar to that from MLF. In all five experiments only minimal or negligible facilitation was evoked during the first three periods of cathodal polarization and the main increase of the EMG potentials became apparent during 4th to 7th periods or after a total of 20–35 min of tDCS (Figs 6A–C and 8C), i.e. only slightly later than facilitation of EMG potentials evoked from MLF. Residual effects of tDCS were observed up to 1 h after the termination of the last period of tDCS though they were not found to be statistically significant. In contrast to facilitation, the depression evoked by anodal tDCS reached a near-maximum level (50–60%) already during the first or the second period of polarization but declined faster once the polarization was completed. In only one of the five experiments (Fig. 6F) did it outlast the final tDCS. These decreases were found to be significant during but not after polarization (for details see Tables 1 and 2). The duration of the effects of anodal tDCS might, however, have been shortened by after-effects of cathodal polarization which in this series of experiments always preceded anodal polarization (see Fig. 5J and K).
Control experiments
All of the results reported above were found in both rat strains used (Sprague–Dawley and Wistar) and no indications were found for differences related to age, weight or sex. All the data were therefore pooled together and in control experiments we focused on the differences between effects of anodal and cathodal tDCS in the rat and in the cat. In an attempt to find an explanation for these differences we tried in particular to relate them to the spatial configuration between the focal and reference electrodes in the rat. Considering that the differences between the responses in the cat and rat might be related to differences in the orientation of subcortical neurons in electric fields in the much smaller rat brain, in two experiments the electric fields during tDCS were altered by placing the reference electrode in contact with the left side of the skull, the left ear lobe, the lower jaw or the chest, while maintaining the same position of the focal electrode over the bregma (on the right side). In both experiments in which the effects of tDCS on EMG responses evoked from the MLF at these different electrode locations were compared, depressive effects of anodal tDCS and facilitatory effects of cathodal tDCS were independent of the space relationships between the focal and reference electrodes. This is illustrated with records obtained at three placements of the reference electrode in the same experiment in Fig. 9.
Figure 9. Qualitatively similar effects of tDCS at different locations of the reference electrode.
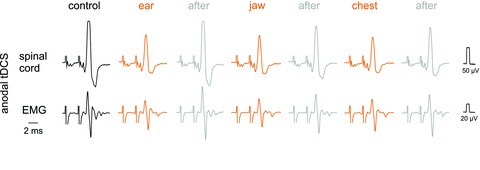
Upper records in each panel are from the surface of the spinal cord in the C1 segment (as in Fig. 5) while lower records are from a neck muscle (as in Fig. 4). The responses were evoked by 27 μA stimuli applied in the MLF. Anodal tDCS was applied through an electrode on the right side of the skull just rostral to the bregma with the reference electrode in contact with the left ear lobe, the lower jaw, or the chest, as indicated. Note that all responses evoked during tDCS were smaller than those evoked either before or after the tDCS periods. The illustrated responses were evoked by the 3rd stimulus, the earlier parts of the records and the shock artefacts having been cropped off.
No systematic analysis of effects of cathodal tDCS was previously performed in the cat, although the depression by cathodal tDCS was found by Bolzoni et al. (2013) in two experiments in which cathodal tDCS followed the anodal tDCS. In further support of differences between effects of anodal and cathodal tDCS in the cat and the rat we have also found depression of indirect volleys from the MLF by cathodal tDCS under conditions when its application in the cat was not preceded by anodal tDCS (M. Baczyk, F. Bolzoni & E. Jankowska, unpublished observations).
Discussion
As indicated in the Introduction, the main aim of the present study was to investigate functional consequences of the relatively modest facilitation of activation of subcortical neurons found in the previous study on cats under neuromuscular blockade. The effects of tDCS in cats consisted of shortening of latencies of descending volleys by only 0.1–0.2 ms and/or of increases in their amplitude by on average 120–160% (Bolzoni et al. 2013) but the degree to which these changes might result in facilitation of motor reactions could not be predicted. The currently reported results show that changes in the amplitude and/or latencies of descending volleys following MLF and RN stimuli in the rat are similarly weak but the effects of tDCS on EMG responses are much more marked. These results therefore indicate that even weak modulation of descending volleys by tDCS (Bolzoni et al. 2013) might have as strong an impact on spinal target cells of rubrospinal, reticulospinal and vestibulospinal neurons in the cat and humans as on neck motoneurons found in the rat in this study. The effects of tDCS on subcortical neurons should thus substantially add to the effects of tDCS on cortical neurons.
We attribute the reported effects of MLF and RN stimulation on neck muscles to actions of reticulospinal and rubrospinal neurons, respectively, having generalized estimates of the effects of such stimuli from the cat to the rat. These estimates were originally based on the measurements showing that 20–50 μA stimuli would have an effect within a radius of only about 0.2–0.5 mm (Gustafsson & Jankowska, 1976). Stimuli applied at locations indicated in Fig. 1A are thus unlikely to have induced action potentials in cells or fibres outside the neighbouring regions of the reticular formation and the contralateral MLF, at least not in the cases of stimuli at the 10 more ventral locations. Stimuli applied at locations indicated in Fig. 1B should similarly primarily activate rubrospinal neurons, or fibres providing input to them. This contention was based not only on estimates of the effective spread of current but also on similar spacial distribution of thresholds of descending volleys with characteristics of volleys evoked from the RN in cats (Baldissera et al. 1972) and rats (Al-Izki et al. 2008). These volleys were evoked at the lowest thresholds from within the nucleus and at higher thresholds (or at smaller amplitudes) at electrode positions only a fraction of a millimetre more dorsal (Fig. 1C) or more lateral.
The arguments for attributing the earliest components of EMG responses analysed in this study to either monosynaptic or disynaptic actions of reticulospinal and disynaptic or trisynaptic actions of rubrospinal neurons on neck motoneurons are summarized in the first section of Results. It could be pointed out here that the most sensitive to the effects of tDCS were most likely the disynaptically or trisynaptically evoked EMG responses that did, to a great extent, depend on temporal facilitation. They required two to three stimuli to appear, were linked to the last stimulus and were facilitated or depressed in a graded manner. The only exceptions were unitary EMG responses illustrated in Fig 3 and possibly those following the 3rd stimulus in Fig. 5.
Facilitation by cathodal tDCS and depression by anodal TDCS
In contrast to the predominantly facilitatory effects of anodal tDCS in humans and cats, subcortical effects of anodal tDCS in the rat were found to be depressive while facilitation was evoked by cathodal tDCS. Opposite effects of anodal polarization on subcortical neurons in the cat and in the rat might be related to several factors. One of these would be the differences in the geometry and size of the brain in the two species and the resulting differences in the current flow during tDCS (for recent discussion of effects of transcranially applied current see Fenrich et al. 2012). Subcortical effects of tDCS in the rat were nevertheless qualitatively similar independently of the location of the reference electrode, whether it was in contact with the contralateral part of the skull, ear lobe, ventral part of the neck, or chest.
It may also be relevant for this issue that some of the previously reported effects of anodal tDCS in the rat were facilitatory, especially those involving changes at the level of the cortex. Thus, anodal tDCS was found to improve rat working memory (de Souza Custódio et al. 2012), increase propagation velocity of the cortical spreading depression (Liebetanz et al. 2006), or increase the cerebral blood flow, while cathodal tDCS resulted in a decrease, both for up to about 30 min (Wachter et al. 2011). Anodal tDCS was also concluded to contribute to the recovery of motor functions after cerebrovascular injury in the rat (Kim et al. 2010). A closer inspection of the results of Kim et al. (their Figs 2–4) nevertheless suggests that some aspects of improvement of motor performance after cerebrovascular injury in their study were in fact as good, or even better after cathodal than after anodal tDCS.
Previously reported effects of polarizing current applied to the surface of the brain are furthermore not as uniform as often referred to. Results of the earliest studies on animals revealed for instance that anodal polarization attenuates, or eliminates surface positive waves and enhances surface negative components of cortical evoked potentials, while cathodal polarization has opposite effects. This was found in the rat together with an enhancement in activity of individual cortical neurons recorded at the same depth as the negative phase of the evoked potentials by positive polarization and a decrease by negative polarization (Bindman et al. 1964). Different components of evoked potentials in humans were also found to be differently affected by tDCS. While anodal tDCS applied over the temporal cortex increased amplitudes of some components of responses to acoustic stimuli, cathodal tDCS over the temporoparietal cortex increased other components (Zaehle et al. 2011). Cells of most likely the same category but different location might likewise be differently affected. Creutzfeldt et al. (1962) reported that while most cortical neurons in the cat were activated by inward currents (surface positive) and inhibited by outward currents (surface negative), neurons located in one of the sulci at a depth exceeding 3 mm often demonstrated the opposite effect (Creutzfeldt et al. 1962). Similarly, Lang et al. (2005) noted that in contrast to other human cortical areas, cathodal tDCS rather than anodal tDCS increased movement-related activity in the left dorsal premotor cortex (Lang et al. 2005).
The facilitatory effects of cathodal rather than anodal tDCS on reticulospinal and rubrospinal neurons in the rat may thus not be as singular and odd as they might appear, even if the reasons for differences of tDCS on cat and rat subcortical neurons are still undefined. In order to elucidate these reasons much more should be known about the mechanisms and sites of actions of tDCS at each particular experimental protocol. For instance, for primarily presynaptic tDCS actions, the excitability of long tract fibres stimulated within the MLF might depend on the relative orientation of the DC current vector with respect to these fibres, as in the in vitro preparation of Kabakov et al. (2012) to a greater extent than the excitability of presynaptic interpositorubral fibre terminals stimulated within the red nucleus in close proximity to rubrospinal neurons. Effects of tDCS related to changes in synaptic transmission, postsynaptic membrane receptors or protein synthesis might on the other hand be more related to the orientation of dendrites, soma and initial parts of the axons of reticulospinal and rubrospinal neurons with respect to the electric fields induced by tDCS, as it was found to be to the somadendritic axis of hippocampal pyramidal cells in which somatic polarization was induced by DC current in the preparations of Bikson et al. (2004) and Ranieri et al. (2012). The same authors also show that presynaptic and postsynaptic effects of tDCS do not need to depend on its polarity in strictly the same way and that they may vary depending on the orientation of neurons and their axons in different parts of the explored nuclei.
With respect to positron emission tomography (PET) of regional cerebral blood flow (rCBF) in humans, it might be relevant that the degree of increases and decreases in rCBF during anodal or cathodal tDCS applied over the motor cortex differed between various cortical and subcortical regions. With respect to RN and the mesencephalic and/or pontine reticular nuclei, which are of particular interest in the context of our studies, an inspection of Figs 3 and 4 in Lang et al. (2005) strongly suggests that rCBF in these structures is increased by anodal tDCS (see Fig. 10 in Bolzoni et al. 2013). It would therefore be of great interest to examine whether cathodal tDCS evokes similar changes in rCBF in corresponding brain regions in the rat.
Long-lasting effects
Some of the effects of tDCS found in this study appeared almost immediately while other effects seemed to develop fairly slowly. In some cases, especially EMG responses evoked from the MLF, facilitation or depression appeared during the first minute, or at least during the first period of application of the polarizing current. Such early effects of tDCS might thus be considered to be at odds with the slowly developing facilitation of descending volleys found previously (Bolzoni et al. 2013). The differences might be species related, as the previous study was performed on cats and the present one on rats, or attributable to the opposite polarity of the effective tDCS in these species. However, the early effects of tDCS in the rat were not a general rule and in several cases both facilitation and depression developed as slowly as in the cat. This was in particular the case for effects of tDCS on all EMG potentials evoked from RN in the rat, weakening the probability of the species difference. We therefore favour the explanation that the earlier expression of tDCS effects on EMG than on descending volleys primarily depends on a higher sensitivity of changes in EMG responses as a measure of activation of reticulospinal neurons.
The increasingly stronger facilitatory and depressive effects on EMG potentials during successive periods of tDCS appear to replicate the time course of slower developing effects of tDCS on descending volleys in the cat. Both might thus be compatible with the accumulation of some effects on the background of previously consolidated changes which outlasted the previous periods of tDCS application. Even when EMG responses evoked after a given tDCS period did not remain as large as those evoked during this period, they changed roughly in parallel with responses evoked during tDCS application. In addition the slowly accumulating changes occurring after the successive tDCS periods seemed to develop according to their own pace as they sometimes continued to increase during 1 or 2 h. The accruing of the after-effects of tDCS was more distinct after the number of tDCS periods increased, in keeping with observations that the duration of after-effects of tDCS in humans depends on the number and length of tDCS applications (Monte-Silva et al. 2010, Fricke et al. 2011).
As judged by the fairly large differences in effects of tDCS in individual experiments, the development and the degree of facilitation and depression might depend on a number of factors, including alterations in the excitability of motoneurons, some general effects of tDCS, or the level of anaesthesia, although these differences were not related to changes in heart rate nor any noticable activation of other muscles. Some differences could also be related to after-effects of previously applied tDCS of opposite polarity, because cathodal tDCS seemed to have weaker effects after a series of anodal polarizations and vice versa. In view of this complex situation it is difficult to interpret the mechanisms of the long-lasting effects before they are experimentally ‘dissected’ and analysed one by one. As indicated by Bolzoni et al. (2013), the effects of tDCS may involve various aspects of activation of subcortical neurons, including excitability of presynaptic elements and transmitter release, actions on postsynaptic membrane receptors, or the excitability of postsynaptic neurons. We may therefore hope that future studies either restrict the number of these possibilities or, on the contrary, show that all of these have to be considered and provide a means for their selective examination.
Acknowledgments
We wish to thank Drs Lars-Gunnar Petterson, Gerta Vrbova and Ingela Hammar for comments on preliminary versions of this paper and Jytte Grännsjö for excellent technical assistance during the experiments and the histological control.
Glossary
- MLF
medial longitudinal fascicle
- RN
red nucleus
- RS
reticulospinal
- tDCS
transcranial direct current stimulation
Additional information
Competing interests
None.
Author contributions
The experiments were performed at the Department of Physiology, University of Gothenburg. All authors contributed to the design of the experiments as well as to the collection, analysis and interpretation of the data and to the drafting of the article, and all approved the final version of the manuscript.
Funding
This work was supported by the National Institutes of Health (grant number R01 NS040863 to E.J.).
References
- Al-Izki S, Kirkwood PA, Lemon RN, Enriquez Denton M. Electrophysiological actions of the rubrospinal tract in the anaesthetised rat. Exp Neurol. 2008;212:118–131. doi: 10.1016/j.expneurol.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Baldissera F, Lundberg A, Udo M. Stimulation of pre- and postsynaptic elements in the red nucleus. Exp Brain Res. 1972;15:151–167. doi: 10.1007/BF00235579. [DOI] [PubMed] [Google Scholar]
- Bikson M, Inoue M, Akiyama H, Deans JK, Fox JE, Miyakawa H, Jefferys JG. Effects of uniform extracellular DC electric fields on excitability in rat hippocampal slices in vitro. J Physiol. 2004;557:175–190. doi: 10.1113/jphysiol.2003.055772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindman LJ, Lippold OC, Redfearn JW. The action of brief polarizing currents on the cerebral cortex of the rat (1) during current flow and (2) in the production of long-lasting after-effects. J Physiol. 1964;172:369–382. doi: 10.1113/jphysiol.1964.sp007425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggio PS, Rigonatti SP, Ribeiro RB, Myczkowski ML, Nitsche MA, Pascual-Leone A, Fregni F. A randomized, double-blind clinical trial on the efficacy of cortical direct current stimulation for the treatment of major depression. Int J Neuropsychopharm. 2008;11:249–254. doi: 10.1017/S1461145707007833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolzoni F, Pettersson L-G, Jankowska E. Evidence for longlasting subcortical facilitation by transcranial direct current stimulation (tDCS) in the cat. J Physiol. 2013;591:3381–3399. doi: 10.1113/jphysiol.2012.244764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunoni AR, Fregni F, Pagano RL. Translational research in transcranial direct current stimulation (tDCS): a systematic review of studies in animals. Rev Neurosci. 2011;22:471–481. doi: 10.1515/RNS.2011.042. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Nitsche MA, Bolognini N, Bikson M, Wagner T, Merabet L, Edwards DJ, Valero-Cabre A, Rotenberg A, Pascual-Leone A, Ferrucci R, Priori A, Boggio PS, Fregni F. Clinical research with transcranial direct current stimulation (tDCS): Challenges and future directions. Brain Stimul. 2012;5:175–195. doi: 10.1016/j.brs.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutzfeldt OD, Fromm GH, Kapp H. Influence of transcortical d-c currents on cortical neuronal activity. Exp Neurol. 1962;5:436–452. doi: 10.1016/0014-4886(62)90056-0. [DOI] [PubMed] [Google Scholar]
- de Souza Custódio JC, Martins CW, Valladão Lugon MD, Fregni F, Nakamura-Palacios EM. Epidural direct current stimulation over the left medial prefrontal cortex facilitates spatial working memory performance in rats. Brain Stimul. 2012;6:261–269. doi: 10.1016/j.brs.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Ziemann U, Lemon RN. State of the art: Physiology of transcranial motor cortex stimulation. Brain Stimul. 2008;1:345–362. doi: 10.1016/j.brs.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in the Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond GB, Vowler SL. Data interpretation: using probability. J Physiol. 2011;589:2433–2435. doi: 10.1113/jphysiol.2011.208793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenrich KK, Weber P, Hocine M, Zalc M, Rougon G, Debarbieux F. Long-term in vivo imaging of normal and pathological mouse spinal cord with subcellular resolution using implanted glass windows. J Physiol. 2012;590:3665–3675. doi: 10.1113/jphysiol.2012.230532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricke K, Seeber AA, Thirugnanasambandam N, Paulus W, Nitsche MA, Rothwell JC. Time course of the induction of homeostatic plasticity generated by repeated transcranial direct current stimulation of the human motor cortex. J Neurophysiol. 2011;105:1141–1149. doi: 10.1152/jn.00608.2009. [DOI] [PubMed] [Google Scholar]
- Ginanneschi F, Del Santo F, Dominici F, Gelli F, Mazzocchio R, Rossi A. Changes in corticomotor excitability of hand muscles in relation to static shoulder positions. Exp Brain Res. 2005;161:374–382. doi: 10.1007/s00221-004-2084-x. [DOI] [PubMed] [Google Scholar]
- Gustafsson B, Jankowska E. Direct and indirect activation of nerve cells by electrical pulses applied extracellularly. J Physiol. 1976;258:33–61. doi: 10.1113/jphysiol.1976.sp011405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabakov AY, Muller PA, Pascual-Leone A, Jensen FE, Rotenberg A. Contribution of axonal orientation to pathway-dependent modulation of excitatory transmission by direct current stimulation in isolated rat hippocampus. J Neurophysiol. 2012;107:1881–1889. doi: 10.1152/jn.00715.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Kim B, Ko Y, Bang M, Kim M, Han T. Functional and histologic changes after repeated transcranial direct current stimulation in rat stroke model. J Korean Med Sci. 2010;25:1499–1505. doi: 10.3346/jkms.2010.25.10.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang N, Siebner HR, Ward NS, Lee L, Nitsche MA, Paulus W, Rothwell JC, Lemon RN, Frackowiak RS. How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain. Eur J Neurosci. 2005;22:495–504. doi: 10.1111/j.1460-9568.2005.04233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laste G, Caumo W, Adachi LN, Rozisky JR, de Macedo IC, Filho PR, Partata WA, Fregni F, Torres IL. After-effects of consecutive sessions of transcranial direct current stimulation (tDCS) in a rat model of chronic inflammation. Exp Brain Res. 2012;221:75–83. doi: 10.1007/s00221-012-3149-x. [DOI] [PubMed] [Google Scholar]
- Liebetanz D, Klinker F, Hering D, Koch R, Nitsche MA, Potschka H, Loscher W, Paulus W, Tergau F. Anticonvulsant effects of transcranial direct-current stimulation (tDCS) in the rat cortical ramp model of focal epilepsy. Epilepsia. 2006;47:1216–1224. doi: 10.1111/j.1528-1167.2006.00539.x. [DOI] [PubMed] [Google Scholar]
- Miranda PC, Faria P, Hallett M. What does the ratio of injected current to electrode area tell us about current density in the brain during tDCS. Clin Neurophysiol. 2009;120:1183–1187. doi: 10.1016/j.clinph.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monte-Silva K, Kuo MF, Liebetanz D, Paulus W, Nitsche MA. Shaping the optimal repetition interval for cathodal transcranial direct current stimulation (tDCS) J Neurophysiol. 2010;103:1735–1740. doi: 10.1152/jn.00924.2009. [DOI] [PubMed] [Google Scholar]
- Morrell F. Effect of anodal polarization on the firing pattern of single cortical cells. Ann N Y Acad Sci. 1961;92:860–876. doi: 10.1111/j.1749-6632.1961.tb40962.x. [DOI] [PubMed] [Google Scholar]
- Paulus W. Transcranial electrical stimulation (tES – tDCS; tRNS, tACS) methods. Neuropsychol Rehabil. 2011;21:602–617. doi: 10.1080/09602011.2011.557292. [DOI] [PubMed] [Google Scholar]
- Peterson BW, Pitts NG, Fukushima K, Mackel R. Reticulospinal excitation and inhibition of neck motoneurons. Exp Brain Res. 1978;32:471–489. doi: 10.1007/BF00239548. [DOI] [PubMed] [Google Scholar]
- Purpura DP, McMurtry JG. Intracellular activities and evoked potential changes during polarization of motor cortex. J Neurophysiol. 1965;28:166–185. doi: 10.1152/jn.1965.28.1.166. [DOI] [PubMed] [Google Scholar]
- Ranieri F, Podda MV, Riccardi E, Frisullo G, Dileone M, Profice P, Pilato F, Di Lazzaro V, Grassi C. Modulation of LTP at rat hippocampal CA3-CA1 synapses by direct current stimulation. J Neurophysiol. 2012;107:1868–1880. doi: 10.1152/jn.00319.2011. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. Neuroscientist. 2011;17:37–53. doi: 10.1177/1073858410386614. [DOI] [PubMed] [Google Scholar]
- Wachter D, Wrede A, Schulz-Schaeffer W, Taghizadeh-Waghefi A, Nitsche MA, Kutschenko A, Rohde V, Liebetanz D. Transcranial direct current stimulation induces polarity-specific changes of cortical blood perfusion in the rat. Exp Neurol. 2011;227:322–327. doi: 10.1016/j.expneurol.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Wilson VJ, Yoshida M. Comparison of effects of stimulation of Deiters’ nucleus and medial longitudinal fasciculus on neck, forelimb, and hindlimb motoneurons. J Neurophysiol. 1969;32:743–758. doi: 10.1152/jn.1969.32.5.743. [DOI] [PubMed] [Google Scholar]
- Zaehle T, Beretta M, Jancke L, Herrmann CS, Sandmann P. Excitability changes induced in the human auditory cortex by transcranial direct current stimulation: direct electrophysiological evidence. Exp Brain Res. 2011;215:135–140. doi: 10.1007/s00221-011-2879-5. [DOI] [PubMed] [Google Scholar]


